Abstract
In this work we have fabricated hydrogen gas sensors based on undoped and 1 wt% multi-walled carbon nanotube (MWCNT)-doped tungsten oxide (WO3) thin films by means of the powder mixing and electron beam (E-beam) evaporation technique. Hydrogen sensing properties of the thin films have been investigated at different operating temperatures and gas concentrations ranging from 100 ppm to 50,000 ppm. The results indicate that the MWCNT-doped WO3 thin film exhibits high sensitivity and selectivity to hydrogen. Thus, MWCNT doping based on E-beam co-evaporation was shown to be an effective means of preparing hydrogen gas sensors with enhanced sensing and reduced operating temperatures. Creation of nanochannels and formation of p-n heterojunctions were proposed as the sensing mechanism underlying the enhanced hydrogen sensitivity of this hybridized gas sensor. To our best knowledge, this is the first report on a MWCNT-doped WO3 hydrogen sensor prepared by the E-beam method.
Keywords: WO3, hydrogen sensor, nanochannels, E-beam evaporation, carbon nanotube
1. Introduction
Hydrogen (H2) is one of the most useful gases, being used in many chemical processes and various industries including aerospace, medical, petrochemical, transportation, and energy [1–3]. In recent years, H2 has attracted a great deal of attention as a potential clean energy source for the next generation of automobiles and household appliances due to its perfectly clean combustion without any release of pollutants or greenhouse gases [4]. However, this low molecular weighted gas can easily leak out and may cause fires or explosions when its concentration in air is between 4% and 75% by volume [5]. Moreover, H2 is a colorless, odorless and tasteless gas that cannot be detected by human senses. Therefore, it is very essential to develop the effective H2 gas sensors for monitoring of H2 leaks.
Tungsten Oxide (WO3) is one of the most widely studied gas-sensing materials due to its fast, high sensitivity response toward NOx [6–9], H2S [10–13], C2H5OH [13,14] CO [15], NH3 [15–19] and O3 [20]. In case of H2 detection, it is well known that H2 molecules are not activated on the smooth WO3 surface of single crystals [21]. Addition of some noble metals such as Pt, Pd, or Au [22–26] to WO3 usually improves the sensitivity and selectivity to H2 gas. These metal doped WO3 films can be prepared by several methods, including screen printing [22], sputtering [23,24] and sol-gel process [25,26].
In the present work, multi-walled carbon nanotube (MWCNT)-doped WO3 thin films fabricated by an electron beam (E-beam) evaporation process and their application for H2 gas sensing are reported for the first time. The E-beam process offers extensive possibilities for controlling film structure and morphology with desired properties such as dense coating, high thermal efficiency, low contamination, high reliability and high productivity. MWCNTs were selected for doping because of their larger effective surface area, with many sites available to adsorb gas molecules, and their hollow geometry that may be helpful to enhance the sensitivity and reduce the operating temperature. Furthermore, MWCNTs were reported to be sensitive to H2, with good recovery times [27].
