Abstract
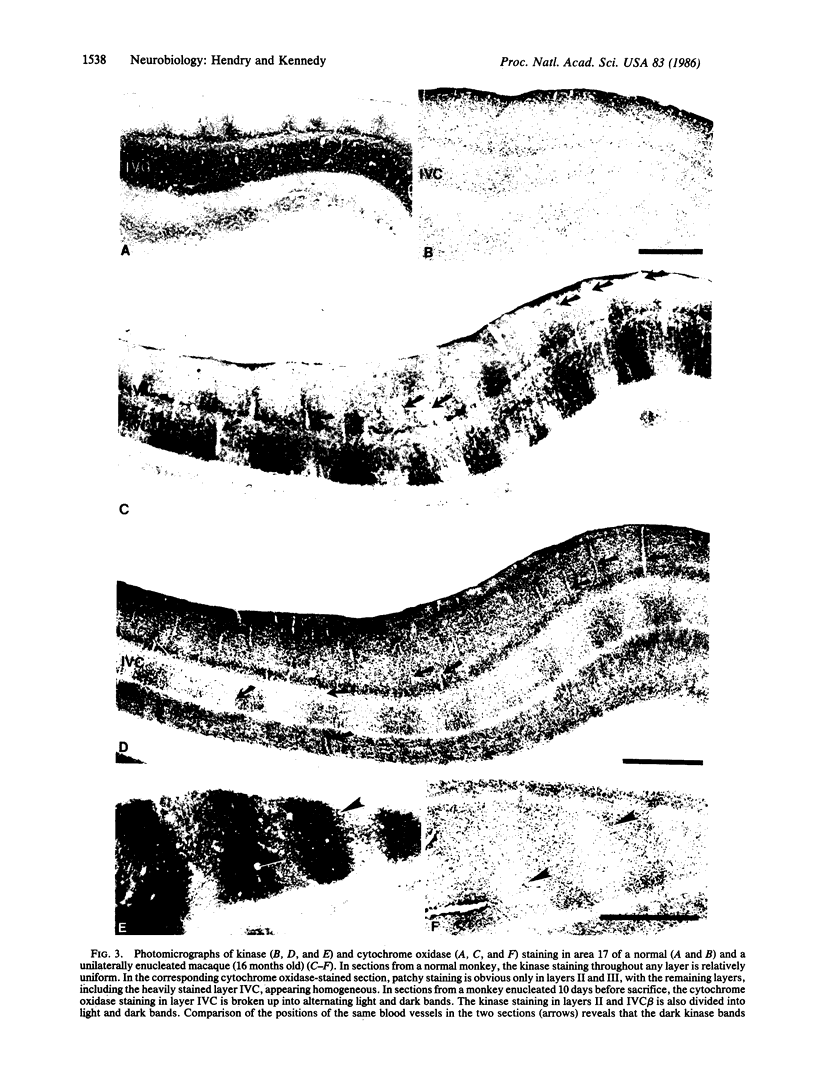
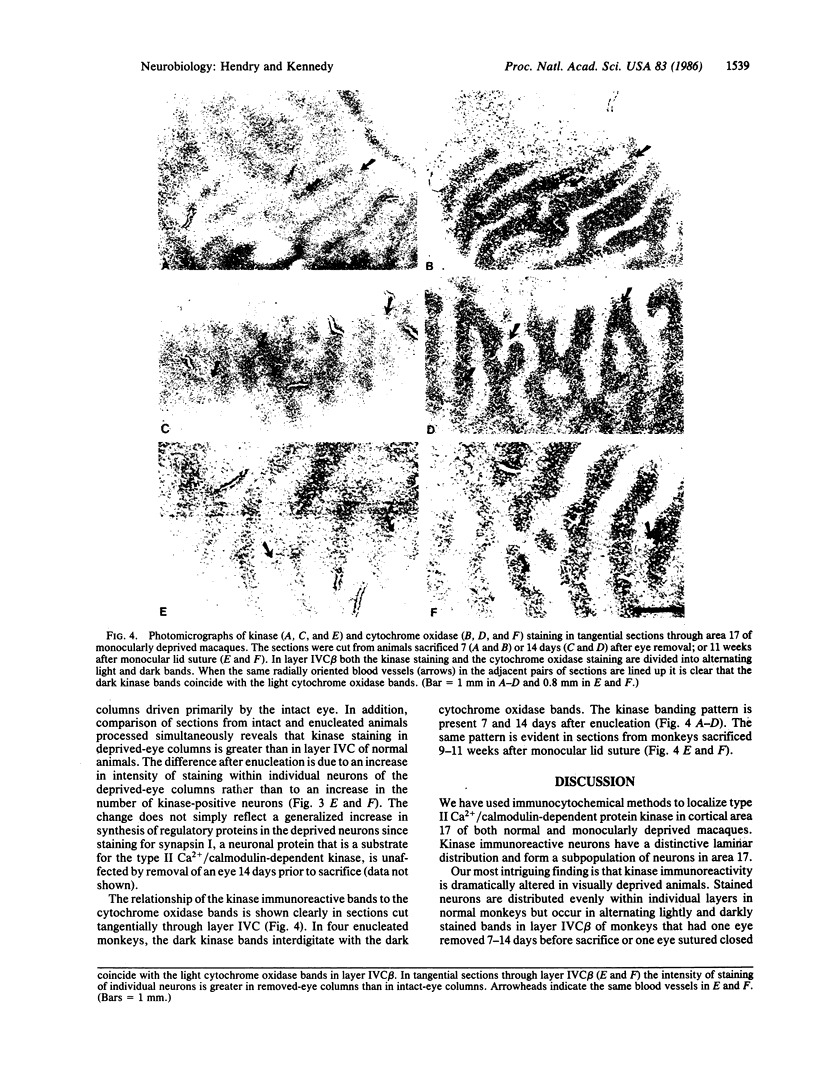
Immunocytochemical methods were used to localize type II Ca2+/calmodulin-dependent protein kinase in the macaque primary visual cortex. Neurons that stain for the kinase include both pyramidal and nonpyramidal cells and they appear to form a subset of cortical neurons. They are densely packed in layers II and IVB, somewhat more sparse in layers III, IVC beta, and VI, and nearly absent in layer V. In normal animals the distribution of kinase-positive cells within each layer is relatively uniform. However, in animals in which one eye is removed 7-14 days before sacrifice or sutured shut for 9 or 11 weeks, the cells in layer IVC beta are divided into alternating lightly and darkly stained bands. Comparison of immunocytochemically stained sections with adjacent sections stained for the mitochondrial enzyme, cytochrome oxidase, reveals that the kinase staining increases in ocular dominance columns originally driven by the removed or closed eye. These findings suggest that either the concentration of type II Ca2+/calmodulin-dependent protein kinase or its accessibility to the antibody probe increases dramatically and selectively in neurons of macaque primary visual cortex that have been deprived of their normal visual input. This may indicate that changing levels of activity in cortical neurons can alter their regulatory machinery.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Technical considerations on the use of horseradish peroxidase as a neuronal marker. Neuroscience. 1977;2(1):141–145. doi: 10.1016/0306-4522(77)90074-4. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Erondu N. E., Kennedy M. B. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983 Oct 25;258(20):12735–12744. [PubMed] [Google Scholar]
- Black I. B., Adler J. E., Dreyfus C. F., Jonakait G. M., Katz D. M., LaGamma E. F., Markey K. M. Neurotransmitter plasticity at the molecular level. Science. 1984 Sep 21;225(4668):1266–1270. doi: 10.1126/science.6147894. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. The fate of axon terminals in visual cortex during trans-synaptic atrophy of the lateral geniculate nucleus. Brain Res. 1971 Nov;34(1):53–60. doi: 10.1016/0006-8993(71)90350-7. [DOI] [PubMed] [Google Scholar]
- Erondu N. E., Kennedy M. B. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985 Dec;5(12):3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote S. L., Morrison J. H. Postnatal development of laminar innervation patterns by monoaminergic fibers in monkey (Macaca fascicularis) primary visual cortex. J Neurosci. 1984 Nov;4(11):2667–2680. doi: 10.1523/JNEUROSCI.04-11-02667.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globus A., Scheibel A. B. Loss of dendrite spines as an index of pre-synaptic terminal patterns. Nature. 1966 Oct 29;212(5061):463–465. doi: 10.1038/212463a0. [DOI] [PubMed] [Google Scholar]
