Abstract
Plant transpiration is considered one of the most important physiological functions because it constitutes the plants evolving adaptation to exchange moisture with a dry atmosphere which can dehydrate or eventually kill the plant. Due to the importance of transpiration, accurate measurement methods are required; therefore, a smart sensor that fuses five primary sensors is proposed which can measure air temperature, leaf temperature, air relative humidity, plant out relative humidity and ambient light. A field programmable gate array based unit is used to perform signal processing algorithms as average decimation and infinite impulse response filters to the primary sensor readings in order to reduce the signal noise and improve its quality. Once the primary sensor readings are filtered, transpiration dynamics such as: transpiration, stomatal conductance, leaf-air-temperature-difference and vapor pressure deficit are calculated in real time by the smart sensor. This permits the user to observe different primary and calculated measurements at the same time and the relationship between these which is very useful in precision agriculture in the detection of abnormal conditions. Finally, transpiration related stress conditions can be detected in real time because of the use of online processing and embedded communications capabilities.
Keywords: smart sensor, transpiration, stomatal conductance, precision agriculture, phytomonitoring, water stress, field programmable gate array
1. Introduction
Plant transpiration is the process in which plants exchange moisture with the atmosphere [1]. This process is carried out when plants perform photosynthesis. While the plants are absorbing the carbon dioxide (CO2) they also lose a certain amount of water and release oxygen O2 [2]. Also, transpiration is performed to maintain temperature equilibrium between plants and their environments, dissipating undesirable heat in the lost water vapor. Plant monitoring commonly includes the estimation of photosynthesis itself, the assimilation or CO2 uptake and water thermodynamic relations such as: transpiration (E), stomatal conductance (Cleaf), vapor pressure deficit (VPD), and leaf-air temperature difference (LATD) [3]. Those variables constitute transpiration dynamic indicators which are often used in agriculture to optimize the available water resources [4].
E is considered one of the most important plant physiological functions because it encompasses plants evolving and adapting to exchange moisture in a very dry atmosphere that can dehydrate or eventually kill the plant [1]. Cleaf is a transpiration variable that represents a quantitative measurement of the stomatal resistance (rs) inverse of plant guard cells plus the inverse boundary resistance (rb) against water vapor flux. Those guard cells act as flux valves to control the water vapor movement from plant to the atmosphere and CO2 movement in an inverse way [5]. LATD is the difference between air temperature (Ta) and leaf temperature (Tleaf) in relation to the global transpiration process which is proportional to E. Furthermore, VPD is also a response variable that is calculated by subtracting air vapor pressure (ei) content from saturation vapor pressure (es). These variables are very important because they can indicate drought stress conditions and condensation problems that may cause dangerous plant diseases [2,6,7].
Because of this, transpiration dynamic measurement is crucial and necessary to establish comparisons and understand plant-soil-atmosphere relationships at leaf, plant, canopy, or community levels as well as their interaction and response to environmental [6], chemical [8], or biological [9] factors that generate different stress conditions. Therefore, continuous monitoring of the aforementioned transpiration dynamics by a single smart sensor system is highly desirable. As a consequence, more accurate measurement methods are required to gather more knowledge about these processes. Relative humidity (RH) capacitive sensors and thermistors are the most commonly utilized sensors to measure these variables in environmental and agricultural research [2,6,10,11]; however, in modern instrumentation the use of intelligent sensors with in situ signal processing capabilities to calculate response variables equations from simple sensor measurements is necessary [12–14]. E and Cleaf calculation is based mainly on water vapor exchange measurements [2,6]. This method consists of