2. Experimental
2.1. Preparation of Materials
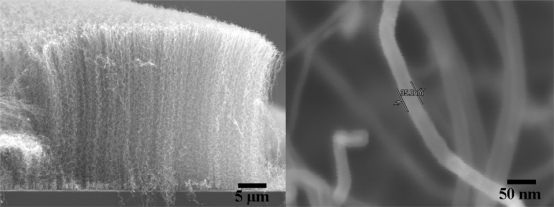
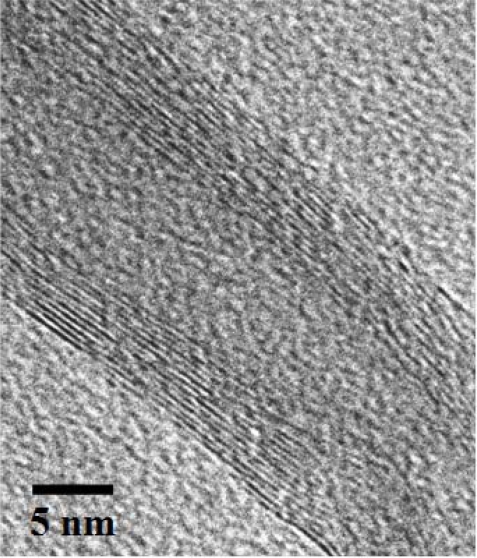
Commercial WO3 powder was obtained from Merck and used without further purification. MWCNTs were grown by the thermal chemical vapor deposition (CVD) process. The catalyst layer of aluminium oxide (10 nm) and stainless steel (5 nm) was deposited on the silicon (100) substrates (Semiconductor Wafer Inc.) using reactive sputtering apparatus. The synthesis of MWCNTs was performed under a flow of acetylene/hydrogen at a ratio of 3.6:1 at 700 °C for 3 min. To obtain high-purity MWCNTs, the water-assisted selective etching technique [28] was applied after each CNT’s growth stage. Water vapor (300 ppm) was introduced into the system by bubbling argon gas through liquid water at room temperature for 3 min. The sequence of acetylene/hydrogen and water vapor flows was repeated for five cycles. Based on the scanning electron microscopic (SEM) image, as shown in Figure 1, the diameter and length of the MWCNTs are ∼35 nm and ∼26 μm, respectively. The electrical conductivity of MWCNTs was ∼75 S/cm, as measured by a four-point probe method at room temperature. In addition, high-resolution transmission electron microscopic (HR-TEM) imaging, as shown in Figure 2, confirms that CNTs are multi-walled, with the width and number of walls being ∼4.6 nm and 14, respectively. Thus, the spacing between two graphitic layers is ∼0.33 nm, which is in good agreement with theoretical and experimental values.
Figure 1.
SEM images of the produced MWCNTs grown by the CVD process.
Figure 2.
High resolution TEM image of the produced MWCNT grown by the CVD process.
2.2. Fabrication of MWCNTs-doped WO3 Thin Film
MWCNT-doped WO3 thin film was fabricated by the E-beam evaporation technique onto Cr/Au interdigitated electrodes on an alumina substrate [29]. The target was prepared by mixing 99 wt% of WO3 powder with 1 wt% of MWCNT powder using a grinder in a mortar for 30 min and then pelletizing with a hydraulic compressor. Deposition was performed at a pressure of 5 × 10−6 Torr in the evaporation chamber. The substrate was rotated and kept at 130 °C during the deposition in order to obtain a homogeneous thin film. The deposition rate was 2 Å/sec and the final film thickness was 150 nm, as controlled by a quartz crystal monitor. After E-beam evaporation, the film was annealed at 500 °C for 3 h in air to stabilize the crystalline structure. In addition, an undoped WO3 thin film was also fabricated using the same conditions for comparison.
2.3. Measurement of Gas Sensing
To evaluate the gas sensing properties of the thus prepared thin films, MWCNT-doped WO3 and undoped WO3 gas sensors were placed inside a stainless steel chamber and the resistance measured using a 8846A Fluke multimeter with 6.5 digit resolution. The gas sensing measurements were made within a dynamic flow system with control of sensor operating temperatures (200–400 °C) under variable gas concentrations (100–50,000 ppm). Hydrogen (H2), ethanol (C2H5OH), methane (CH4), acetylene (C2H2), and ethylene (C2H4) were used to test the sensing properties and selectivity of the thin films. The sample gas flow time and the clean air reference flow time were fixed at 5 min and 15 min, respectively. It should be noted that these switching interval was selected so that the resistance change is at least 90% of the saturated value. The sensor resistances were sampled and recorded every second using LabVIEW with a USB DAQ device for subsequent analyses.
