Abstract
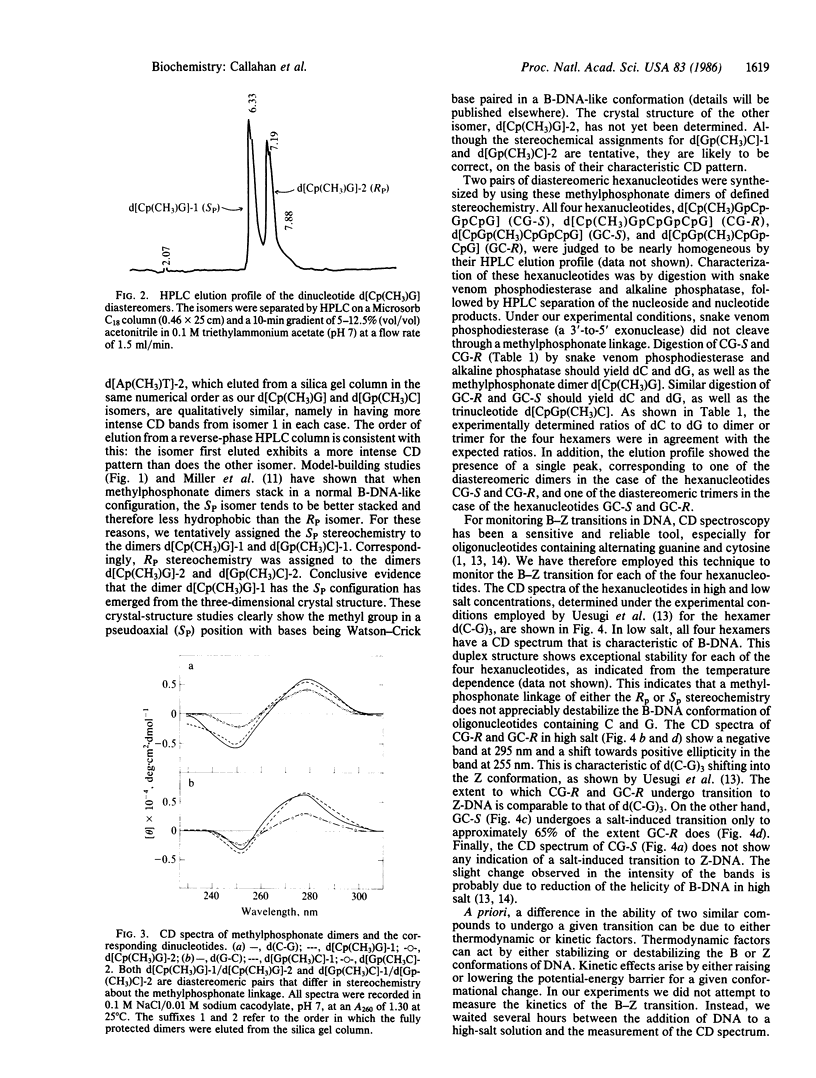
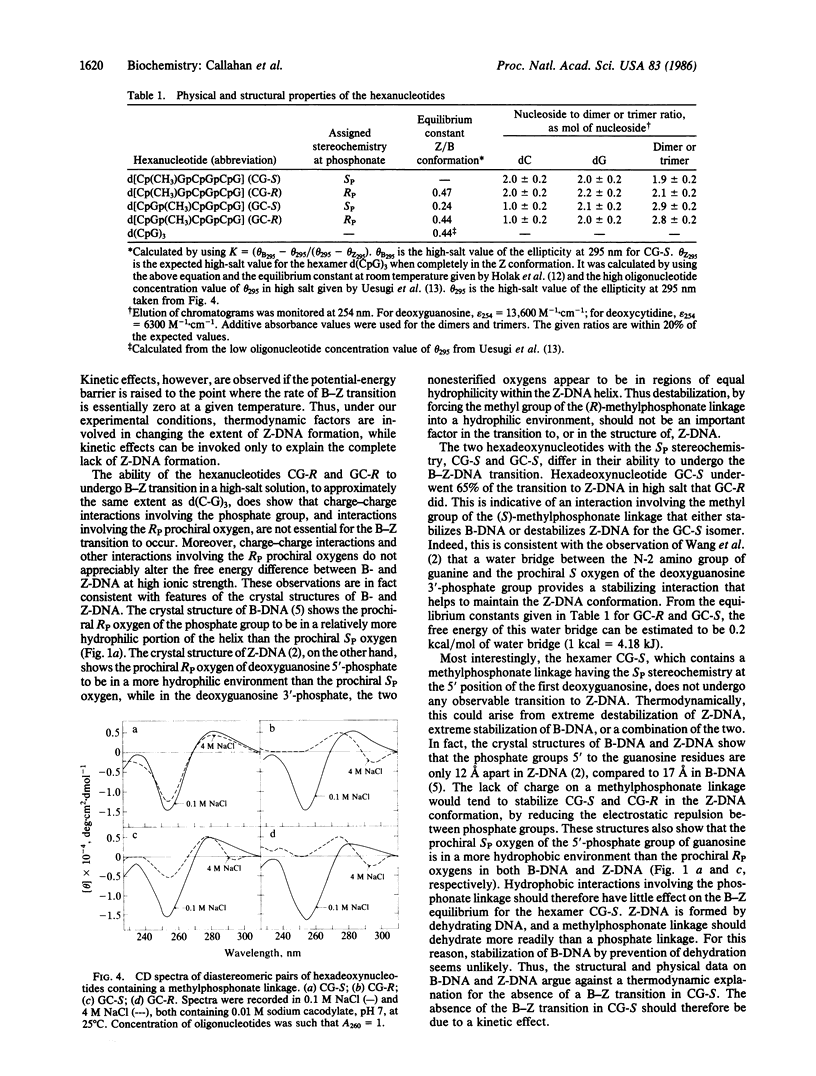
The simulation of the B--Z-DNA transition by using space-filling models of the dimer d(C-G) shows the possibility of hydrogen-bond formation between the N-2 amino group of the partially rotated guanine and one of the 5'-phosphate oxygens of deoxyguanylic acid. To probe the importance of this postulated interaction, analogs of the hexamer d(C-G)3 were synthesized. These analogs contained a methylphosphonate linkage, of distinct stereochemistry, which replaced the first 5'-phosphate linkage of deoxyguanosine. The CD spectra in high salt concentration showed that the hexamer containing a methylphosphonate linkage with the RP stereochemistry formed Z-DNA to the same extent as d(C-G)3, whereas the hexamer containing a methylphosphonate linkage with the SP stereochemistry did not form Z-DNA. These results are consistent with a mechanism in which an interaction between the N-2 amino group of guanine and the prochiral SP oxygen of deoxyguanosine 5'-phosphate kinetically controls the formation of Z-DNA. A water bridge between the N-2 amino group of guanine and the 3'-phosphate oxygen of deoxyguanylic acid has been implicated in the stabilization of Z-DNA. To probe the importance of this water bridge, two additional analogs of the hexamer d(C-G)3 were synthesized. These analogs contained a methylphosphonate linkage, of distinct stereochemistry, that replaced the first deoxyguanosine 3'-phosphate. The CD spectra showed that