temporally isolating a plant leaf sample in a miniature gas exchange chamber which is often used for photosynthesis measurements [15]. An air flow is introduced into the leaf chamber to measure the intake ei and the amount of leaf out vapor (eo). The absolute amount of water is calculated using RH sensors and vapor curve equations from Mollier diagrams by expressing, E and Cleaf as vapor mass for each surface unit of each time unit [6,16,17]. Previous monitoring systems have used this technique to obtain E and Cleaf from air relative humidity (RHa) and Ta [18,19]. Temperature, light, carbon, and RH measurements contain merged transpiration and photosynthesis dynamics information; therefore, the extraction of those response variables is desirable for precision agriculture applications. Previously, Ta and RH sensors have been used in data acquisition systems for environmental monitoring and greenhouse climate controllers [2]. More advanced applications involve offline crop water stress detection based on E behavior analysis [20]. Forestry research has also used transpiration dynamics to investigate the properties of trees [21]. Intelligent irrigation has been investigated in order to schedule irrigation cycles according to the speaking plant concept approach, better known as phytomonitoring technique [20,22]. It takes into consideration the plant as the final user of the irrigation line, activating water delivery when plant has an excessive E. VPD has been studied in greenhouse climate controller design also in order to determine when RH is near to dew point to avoid excessive fogging and consequently leaf condensation that leads to plant diseases [7]. However, those systems do not fuse their sensors data with other transpiration-related response variables such as ambient light and LATD nor do they have online in situ signal processing capabilities to make real-time decisions. Consequently, it involves having an agricultural expert technician to manually download data to be analyzed offline with at least a one day delay [22]. In precision agriculture, a one day delay can sometimes represent the loss of the total crop. It makes necessary the development of a real time transpiration dynamics intelligent sensor to early detect stress and disorder conditions.
The contribution of this project is to develop a smart sensor capable of estimating plant transpiration dynamic variables: E, Cleaf, LATD, and VPD, through the fusion of five primary low-cost sensors: two RH capacitive sensors, two Resistance Temperature Detector (RTD) sensors, one light quantum sensor, average atmospheric pressure data, and fixed volumetric air flow. All the aforementioned instrumentation was embedded into a smart sensor system using an aluminum/acrylic leaf chamber with automatic open/close mechanism based on a miniature servomotor to perform temporal leaf isolation cycles. A vacuum pump is used to generate the air flow through the leaf chamber. Transpiration dynamic response variables are extracted from the primary sensors and its computation is performed in situ using digital signal processing techniques such as: average decimation filters, infinite-impulse-response (IIR) filters, polynomial fitting, and the corresponding E, Cleaf, LATD, and VPD equations. The light sensor is fused as a reference to understand daylight information which is related to the beginning of daily transpiration dynamic processes. The data acquisition systems, aforementioned computations, data communication and leaf chamber servomotor/vacuum pump control system are implemented in a field programmable gate array (FPGA) as an embedded smart sensor approach.
2. Background
2.1. Plant Transpiration Water-Atmosphere Scheme
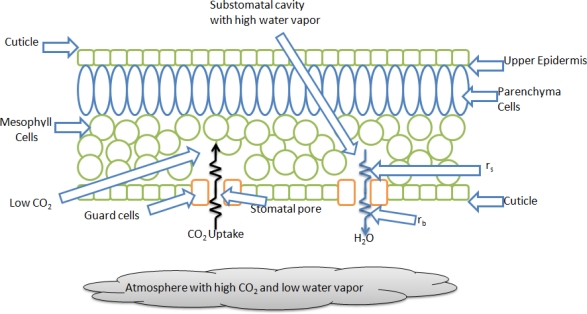
In Figure 1, a plant leaf cut scheme is shown where it can be noticed that the different plant tissues (parenchyma, mesophyll and guard cells) which have low CO2 contents and a high amount of water. The atmosphere, presented as a gray cloud constitutes a relatively dry environment that can eventually dehydrate or even kill the entire plant if the environmental conditions are not adequate [1]. The stomata guard cells which are the orange ones in Figure 1, are the plant system that controls the stomatal pores open and close process to balance the CO2 and water fluxes between the plant and its environment. Here rb is the boundary resistance and rs is the stomatal resistance.
Figure 1.
Leaf cut water scheme, showing CO2 and H2O flows.