3. Results and Discussion
3.1. Characterization of Thin Films
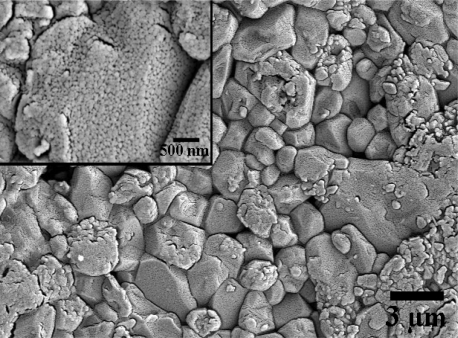
Surface morphology, particle size and crystalline structure of the films were characterized by SEM and TEM. Figure 3 shows the SEM surface morphology of MWCNT-doped WO3 thin film deposited on an alumina substrate. It was seen that the film coated on the rough alumina substrate has approximate grain sizes ranging from 40 to 80 nm.
Figure 3.
SEM image of MWCNT-doped WO3 thin films on alumina substrate.
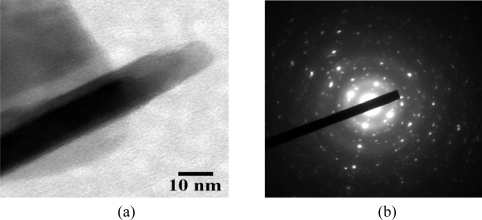
The nanometer grain size together with the roughness of the alumina substrate can enhance the gas sensitivity of thin films [30,31] because more gas adsorption sites are available due to the increased surface area and porosity. With the SEM resolution, CNT structure cannot be observed on the thin film surface. Therefore, TEM characterization was used to confirm CNT inclusion into the WO3 film. It should be noted that copper TEM grid samples were loaded inside the evaporation chamber for sample deposition at the same time as coating on the Cr/Au interdigitated electrodes. TEM observation clearly shows CNT inclusion into the nanocrystalline WO3, while the electron diffraction pattern exhibits polycrystalline phase in the film, as shown in Figure 4a,b, respectively.
Figure 4.
(a) High-resolution TEM image and (b) corresponding selected area diffraction pattern of MWCNT-doped WO3 thin film.
The film morphology obtained in our study is in accordance with observations on nanocrystalline WO3 films grown by other methods [32,33]. Doping of CNT does not change the phase or surface morphology of the film, but it may help form nanochannels in WO3 films, leading to the enhancement of the sensitivity and reduction of the operating temperature.
3.2. Sensing Properties of Thin Films
The sensor response (S) of the thin films is defined as the percentage of resistance change:
| (1) |
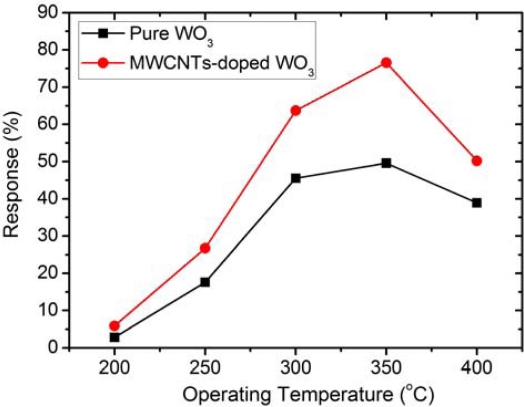
where R0 and R are the resistance of the thin films in pure air and test gas, respectively. Figure 5 shows the response of the undoped WO3 and MWCNT-doped WO3 thin films to 1,000 ppm H2 at varying operating temperatures. It can be seen that the response of the films increases as the operating temperature increases up to 350 °C, and then decreases. The gas-sensing response increases with temperature in the 200–350 °C range because thermal energy helps the reactions involved overcome their respective activation energy barriers [34,35]. However, if the operating temperature becomes too high (i.e., >350 °C), the adsorbed oxygen species at the sensing sites on the film surface will be diminished and less available to react with H2 molecules [36], thereby limiting the film’s response.
Figure 5.
Sensing response to H2 (1,000 ppm) at different operating temperatures.
At any operating temperature, the sensor response of the MWCNT-doped WO3 thin film is higher than that of the undoped WO3 thin film. Specifically, at the optimum operating temperature (350 °C), MWCNT-doped WO3 thin film yields a 26.9 % higher response than the undoped one. The doped sensor prepared in this work also shows higher response than the WO3 films prepared by the sol–gel process [25].
