Abstract
Background
It is possible that psychopathological differences exist between the restricting and bulimic forms of anorexia nervosa. We investigated localized differences of brain blood flow of anorexia nervosa patients using SPECT image analysis with statistic parametric mapping (SPM) in an attempt to link brain blood flow patterns to neurophysiologic characteristics.
Methods
The subjects enrolled in this study included the following three groups: pure restrictor anorexics (AN-R), anorexic bulimics (AN-BP), and healthy volunteers (HV). All images were transformed into the standard anatomical space of the stereotactic brain atlas, then smoothed. After statistical analysis of each brain image, the relationships among images were evaluated.
Results
SPM analysis of the SPECT images revealed that the blood flow of frontal area mainly containing bilateral anterior cingulate gyri (ACC) was significantly decreased in the AN-R group compared to the AN-BP and HV groups.
Conclusions
These findings suggest that some localized functions ofthe ACCare possibly relevant to the psychopathological aspects of AN-R.
Background
A number of studies have documented important and reliable differences between AN-R and AN-BP [1]. Restrictive patients are distinguished by the presence of interrelated psychological characteristics; a distortion of body image, misperception of internal sensations, and an underlying sense of ineffectiveness [2], or have a weight phobia based on the preoccupation to maintain a subpubertal body weight and to avoid weight gain [3].
Evidence from non-invasive techniques has encouraged the belief that modern neurobiological approaches can be used in the quest for understanding complex frontal cortical function. Positron emission tomography (PET) study has described relative regional hypometabolism in frontal and parietal area in the patients with anorexia nervosa [4]. A functional MRI study revealed that visual stimuli of high calorie foods increased regional cerebral blood flow (rCBF) in ACC, insula and paralimbic area of patients with anorexia nervosa [5]. Using the ROI (region of interest) we previously reported decreases at rest and increases after food intake in %change of blood flow over a wide range of the cerebral cortex, primarily the frontal lobes, in anorexic patients [6]. We have recently demonstrated that AN-BP subjects may show specific activation in right anterior cortical regions using the same ROI method [7].
It is, however, difficult to select ROIs and analyze large areas of the human brain using the ROI method of SPECT examinations. Contrary to this, the statistic parametric mapping (SPM) 96 method allows for a better ability to SPECT studies assessing brain functions in large brain areas, and may be as informative as PET or fMRI.
The SPM 96 method, which was developed to analyze focal changes inregional cerebral blood flow (rCBF), is an automated and objective approach. We apply this approach to SPECT image data sets, specifying the regional abnormality in rCBF in more detail.
Subjects and Methods
Seven female AN-R patients and seven female AN-BP patients without a history of substance abuse or dependency, all of whom met the DSM-4 criteria for these respective disorders, participated in the present study. All subjects had been free of all psychoactive medications for at least five days. Seven healthy and drug-free HV without brain lesions and without any history of psychiatric disorders such as eating, mood, or anxiety disorders, or schizophrenia, served as age and sex-matched controls. Written consent was obtained after the subjects had been informed about the radiation doses and the procedures involved. All subjects were right-handed and had no abnormal neurological findings. XCT scans revealed no mass lesions or any major structural differences among the patients. For ethical reasons, volunteers were not required to undergo XCT scans. Each SPECT examination was performed before breakfast. After antecubital vein acquisition, the subject was asked to close her eyes and maintain a restful state during the subsequent examination. PAO, which is developed as a technetium tracer for use in measuring cerebral blood flow, was intravenously injected as a bolus (555- 720 MBq). Characteristics of PAO include excellent blood-brain barrier permeability, peak levels of brain distribution within 5 minutes after intravenous injection, and maintenance of steady-state levels over a subsequent period of a several hours. The SPECT data were acquired once, at 5-25 min after injection of PAO, with a triple-head rotating gamma camera with fanbeam high-resolution collimators (PRISM3000, Shimazu co) 140 Kev +/- 10 % of a photo window having 90 projections with 360 degree rotation (128 x 128 matrix). Each scan was performed in order to obtain transverse images parallel to the intercommisural line (AC-PC line). SPM96 for unix was installed to PC/AT machine (Pentiumja <2> 350 MHz, Memory 256 M, OS: Linux) in which MATLAB 4.2c had been installed. All images were transmitted to this PC/AT machine via network. The SPECT data were transformed into a standard stereostatic space (PET temple: Boudig box MNI) and the images were smoothed with an isotropic Gaussion filter (FWHM: 12 mm). The stereotactically normalized regional CBF images were then adjusted for individual difference in global blood flow (images scaled to an overall meanfor CBF: 50 ml/100 g/min) using a proportional scaling. Finally, comparisons (t-statistics) between the control vs. AN-R and vs. AN-BP were performed on a voxel-by-voxel basis for all voxels common to all subjects. Statistics across the whole brain were displayed as Z scores. The subsets of voxels exceeding a threshold of p < 0.001 and the size of cluster > 550 voxels were displayed as a volume image rendered in three orthogonal projection.