2.2. Transpiration Process
As was aforementioned, E is a function that depends primarily on the difference between ei and eo. However, primary humidity sensors provide relative humidity measurement values [6] and need to be converted into ei and eo. First, it is necessary to determine es in order to know the maximum amount of water that air can contain at a specified Ta by using vapor curves in the Mollier thermodynamic diagrams [2] or by using the simplified equation (1) as was previously reported [19]. Then ei and eo can easily be obtained with (2) and (3), where RHi is air input RH and RHo is leaf chamber output RH.
| (1) |
| (2) |
| (3) |
In order to estimate E; it is necessary to calculate another important factor, W which is the mass flow rate per leaf area, expressed in mol/m2/s for open flow systems. W equation is stated in (4), where P is the atmospheric pressure in Bar, V is the volumetric air flow in liters per minute (lpm), TaK is air temperature in Kelvin (K) and A is leaf area in cm2, which is often used the effective leaf chamber area in transpiration and photosynthesis measurement systems [19]. The 2005.39 constant is an adjusted coefficient to change mass units to mol, surface to m2 and time from minutes to seconds:
| (4) |
Once ei, eo and W were estimated, E can be calculated as established in (5) and expressed in mg/m2/s:
| (5) |
2.3. Stomatal Conductance
Stomatal conductance is another important transpiration dynamic variable that represents the guard cells vapor conductivity [1,2,5]. It can be estimated from primary temperature and RH sensors data. The first step is to calculate the leaf saturation vapor pressure eleaf as a function of Tleaf. For this purpose, (1) can be used to calculate eleaf substituting Ta by Tleaf to obtain (6). rb is considered a constant of 0.3 m2s/mol. Once eleaf is obtained, stomatal conductance (Cleaf) can be calculated by using (7), expressing the result in mmol/m2/s as was previously utilized [19]:
| (6) |
| (7) |
2.4. Vapor Pressure Deficit
VPD is a variable that represents the margin between air vapor pressure and air saturation vapor pressure. If air RH is low, VPD has a large margin; but if RH is high VPD is low and it is easy to get undesirable condensation conditions [6]. To calculate VPD it is necessary to subtract ei from es. The most common units to represent VPD are kPa [2]:
| (8) |
3. Smart Sensor Methodology
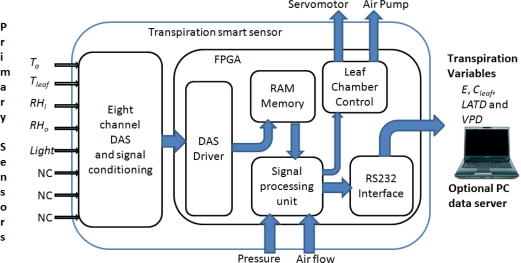
The proposed methodology for the smart sensor can be seen in Figure 2. It consists of the following stages: primary sensors, data acquisition system (DAS), FPGA-based digital signal processing (DSP), RAM memory to storage sensors measurements, RS232 data communication module, and leaf chamber mechanism control system. In the first stage, five primary sensor signals are obtained from two RTD temperature sensors, two RH capacitive sensors and one light quantum sensor. The second stage consists of an eight channel DAS capable of acquiring the signals of the five primary sensors and leaving the last three channels disconnected (NC). The signal processing stage is carried out on a FPGA-based hardware signal processing (HSP) unit, as reported by [23] for CNC and [24] for robotics vibration applications. Atmospheric pressure is provided by external smart sensor input. Volumetric air flow is fixed at a constant 0.9 lpm flow rate by using a passive flow limiter. Data communication is carried out via RS232 interface embedded in the FPGA unit to send the measurement to a data server PC or another system. Finally, the leaf chamber opening mechanism and vacuum pump is controlled by the FPGA HSP unit.
Figure 2.
Transpiration smart sensor architecture.
3.1. Transpiration Smart Sensing Cycle Methodology
Transpiration smart sensing cycle methodology is shown in Figure 3. Here, the green blocks represent the open leaf chamber stages and red blocks represent the closed leaf chamber; therefore, isolating the plant leaf. Each measurement starts with the activation of the air vacuum pump and closing the leaf chamber by the servomotor controller to isolate the plant sample. The smart sensor performs a 1 min delay to wait pneumatic line flow steady state. After that, the data acquisition starts by measuring cycles of 1 Hz sampling frequency from the five primary sensors. This process is repeated to acquire 64 samples from each sensor in each transpiration measurement cycle. Once sufficient data has been acquired, the computation of transpiration dynamics are performed, data can be transferred, the leaf chamber is opened and vacuum pump is turned off to save energy. Finally, another delay is carried out to complete the 15 min duration of the entire transpiration smart sensing process. This measurement period was selected because in commercial equipment, the fastest acquisition period is 15 min and this is necessary to establish the same sampling frequency to compare both sensing techniques.