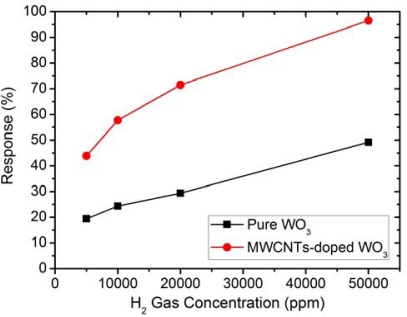
One major advantage of MWCNT-doped WO3 thin film is that the sensor can be operated at a lower operating temperature (250 °C), especially if this sensor is used to measure the H2 gas at higher concentrations (5,000–50,000 ppm). As shown in Figure 6, at such a concentration range, there are sufficient numbers of H2 molecules available to react with the surface oxygen adsorption sites. It is also well-known that MWCNTs contribute to the reduction of sensor resistance of metal oxides [37] and the activation energy between the WO3 surface and H2 gas. The details of the sensing mechanisms of MWCNT-doped WO3 thin films will be discussed in the next section.
Figure 6.
Sensing response of the undoped WO3 and MWCNT-doped WO3 thin films to high H2 concentrations (5,000–50,000 ppm) at the operating temperature of 250 °C.
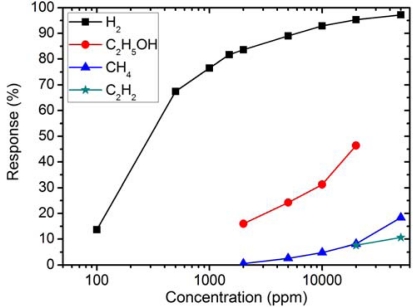
To demonstrate the selectivity of the MWCNT-doped WO3 thin film, its sensing response (at the operating temperature of 350 °C) to various gas vapors, namely H2, C2H5OH, CH4, and C2H2, was measured and plotted (Figure 7). It can be seen that MWCNT-doped WO3 thin film exhibits a strong response to H2, and much weaker responses to C2H5OH, CH4, and C2H2. In particular, this thin film was found to be insensitive to C2H4 at the optimum operating temperature of 350 °C. It is therefore concluded that the MWCNT-doped WO3 thin film exhibits high selectivity to H2.
Figure 7.
Sensing response of MWCNT-doped WO3 thin film at the operating temperature of 350 °C to various concentrations of different gas vapors.
3.3. Sensing Mechanism of MWCNTs-doped WO3 Thin Film
It is well known that WO3 is an n-type semiconductor while CNT is a p-type semiconductor. MWCNT-doped WO3 thin film can be either p-type or n-type semiconductors depending on the quantity of MWCNTs and the operating temperature [38]. In this work, the produced MWCNTs-doped WO3 thin film behaves as an n-type semiconductor since the electrical conductivity of the film increases when reducing gases, i.e., H2, are absorbed by its surface. Doping of MWCNTs into the WO3 matrix can introduce nanochannels and form p-n heterojunctions in the thin film. These nanochannels play an important role for gas diffusion. The gas molecules can easily transport into the gas-sensing layers leading to increasing sensitivity [39,40]. In addition, MWCNT-doped WO3 thin film p-n heterojunctions could be formed at the interface between WO3 and the MWCNTs [38,41]. When H2 gas is exposed to MWCNT-doped WO3 thin film, the widths of the depletion layers at the p-n heterojunctions can be modulated. The potential barriers at the interfaces or inside the WO3 may be changed. This change of the depletion layer in the p–n heterojunctions of MWCNT-doped WO3 thin film may explain the enhanced response of the film at low operating temperatures. Various oxygen species chemisorbed at the thin film surface such as O2−, O2−, and O− are available for catalytic reactions with H2, thus depending on the temperature at the metal oxide surface [42]. At the operating temperature range of 200–400 °C, O− is commonly chemisorbed. Consequently, the chemical reaction underlying the H2 gas sensing in this study is given by [43]:
| (2) |
The adsorbed O− on the thin film surface reacts with the H2 gas yielding H2O and releasing electrons which contribute to the current increase through the thin film that causes the electrical conductivity to increase.