Results
Table 1 summarizes clinical and physiological characteristics of the subjects. An analysis of variance (ANOVA) observed a number of significant differences between the AN-R, AN-BP and HV groups. The BMI and hematocrits in both the AN-R and AN-BP groups were significantly lower than those in the HV groups [F (2,18) = 35.38 and 9.37, p < 0.01: AN-R and AN-BP vs. HV, p < 0.05].
Table 1.
Clinical and physiological characteristics: (Mean +/- SD)
| AN-R (n = 7) | AN-BP (n = 7) | HV (n = 7) | |
| Body mass index | 12.8 +/- 2.1* | 14.5 +/- 1.3* | 20.0 +/- 1.4 |
| Hematocrit (%) | 39.0 +/- 3.1* | 37.4 +/- 2.6* | 42.8 +/- 1.3 |
| Blood Sugar (mg/dl) | 64.4 +/- 8.7 | 58.7 +/- 2.9 | 69.4 +/- 12.3 |
AN-R = restrictive anorexia nervosa patients
AN-BP = anorexia nervosa patients with binge/purge eating
HV = healthy volunteers
*Significantly different from the control group (p < 0.05), by ANOVA with Tukey-HSD
SPM analysis of SPECT images revealed that the blood flow of the bilateral anterior parts of cingulate gyri (ACC: Brodmann's areas 24) and parts of frontal regions (parts of areas 8, 9,10 and 32) were significantly decreased in the AN-R group in comparison with that in the HV (p < 0.05) (Figure 1). In comparison with the AN-BP group, SPM analysis showed significantly decreased areas within almostthe same bilateral anterior parts of the frontal lobes (Figure 2). On the other hand, comparing with the HV or AN-R group, SPM analysis found no significant change of rCBF in the AN-BP group.
Figure 1.
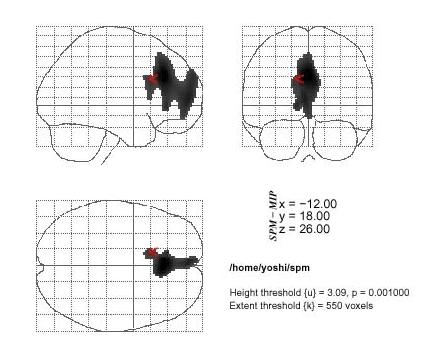
The statistical parametric mapping (SPM) of areas of decreased rCBF in the anorexia nervosa restricter group. Decreased of rCBF are shown in the bilateral anterior parts of cingulate gyri (ACC: Brodmann's areas 24) and parts of frontal regions (parts of areas 8, 9,10 and 32) when compared to the healthy controls. (p < 0.001, extent = 550)
Figure 2.
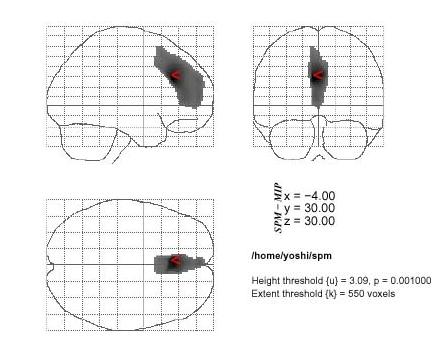
The statistical parametric mapping (SPM) of areas of decreased rCBF in the anorexia nervosa restricter group. Decreased of rCBF are also shown in the bilateral anterior parts of cingulate gyri (ACC: Brodmann's areas 24) and parts of frontal regions (parts of areas 8, 9,10 and 32) when compared to the anorexia nervosa patients with binge/purge eating (p < 0.001, extent = 550)
We compared adjusted rCBF among the three groups in the anterior frontal region, where the greatest reduction in rCBF (Z-scores) occurred, and found that the AN-R group showed the lowest rCBF value in this region (Figure 3).
Figure 3.
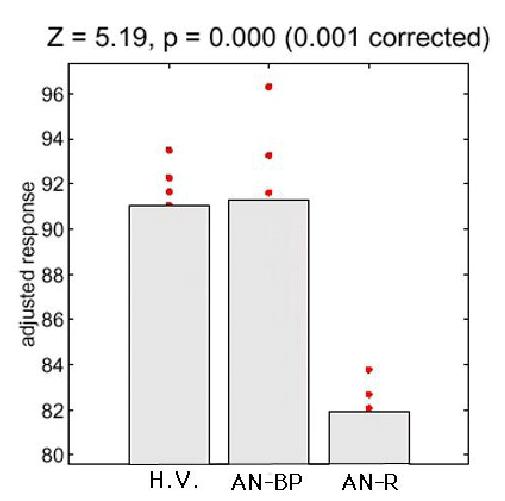
Comparison of mean values and distributions of adjusted rCBF (response) of the voxels where magnitude of Z-scores showed the greatest reduction in the anterior brain area. The AN-R group shows evidently the lowest values of adjusted rCBF among three groups.