Figure 3.
Block diagram of transpiration smart sensing cycle.
3.2. Transpiration Methodology
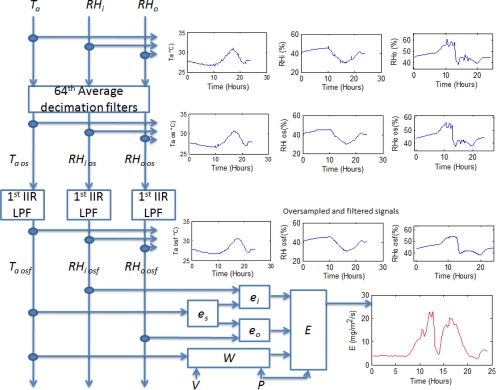
In order to calculate plant transpiration E, an FPGA-based signal processing methodology is proposed and described in Figure 4. Average decimation filters of 64th order are applied to all the primary sensors signals Ta, RHi, and RHo in order to reduce undesired quantization noise as stated in (9), (10), and (11) and presented by [14]. Once Ta os, RHi os, and RHo os were estimated, a 1st order IIR low-pass filter (LPF) with cut-off frequency (fc) of 1/3600 Hz is applied to obtain the filtered versions of the decimated signals known as Ta osf, RHi osf, and RHo osf. To gather E, equations (1) to (5) are computed from the filtered signals as presented in Figure 4.
| (9) |
| (10) |
| (11) |
Figure 4.
Transpiration estimation signal processing methodology.
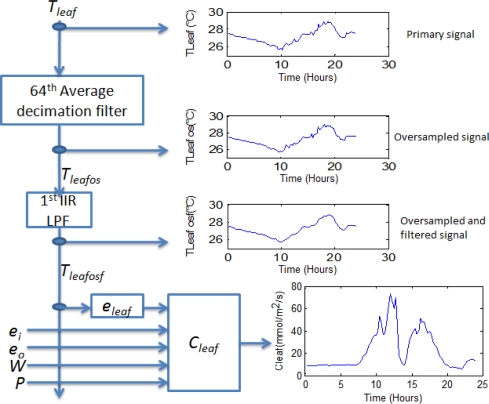
3.3. Stomatal Conductance Estimation Methodology
Stomatal conductance is advantageous because it utilizes certain factors previously calculated in the transpiration stage. A FPGA-based signal processing methodology is proposed and described in Figure 5.
Figure 5.
Stomatal conductance estimation signal processing methodology.
Average decimation filters of 64th order are applied to Tleaf sensor signals to reduce undesired quantization noise in the same manner as the previous stage according to (12). Once Tleaf os was estimated, a 1st order IIR low-pass filter (LPF) stage with fc = 1/3600 Hz is applied to obtain its filtered version Tleaf osf. Consequently, eleaf is calculated as stated in (6) and introduced in (7) to obtain Cleaf :
| (12) |
3.4. Vapor Pressure Deficit Methodology
VPD estimation is very simple once es and ei are calculated in the transpiration calculation stage. VPD is obtained from the subtraction stated in (13). VPD implementation can be noted in Figure 6:
| (13) |
Figure 6.
VPD, LATD and Light estimation signal processing methodology.