4. Conclusions
MWCNT-doped WO3 thin film was successfully prepared by the E-beam evaporation technique. The 1 wt% MWCNT-doped WO3 thin film exhibits n-type semiconductor behavior of the polycrystalline phase. Doping with MWCNTs does not significantly change any phase or surface morphology of the film, but it introduces nanochannels and form p-n heterojunctions in the WO3 matrix. The MWCNT-doped WO3 thin film exhibits high selectivity and sensitivity to H2 over a relatively wide range of concentrations (100–50,000 ppm). Moreover, it can operate at a relatively low temperature. This should be useful for developing high performance H2 gas sensors. To our best knowledge, this is the first report on MWCNT-doped WO3 hydrogen sensors prepared by the E-beam method.
Acknowledgments
Mahidol University and the National Science and Technology Agency are gratefully acknowledged for supports of this research. C.W. acknowledges the Commission on Higher Education for a Ph.D. scholarship under the program “Strategic Scholarships for Frontier Research Network”. T.K. expresses his great gratitude to the Thailand Research Fund (BRG5180023) for a research career development grant.
References
- 1.Korotcenkov G, Han SD, Stetter JR. Review of electrochemical hydrogen sensors. Chem. Rev. 2009;109:1402–1433. doi: 10.1021/cr800339k. [DOI] [PubMed] [Google Scholar]
- 2.Moriarty P, Honnery D. Hydrogen's role in an uncertain energy future. Int. J. Hydrogen. Energ. 2009;34:31–39. [Google Scholar]
- 3.Momirlan M, Veziroglu TN. The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet. Int. J. Hydrogen. Energ. 2005;30:795–802. [Google Scholar]
- 4.Árnason B, Sigfússon TI. Iceland—A future hydrogen economy. Int. J. Hydrogen. Energ. 2000;25:389–394. [Google Scholar]
- 5.Carcassi MN, Fineschi F. Deflagrations of H2-air and CH4-air lean mixtures in a vented multi-compartment environment. Energy. 2005;30:1439–1451. [Google Scholar]
- 6.Penza M, Tagliente MA, Mirenghi L, Gerardi C, Martucci C, Cassano G. Tungsten trioxide (WO3) sputtered thin films for a NOx gas sensor. Sens. Actuat. B Chem. 1998;50:9–18. [Google Scholar]
- 7.Wang X, Miura N, Yamazoe N. Study of WO3-based sensing materials for NH3 and NO detection. Sens. Actuat. B Chem. 2000;66:74–76. [Google Scholar]
- 8.Kim TS, Kim TB, Yoo KS, Sung GS, Jung HJ. Sensing characteristics of dc reactive sputtered WO3 thin films as an NOx gas sensor. Sens. Actuat. B Chem. 2000;62:102–108. [Google Scholar]
- 9.Sawicka KM, Prasad AK, Gouma PI. Metal oxide nanowires for use in chemical sensing applications. Sens. Lett. 2005;3:31–35. [Google Scholar]
- 10.Tao WH, Tsai CH. H2S sensing properties of noble metal doped WO3 thin film sensor fabricated by micromachining. Sens. Actuat. B Chem. 2002;81:237–247. [Google Scholar]
- 11.Frühberger B, Grunze M, Dwyer DJ. Surface chemistry of H2S-sensitive tungsten oxide films. Sens. Actuat. B Chem. 1996;31:167–174. [Google Scholar]
- 12.Hoel A, Reyes LF, Heszler P, Lantto V, Granqvist CG. Nanomaterials for environmental applications: Novel WO3-based gas sensors made by advanced gas deposition. Curr. Appl. Phys. 2004;4:547–553. [Google Scholar]