- Goldenring J. R., Gonzalez B., McGuire J. S., Jr, DeLorenzo R. J. Purification and characterization of a calmodulin-dependent kinase from rat brain cytosol able to phosphorylate tubulin and microtubule-associated proteins. J Biol Chem. 1983 Oct 25;258(20):12632–12640. [PubMed] [Google Scholar]
- Hendrickson A. E., Hunt S. P., Wu J. Y. Immunocytochemical localization of glutamic acid decarboxylase in monkey striate cortex. Nature. 1981 Aug 13;292(5824):605–607. doi: 10.1038/292605a0. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. E., Wilson J. R. A difference in [14C]deoxyglucose autoradiographic patterns in striate cortex between Macaca and Saimiri monkeys following monocular stimulation. Brain Res. 1979 Jul 13;170(2):353–358. doi: 10.1016/0006-8993(79)90113-6. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. E., Wilson J. R., Ogren M. P. The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in Old World and New World primates. J Comp Neurol. 1978 Nov 1;182(1):123–136. doi: 10.1002/cne.901820108. [DOI] [PubMed] [Google Scholar]
- Hendry S. H., Jones E. G., Emson P. C. Morphology, distribution, and synaptic relations of somatostatin- and neuropeptide Y-immunoreactive neurons in rat and monkey neocortex. J Neurosci. 1984 Oct;4(10):2497–2517. doi: 10.1523/JNEUROSCI.04-10-02497.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. C. Cytochrome oxidase patches: a new cytoarchitectonic feature of monkey visual cortex. Philos Trans R Soc Lond B Biol Sci. 1984 Jan 17;304(1119):199–253. doi: 10.1098/rstb.1984.0021. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Hubel D. H. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature. 1981 Aug 20;292(5825):762–764. doi: 10.1038/292762a0. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J Comp Neurol. 1972 Dec;146(4):421–450. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C., Des Rosiers M. H., Sakurada O., Shinohara M., Reivich M., Jehle J. W., Sokoloff L. Metabolic mapping of the primary visual system of the monkey by means of the autoradiographic [14C]deoxyglucose technique. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4230–4234. doi: 10.1073/pnas.73.11.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B. Experimental approaches to understanding the role of protein phosphorylation in the regulation of neuronal function. Annu Rev Neurosci. 1983;6:493–525. doi: 10.1146/annurev.ne.06.030183.002425. [DOI] [PubMed] [Google Scholar]