3.5. Leaf-Air Temperature Difference Methodology
LATD calculation requires subtracting the filtered air temperature and filtered leaf temperature. As occurred in previous calculation stages, once Ta osf and Tleaf osf was calculated, LATD computation is simple by using (14), as demonstrated in Figure 6:
| (14) |
3.6. Ambient Light Smart Sensor Methodology
To measure ambient light in a smoother signal manner, the same average decimation filter (15) plus 1st order IIR LPF with fc = 1/3600 Hz was applied to the signal light in order to obtain its improved version lightosf:
| (15) |
4. Experimental Setup
4.1. Experiment Setup
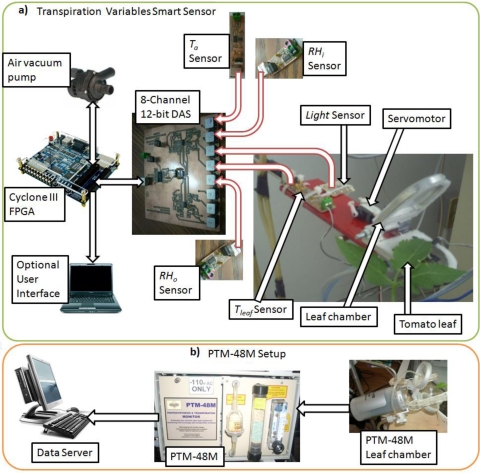
The experimental setup can be seen in Figure 7, which shows the development of a smart sensor setup and the commercial Phytech PTM-48M, see Figure 7a and Figure 7b used for performance comparisons. For this experiment, tomato (Lycopersicon esculentum) plants were chosen as biological material to measure transpiration responses in the proposed smart sensor testing. The proposed smart sensor consists on an instrumentation platform capable of measuring Ta, Tleaf, RHi, RHo and Light. To measure temperature readings, Honeywell Pt1000 RTD primary sensors were used which have a measurement range from −200 °C to 540 °C, but are configured for a 0 to 65 °C range with an accuracy rate of ±0.3 °C considered suitable for plant temperature ranges [10]. A RTD-signal conditioning system was developed to convert the resistance variation into a 0 to 5 volt format. For RH measurements, Honeywell HIH-4000 capacitive RH sensors with a range from 0 to 95% RH and accuracy of ±2.5% were selected and connected through a developed RH-signal conditioning system [11]. Ambient light measurement is achieved by using an OSRAM SFH-5711 light sensor with range from 0 to 100,000 lux and an accuracy of ±0.04% of its measured value, providing a 0 to 50 μA current signal [25], converted into 0 to 5 V by a designed light-signal conditioning system. The results are suitable in order to measure light intensities in rooms from total darkness to complete sunlight. Each primary sensor reading passes through a 2nd order analog anti-alias LPF with 20 Hz cut-off frequency embedded in the proposed smart sensor. An eight channel 12-bit data acquisition system based on the Burr Brown ADS7844 analog to digital converter instrumentation platform was developed to read the primary sensors [26]. An FPGA based hardware digital signal processing unit was utilized to compute the transpiration response variables from the primary sensors readings based on an Altera Cyclone III EP3C16F484C6N device with 16,000 LE [27]. For the open/close leaf chamber mechanism, a miniature servomotor model E-Sky 000155 was utilized because of its low power consumption. The air flow was induced using a dc-motor piston based vacuum pump. The FPGA IP core was implemented in VHDL language, integrating the DAS driver, leaf chamber motors control, signal processing unit and communications blocks.
Figure 7.
(a) Transpiration smart sensor experimental setup. (b) Phytech PTM-48M setup.
The experiment was designed to monitor transpiration variables every 15 min to compare the proposed smart sensor with a Phytech PTM-48M photosynthesis and transpiration monitor configured at its fastest sampling period which is 15 min [18]. Both were connected to the same tomato plant to prove the effectiveness of the proposed smart sensor. The experiment ran for 24 hours beginning at 12:00AM and finishing at 12:00AM of the next day. It permits the acquisition of four measurement cycles per hour for a total of 96 measurements every 24 h. As was aforementioned, each measurement cycle acquires 64 samples from each primary sensor at a sampling frequency of 1 Hz to apply the 64th averaging decimation filters. The data can be sent to a data server for massive storage via an Analog Devices ADM3232 RS232 transceiver [28].