- 13.Ionescu R, Hoel A, Granqvist CG, Llobet E, Heszler P. Low-level detection of ethanol and H2S with temperature-modulated WO3 nanoparticle gas sensors. Sens. Actuat. B Chem. 2005;104:132–139. [Google Scholar]
- 14.Li X, Zhang G, Cheng F, Guo B, Chen J. Synthesis, characterization, and gas-sensor application of WO3 nanocuboids. J. Electrochem. Soc. 2006;153:133–137. [Google Scholar]
- 15.Xu Y, Tang Z, Zhang Z, Ji Y, Zhou Z. Large-scale hydrothermal synthesis of tungsten trioxide nanowires and their gas sensing properties. Sens. Lett. 2008;6:938–941. [Google Scholar]
- 16.Neri G, Micali G, Bonavita A, Ipsale S, Rizzo G, Niederberger M, Pinna N. Tungsten oxide nanowires-based ammonia gas sensors. Sens. Lett. 2008;6:590–595. [Google Scholar]
- 17.Llobet E, Molas G, Molinàs P, Calderer J, Vilanova X, Brezmes J, Sueiras JE, Correig X. Fabrication of highly selective tungsten oxide ammonia sensors. J. Electrochem. Soc. 2000;147:776–779. [Google Scholar]
- 18.Balázsia C, Wang L, Zayim EO, Szilágyid IM, Sedlackováe K, Pfeifera J, Tótha AL, Goumab PI. Nanosize hexagonal tungsten oxide for gas sensing applications. J. Eur. Ceram. Soc. 2008;28:913–917. [Google Scholar]
- 19.Wang U, Pfeifer J, Balazsi C, Gouma PI. Synthesis and sensing properties to NH3 of hexagonal WO3 metastable nanopowders. Mater. Manuf. Process. 2007;22:773–776. [Google Scholar]
- 20.Berger O, Hoffmann T, Fischer WJ, Melev V. Tungsten-oxide thin films as novel materials with high sensitivity and selectivity to NO2, O3, and H2S. Part II: Application as gas sensors. J. Mater. Sci.: Mater. Electron. 2004;15:483–493. [Google Scholar]
- 21.Aroutiounian V. Metal oxide hydrogen, oxygen, and carbon monoxide sensors for hydrogen setups and cells. Int. J. Hydrogen. Energ. 2007;32:1145–1158. [Google Scholar]
- 22.Ahmad A, Walsh J. Development of WO3-based thick-film hydrogen sensors. ECS Trans. 2006;3:141–152. [Google Scholar]
- 23.Ippolito SJ, Kandasamy S, Kalantar-zadeh K, Wlodarski W. Hydrogen sensing characteristics of WO3 thin film conductometric sensors activated by Pt and Au catalysts. Sens. Actuat. B Chem. 2005;108:154–158. [Google Scholar]
- 24.Hsu WC, Chan CC, Peng CH, Chang CC. Hydrogen sensing characteristics of an electrodeposited WO3 thin film gasochromic sensor activated by Pt catalyst. Thin Solid Films. 2007;516:407–411. [Google Scholar]
- 25.Fardindoost S, Iraji-zad A, Rahimi F, Ghasempour R. Pd doped WO3 films prepared by sol–gel process for hydrogen sensing. Int. J. Hydrogen. Energ. 2010;35:854–860. [Google Scholar]
- 26.Nakagawa H, Yamamoto N, Okazaki S, Chinzei T, Asakura S. A room-temperature operated hydrogen leak sensor. Sens. Actuat. B Chem. 2003;93:468–474. [Google Scholar]
- 27.Samarasekara P. Hydrogen and methane gas sensors synthesis of multi-walled carbon nanotubes. Chin. J. Phys. 2009;47:361–369. [Google Scholar]
- 28.Zhu L, Xiu Y, Hess DW, Wong CP. Aligned carbon nanotube stacks by water-assisted selective etching. Nano Lett. 2005;5:2641–2645. doi: 10.1021/nl051906b. [DOI] [PubMed] [Google Scholar]