- Kosofsky B. E., Molliver M. E., Morrison J. H., Foote S. L. The serotonin and norepinephrine innervation of primary visual cortex in the cynomolgus monkey (Macaca fascicularis). J Comp Neurol. 1984 Dec 1;230(2):168–178. doi: 10.1002/cne.902300203. [DOI] [PubMed] [Google Scholar]
- LaVail J. H., LaVail M. M. The retrograde intraaxonal transport of horseradish peroxidase in the chick visual system: a light and electron microscopic study. J Comp Neurol. 1974 Oct 1;157(3):303–357. doi: 10.1002/cne.901570304. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Thalamic inputs to cytochrome oxidase-rich regions in monkey visual cortex. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6098–6101. doi: 10.1073/pnas.79.19.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., McGuinness T. L., Leonard C. S., Sugimori M., Greengard P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc Natl Acad Sci U S A. 1985 May;82(9):3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J. S., Lund R. D., Hendrickson A. E., Bunt A. H., Fuchs A. F. The origin of efferent pathways from the primary visual cortex, area 17, of the macaque monkey as shown by retrograde transport of horseradish peroxidase. J Comp Neurol. 1975 Dec 1;164(3):287–303. doi: 10.1002/cne.901640303. [DOI] [PubMed] [Google Scholar]
- Maunsell J. H., van Essen D. C. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983 Dec;3(12):2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Distinct forebrain and cerebellar isozymes of type II Ca2+/calmodulin-dependent protein kinase associate differently with the postsynaptic density fraction. J Biol Chem. 1985 Jul 25;260(15):9039–9046. [PubMed] [Google Scholar]
- Nestler E. J., Greengard P. Protein phosphorylation in the brain. Nature. 1983 Oct 13;305(5935):583–588. doi: 10.1038/305583a0. [DOI] [PubMed] [Google Scholar]
- Ouimet C. C., McGuinness T. L., Greengard P. Immunocytochemical localization of calcium/calmodulin-dependent protein kinase II in rat brain. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5604–5608. doi: 10.1073/pnas.81.17.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F. Structural changes in the area striata of the mouse after enucleation. Exp Brain Res. 1968;5(4):274–292. doi: 10.1007/BF00235903. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H., Lam D. M. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res. 1974 Oct 18;79(2):273–279. doi: 10.1016/0006-8993(74)90416-8. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979 Jul 27;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Purification and characterization of the brain calmodulin-dependent protein kinase (kinase II), which is involved in the activation of tryptophan 5-monooxygenase. Eur J Biochem. 1983 Apr 15;132(1):15–21. doi: 10.1111/j.1432-1033.1983.tb07319.x. [DOI] [PubMed] [Google Scholar]