4.2. Primary Sensor Signal Improvement Results
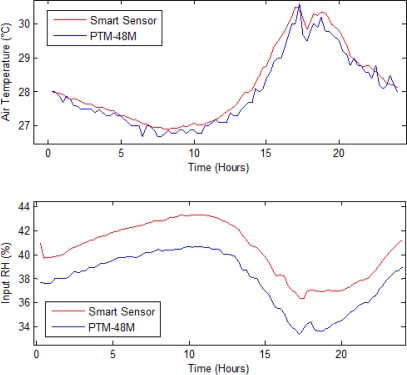
In this subsection, a comparison between the Ta and RHi readings from the proposed smart sensor and the commercial PTM-48M is presented. Figure 8 illustrates this comparison where blue signals correspond to PTM-48M readings and red ones to the proposed smart sensor primary readings. Here, it can be noted that a similar tendency occurs for both measurements, but a lower amount of noise in the red signals is present due to the 64-sample average decimation filters and 1st order IIR filters that reduces undesirable variations. In this manner, the filtering advantages of the smart-sensor signal processing can be clearly noticed. RHo, Tleaf, and Light readings are not compared because PTM-48M does not provide RHo measurement in the data output table and does not have Tleaf and Light sensors.
Figure 8.
Primary sensors signal comparison. The proposed smart sensor signals are in red and Phytech PTM-48M readings in blue.
4.3. Transpiration Results and Comparison
This subsection shows a comparison between the proposed smart sensor transpiration estimation and the reference PTM-48M. In Figure 9, this comparison is represented by using blue for the PTM-48M transpiration signal and red for the smart-sensor transpiration estimation signal. Here, it is noteworthy that a similar transpiration signal behavior between both instruments occurs.
Figure 9.
Comparison between the developed smart sensor and the PTM-48M transpiration estimations.
4.4. Fused Transpiration Dynamics Smart Sensing Results
The proposed smart sensor fuses Ta, Tleaf, RHi, Rho and Light measurements in a single device which is considered highly desirable for precision agriculture applications to be able to monitor different environmental factors that can affect the crops. In Figure 10, the monitoring results of the primary sensors in this experiment are presented. All of these were previously oversampled 64 times for the average filtering process and passed through an IIR 1st order LPF with a cut-off frequency of fc = 1/(3600). As it can be seen, the amount of noise in these primary signals is very low.
Figure 10.
Primary sensor readings of the proposed smart sensor.
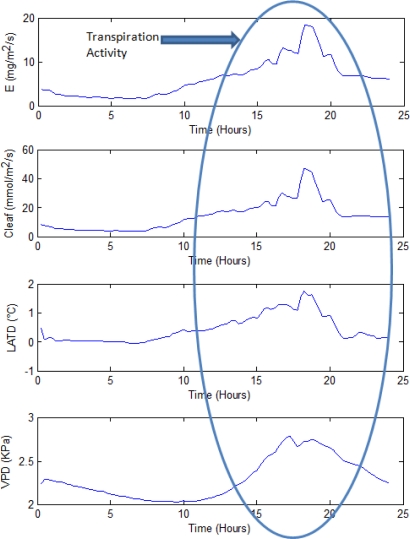
In Figure 11, transpiration dynamics response variables (E, Cleaf, LATD, and VPD) can be observed. These parameters share a similar dynamic behavior because they are related to the entire photosynthesis and transpiration processes which involve different phenomena.
Figure 11.
Transpiration dynamics results.
The computation of the required equations to obtain these results is achieved using FPGA reconfigurability and open architecture that permits implementation of any digital system such as data acquisition, memory management, signal processing, and data communication. The developed smart sensor fuses five primary sensors and can measure the necessary environmental variables to calculate transpiration dynamics and permits the user to observe and record different primary and response measurements at the same time and the relationship between these. This is very useful in precision agriculture in order to detect abnormal conditions. In contrast, commercial equipment as noted in [18,19] can measure time series of transpiration information; however, they do not provide the readings for all the primary sensors. In some cases, they are not equipped with the necessary sensors. The FPGA-based unit permits improvements of the primary sensor signals by oversampling and digital filtering that is consequently reflected in superior accuracy, and overall transpiration variables signal quality. The integration of these elements merge different variables at the same time that can be acquired and used to take specific control actions by communicating these transpiration measured values to other systems via RS232 interface like PC data servers, an irrigation controller, or a climatic control unit due to the online processing and remote communications capabilities of the proposed smart sensor. Taken together they constitute a smart sensor solution to monitor transpiration variables in precision agriculture applications by using a single FPGA-based system.