- 29.Wongchoosuk C, Wisitsoraat A, Tuantranont A, Kerdcharoen T. Portable electronic nose based on carbon nanotube-SnO2 gas sensors and its application for detection of methanol contamination in whiskeys. Sens. Actuat. B Chem. 2010;147:392–399. [Google Scholar]
- 30.Ansari ZA, Ansari SG, Ko T, Oh JH. Effect of MoO3 doping and grain size on SnO2-enhancement of sensitivity and selectivity for CO and H2 gas sensing. Sens. Actuat. B Chem. 2002;87:105–114. [Google Scholar]
- 31.Lee DS, Nam KH, Lee DD. Effect of substrate on NO2-sensing properties of WO3 thin film gas sensors. Thin Solid Films. 2000;375:142–146. [Google Scholar]
- 32.Hussain OM, Swapnasmitha AS, John J, Pinto R. Structure and morphology of laser-ablated WO3 thin films. Appl. Phys. A: Mater. Sci. Process. 2005;81:1291–1297. [Google Scholar]
- 33.Ashrit PV. Dry lithiation study of nanocrystalline, polycrystalline and amorphous tungsten trioxide thin-films. Thin Solid Films. 2001;385:81–88. [Google Scholar]
- 34.Shinde VR, Gujar TP, Lokhande CD. LPG sensing properties of ZnO films prepared by spray pyrolysis method: Effect of molarity of precursor solution. Sens. Actuat. B Chem. 2007;120:551–559. [Google Scholar]
- 35.Zeng Y, Zhang T, Wang L, Kang M, Fan H, Wang R, He Y. Enhanced toluene sensing characteristics of TiO2-doped flowerlike ZnO nanostructures. Sens. Actuat. B Chem. 2009;140:73–78. [Google Scholar]
- 36.Sahay PP, Nath RK. Al-doped zinc oxide thin films for liquid petroleum gas (LPG) sensors. Sens. Actuat. B Chem. 2008;133:222–227. [Google Scholar]
- 37.Liang YX, Chen YJ, Wang TH. Low-resistance gas sensors fabricated from multiwalled carbon nanotubes coated with a thin tin oxide layer. Appl. Phy. Lett. 2004;85:666–668. [Google Scholar]
- 38.Bittencourt C, Felten A, Espinosa EH, Ionescu R, Llobet E, Correig X, Pireaux JJ. WO3 films modified with functionalised multi-wall carbon nanotubes: Morphological, compositional and gas response studies. Sens. Actuat. B Chem. 2006;115:33–41. [Google Scholar]
- 39.Sakai G, Matsunaga N, Shimanoe K, Yamazoe N. Theory of gas-diffusion controlled sensitivity for thin film semiconductor gas sensor. Sens. Actuat. B Chem. 2001;80:125–131. [Google Scholar]
- 40.Hieu NV, Duc NAP, Trung T, Tuan MA, Chien ND. Gas-sensing properties of tin oxide doped with metal oxides and carbon nanotubes: A competitive sensor for ethanol and liquid petroleum gas. Sens. Actuat. B Chem. 2010;144:450–456. [Google Scholar]
- 41.Wei BY, Hsu MC, Su PG, Lin HM, Wu RJ, Lai HJ. A novel SnO2 gas sensor doped with carbon nanotubes operating at room temperature. Sens. Actuat. B Chem. 2004;101:81–89. [Google Scholar]
- 42.Cheong HW, Lee MJ. Sensing characteristics and surface reaction mechanism of alcohol sensors based on doped SnO2. J. Ceram. Process. Res. 2006;7:183–191. [Google Scholar]
- 43.Lupan O, Ursaki VV, Chai G, Chow L, Emelchenko GA, Tiginyanu IM, Gruzintsev AN, Redkin AN. Selective hydrogen gas nanosensor using individual ZnO nanowire with fast response at room temperature. Sens. Actuat. B Chem. 2010;144:56–66. [Google Scholar]