The lack of difference in physiological characteristics (hematocrit and blood glucose levels) between the AN-R and AN-BP groups suggests that some alteration of brain function may be occuring in patients with AN-R.
Discussion
In the present study, we observed significant decreases of rCBF mainly in the bilateral ACC only in the AN-R group.Ina fMRI study, the AN group showed higher level of anxiety along with increased blood flow in response to the contrasting stimuli, especially in the left insula, ACC and left amygdala-hippocampal region [5]. These results provide evidence that increase in rCBF is associated with anxiety and in physiological arousal, and because the changes were caused merely by sight stimulus, there might be a disease specific response of the brain function. Therefore, this is possiblethat the ACC is one of the basic brainregions influencing eating behaviors of anorexic patients, though the subdivision of the subjects and brain imaging techniques are different from our study.
The ACC has been proposed to be an important component of frontal attention control systems. In afMRI study with elaborate task paradigms to investigate the cognitive process of selection in the ACC, the ACC was found to be activated by early selection following somatosenseory stimuli surely without subsequent motor acts [9]. In a series of PET experiments aimed to investigate the neural basis of emotion and feeling, the authors mentioned that emotions engaged structures related to the representation and/or regulations of organism state, such as the insular cortex, secondary somatosensory cortex, cingulated cortex and so on. In particular, in cingulate, insula and secondary somatosensory cortex, signals from brain stem and hypothalamus in addition to direct sensory signals from the organism are more refined and probably accessible to consciousness [10]. Since the ACC is reported to be very important in controlling a wide range of higher brain functions, the disturbance of this area might produce a rather serious damage to the perception/conception and emotion regulation in the patients with anorexia nervosa.
The present study showed that not only the ACC but also other parts of frontal regions had decreased rCBF. Lesions to the frontal regions including the ACC have often been reported to lead to disorders of mood regulation in human. Two rather distinct types of mood change following frontal lesions have been observed [11]. In animal experiments, it has been reported that the removal of the prefrontal cortex in monkeys produced monomaniacal tendencies when subjects were confronted by a variety of problem-solving and instrumental situations [12]. In a course of extinction and discrimination reversal tasks in monkeys, the perseverance of bar-pressing was selectively related to the frontal cortex [13].
Though the connections between the ACC and the prefrontal regions in human is still unknown, the meta-analysis of PET studies regarding the function and connectivity of the human ACC demonstrated that several frontal-lobe gyri were consistently linked with changes in activity in the ACC. That is because blood-flow changes in most subdivisions of the frontal cortex occurred more often in PET study subtractions that also showed changes in the anterior cingulate gyri [14]. The authors explain that such co-activation may reflect the transmission of information computed in the prefrontal cortex to the ACC, where it is modulated by non-specific arousal systems as it is forwarded to motor channels [15].
Interestingly, a recent PET study on eating disorders also reported that the underweight anorectic group showed a global hypometbolism and absolute as well as relative hypometabolism in cortical region with the most significant differences found in the frontal and the parietal cortices. They suggested that this reduction of glucose metabolism might be the consequence of neurophysiological or morphological aspects of anorexia nervosa [16].
Finally, reported findings suggest that decreases of rCBF in the frontal regions mainly containing the ACC are associated with characteristics of clinical symptoms such as relentless pursuit of thinness or disturbed body image in patients with restrictive anorexia nervosa. Since recently several investigators have classified eating disorders on the basis of the presence or absence of binge/purge eating [17, 18], the present findings regarding the reduction of rCBF in ACC areas in AN-R may have important implications for identifying biological differences between different forms of eating disorders.
Conclusion
By using SPM analysis we have demonstrated a decreased area of rCBF in frontal lobe regions mainly containing the ACC in restrictive anorexia nervosa patients. The ACC is thought to be very important in regulating a wide range of human brain functions such as refinement of signals from organs, cognitive process of selection following somatosensory stimuli, mood regulation, and so forth. The present findings therefore suggest that disturbed higher brain function may have an important role in producing the clinical symptoms of the patients with restrictive anorexia nervosa.
Competing interests
None declared
Pre-publication history
The pre-publication history for this paper can be accessed here:
http://www.biomedcentral.com/content/backmatter/1471-244X-1-2-b1.pdf
Acknowledgments
Acknowledgements
This paper was presented at the 41th annual meeting of the Japanese Society of Psychosomatic Medicine, Tokyo Japan, June 22, 2000. This work was supported by a Research Grant from the Japanese Ministry of Health and Welfare.