5. Conclusions
In this investigation the development of a novel smart sensor that can estimate plant-transpiration dynamic variables as: transpiration, stomatal conductance, leaf-air temperature difference, and water vapor deficit in real time is presented. This smart sensor fuses five primary sensors: two temperature sensors, two relative humidity sensors and one light sensor. To show the effectiveness of the proposed smart sensor, it was compared with a commercial Phytech PTM-48M transpiration monitoring system. Results show that the proposed sensor can obtain very similar results compared to the reference system with less noise due to the digital filtering applied to the primary measurements. The transpiration dynamics variables are calculated in real time from the primary sensor data providing very useful information related to the plant transpiration which is valuable to schedule irrigation, prevent diseases, and detect drought conditions in precision agriculture. Similar behavior of the estimated transpiration variables shows the relationship between these and how they depend on the primary sensor readings. The necessary computations in order to obtain the transpiration dynamics are computed in a low-cost FPGA platform in which parallel architecture is utilized to implement the transpiration equations. This permits the integration of the data communication, memory management data acquisition and signal processing in a single embedded sensor which can be used to monitor plant transpiration variables and their relationships in a wide range of precision agriculture applications. Finally, transpiration related stress conditions can be detected in real time because of the online processing and communications capabilities. All of which constitutes a very useful precision agriculture smart sensor.
Acknowledgments
This project was partially supported by CONACYT scholarship 207684, FOMIX-QRO-2008-CO2-101490 and FOMIX-QRO-2008-CO2-102123 projects. The authors wish to thank HSP Digital research group in general but specially by the assistance in the aluminum leaf chamber machining process. Also thanks to Altera Corp. for the donation of an Altera DE2-70 3 in 1 FPGA development kit used at the beginning of this project. They also wish to thank Texas Instruments Inc. and Analog Devices Inc. for donating analog electronics samples which were tested in the data acquisition system development. Finally, the authors wish to acknowledge Silvia C. Stroet of the Engineering Faculty at Universidad Autonoma de Queretaro for editing the English content of this document.
References
- 1.Taiz L, Zeiger E. Plant Physiology. 4th ed. Sinauer Associates; Sunderland, MA, USA: 2006. [Google Scholar]
- 2.Bakker JC, Bot GPA, Challa H, Braak NJ. Greenhouse Climate Control: An Integrated Approach. Wageningen Academic Publishers; Wageningen, The Netherlands: 2001. [Google Scholar]
- 3.Field CB, Ball JT, Berry JA. Photosynthesis: Principles and Field Techniques Plant Physiological Ecology: Field Methods and Instrumentation. Chapman and Hall; London, UK: 1991. pp. 209–253. [Google Scholar]
- 4.Bittelli M. Measuring soil water potential for water management in agriculture: A review. Sustainability. 2010;2:1226–1251. [Google Scholar]
- 5.Hubbard RM, Ryan MG, Stiller V, Sperry JS. Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant Cell Environ. 2001;24:113–121. [Google Scholar]
- 6.Kamp PGH, Timmerman GJ. Computerized Environmental Control in Greenhouses: A Step by Step Approach. IPC Plant; Ede, The Netherlands: 1996. [Google Scholar]
- 7.Prenger JJ, Ling PP. Greenhouse Condensation Control Fact Sheet (Series) AEX-800. Ohio State University Extension; Columbus, OH, USA: 2000. [Google Scholar]
- 8.Marschner H. Mineral Nutrition of Higher Plants. Academic Press; San Diego, CA, USA: 1995. [Google Scholar]
- 9.Stout MJ, Fidantsef AL, Duffey SS, Bostock RM. Signal iinteractions in pathogen and insect attack: Systemic plant-mediated interactions between pathogens and hervibores of the tomato, lycopersicon esculentum. Physiol. Molec. Plant. Pathol. 1999;54:115–130. [Google Scholar]
- 10.HEL-700 Pt1000 RTD Sensor Data Sheet. Honeywell; Golden Valley, MN, USA: 2010. [Google Scholar]
- 11.HIH-4000 RH Sensor Data Sheet. Honeywell; Golden Valley, MN, USA: 2010. [Google Scholar]
- 12.Rivera J, Herrera G, Chacón M, Acosta P, Carrillo M. Improved progressive polynomial algorithm for self-adjustment and optimal response in intelligent sensors. Sensors. 2008;8:7410–7427. doi: 10.3390/s8117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez W. A survey on optimal signal processing techniques applied to improve the performance of mechanical sensors in automotive applications. Sensors. 2007;7:84–102. [Google Scholar]
- 14.Rangel-Magdaleno JJ, Romero-Troncoso RJ, Osornio-Rios RA, Cabal-Yepez E. Novel oversampling technique for improving signal-to-quantization noise ratio on accelerometer-based smart jerk sensors in CNC applications. Sensors. 2009;9:3767–3789. doi: 10.3390/s90503767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millan-Almaraz JR, Guevara-Gonzalez RG, Romero-Troncoso RJ, Osornio-Rios RA, Torres-Pacheco I. Advantages and disadvantages on photosynthesis measurement techniques: A review. Afr. J. Biotechnol. 2009;8:7340–7349. [Google Scholar]
- 16.Strobel BR, Stowell RR. Using a Psychrometric Chart to Describe Air Properties Fact Sheet AEX-120-99. Ohio State University Extension; Columbus, OH, USA: 1999. [Google Scholar]
- 17.Schmidt U. Microclimate control in greenhouses based on phytomonitoring data and Mollier phase diagram. Acta Hortic. 2005;691:125–132. [Google Scholar]
- 18.PTM-48M User Manual. Phytech Inc; Yad Mordechai, Israel: 2005. [Google Scholar]
- 19.CI-340 Hand-held Photosynthesis System Instruction Manual. CID Inc; Camas, WA, USA: 2008. [Google Scholar]
- 20.Escalona L, Flexas J, Medrano H. Comparison of heat balance and gas exchange methods to measure transpiration in irrigated and water stressed grapevines. Acta Hortic. 2000;526:145–156. [Google Scholar]
- 21.Schulze ED. A new type of climatized gas exchange chamber for net photosynthesis and transpiration measurements in the field. Oecologia. 1972;10:243–251. doi: 10.1007/BF00368966. [DOI] [PubMed] [Google Scholar]
- 22.Ton Y, Kopyt M, Nilov N. Phytomonitoring technique for tuning irrigation of vineyards. Acta Hortic. 2004;646:133–139. [Google Scholar]
- 23.Trejo-Hernandez M, Osornio-Rios RA, Romero-Troncoso RJ, Rodriguez-Donate C, Dominguez-Gonzalez A, Herrera-Ruiz G. FPGA-based fused Smart-sensor for Tool-wear area quantitative estimation in CNC machine inserts. Sensors. 2010;10:3373–3388. doi: 10.3390/s100403373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Donate C, Morales-Velazquez L, Osornio-Rios RA, Herrera-Ruiz G, Romero-Troncoso RJ. FPGA-Based fused smart sensor for dynamic and vibration parameter extraction in industrial robot links. Sensors. 2010;10:4114–4129. doi: 10.3390/s100404114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.SFH-5711 Data Sheet. OSRAM Opto Semiconductors Inc; Munich, Germany: 2007. [Google Scholar]
- 26.ADS7844 Data Sheet. Burr Brown Corp; Dallas, TX, USA: 2003. [Google Scholar]
- 27.Cyclone III Hand Book Volume 1. Altera Corp; San Jose, CA, USA: 2010. [Google Scholar]
- 28.ADM3232 Data Sheet. Analog Devices Inc; Norwood, MA, USA: 2008. [Google Scholar]