Contributor Information
Tetsuro Naruo, Email: naruo@m.kufm.kagoshima-u.ac.jp.
Yoshiaki Nakabeppu, Email: yoshi@m3.kufm.kagoshima-u.ac.jp.
Daisuke Deguchi, Email: degu@m.kufm.kagoshima-u.ac.jp.
Nobuatsu Nagai, Email: jaga@mkufm.kagoshima-u.ac.jp.
Junko Tsutsui, Email: Jun@m.kufm.kagoshima-u.ac.jp.
Masayuki Nakajo, Email: nakajo@m.kagoshima-u.ac.jp.
Shin-ichi Nozoe, Email: snozoe@m.kufm.kagoshima-u.ac.jp.
References
- Casper RC, Eckert ED, HalmI KA, Goldberg SC, Davis JM. Bulimia;its incidence and clinical importance in patients with anorexia nervosa. Arch Gen Psychiat. 1980;37:1030–1035. doi: 10.1001/archpsyc.1980.01780220068007. [DOI] [PubMed] [Google Scholar]
- Bruch H. Four decades of eating disorders. Handbook of psychotherapy for anorexia nervosa and bulimia. Edited by Garner DM and Garfinkel PE New York: The Guilford Press; 1985. pp. 9–15.
- Crisp AH. Diagnosis and outcome of anorexia nervosa: the St. George's view. Proce Royal Soc Med. 1977;70:464–470. doi: 10.1177/003591577707000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvenne V, Lotstra F, Goldman S, Biver F, DeMaertelaer V, Appelboom-Fondu J, Schoutens A, Bidaut L, Luxen A, Mendelwicz J. Brain hypometabolism of glucose in anorexia nervosa: a PET scan study. Biol Psychiatry. 1995;37:161–169. doi: 10.1016/0006-3223(94)00189-A. [DOI] [PubMed] [Google Scholar]
- Ellison Z, Foong J, Howard R, Bullmore E, Williams S, Treasure J. Functional anatomy of calorie fear in anorexia nervosa. Lancet. 1998;352:1192. doi: 10.1016/S0140-6736(05)60529-6. [DOI] [PubMed] [Google Scholar]
- Nozoe S, Naruo T, Yonekura R, Nakabeppu Y, Soejima Y, Nagai N, Nakajo M. Comparison ofregional cerebral blood flow in patients with eating disorders. Brain Res Bull. 1996;36:251–255. doi: 10.1016/0361-9230(94)00199-B. [DOI] [PubMed] [Google Scholar]
- Naruo T, Nakabeppu Y, Sagiyama K, Munemoto T, Deguchi D, Nakajo M, Nozoe S. Characteristic regional cerebral blood flow patterns in anorexia nervosa patients with binge/purge behavior. Am J Psychiatry. 2000;157:1520–1522. doi: 10.1176/appi.ajp.157.9.1520. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worseley KJ, Poline JP, Frith CD, Frackowiack RSJ. Statistical parametric maps in functional imaging; a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Nakamura N, Yamamoto T, Saito T, Fujita H. Analysis of activation in anterior cingulated cortex during cognitive process of selection following somatosnesory stimuli: fMRI study with elaborate task paradigms. Mag Reson Imaging. 2000;18:397–404. doi: 10.1016/S0730-725X(00)00129-6. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Garabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Blumer D, Benson DF. Personality changes with frontal and temporal lobe lesions. In Psychiatric aspects of neurological disease. Edited by Benson DF and Blummer D New York and London: Grune and Stratton, 1975. pp. 22–30.
- Mishkin M. Perseverance of centralsets after frontal lesions in monkeys. In The frontal granulacortex and behavior Edited by WarrenJM and AkertK New York, McGraw-Hill; 1992. pp. 219–241.
- Butter CM. Perseveration in extinction and indiscrimination reversal tasks following selective frontal ablations in macaca mulatta. Physiol Behav. 1969;4:163–171. doi: 10.1016/0031-9384(69)90075-4. [DOI] [Google Scholar]
- Koski L, Paus T. Functional connectivity of the anterior cingulate gyri within the human frontal lobe: a brain-mapping meta-analysis. Exp Brain Res. 2000;133:55–65. doi: 10.1007/s002210000400. [DOI] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E. Role of the human anterior cingluate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol. 1993;70:453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- Garfinkel P, Kennedy SH, Kaplan AS. Views on classification and diagnosis of eating disorders. Can J Psychiatry. 1995;40:445–456. doi: 10.1177/070674379504000805. [DOI] [PubMed] [Google Scholar]
- Beebe DW. Bulimia nervosa and depression: a theoretical and clinical appraisal in light of the binge-purge cycle. Bri J Clinical Psychol. 1994;33:259–276. doi: 10.1111/j.2044-8260.1994.tb01123.x. [DOI] [PubMed] [Google Scholar]