the hexamer containing a methylphosphonate linkage of the RP stereochemistry underwent the transition to Z-DNA to the same extent as d(C-G)3, whereas the hexamer containing a methylphosphonate linkage of the SP stereochemistry underwent the transition to Z-DNA to a 35% lesser extent. Thus the water bridge involving the prochiral SP oxygen provides modest stabilization energy for Z-DNA. These studies, therefore, suggest that the B--Z-DNA transition is regulated both thermodynamically and kinetically through hydrogen-bond interactions involving phosphate oxygens and the N-2 amino group of guanine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Riftina F. Synthesis and enzymatic properties of deoxyribooligonucleotides containing methyl and phenylphosphonate linkages. Nucleic Acids Res. 1979 Jul 11;6(9):3009–3024. doi: 10.1093/nar/6.9.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R., Conner B. N., Wing R. M., Fratini A. V., Kopka M. L. The anatomy of A-, B-, and Z-DNA. Science. 1982 Apr 30;216(4545):475–485. doi: 10.1126/science.7071593. [DOI] [PubMed] [Google Scholar]
- Efimov V. A., Reverdatto S. V., Chakhmakhcheva O. G. New effective method for the synthesis of oligonucleotides via phosphotriester intermediates. Nucleic Acids Res. 1982 Nov 11;10(21):6675–6694. doi: 10.1093/nar/10.21.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney B. L., Marky L. A., Jones R. A. The influence of the purine 2-amino group on DNA conformation and stability. Synthesis and conformational analysis of d[T(2-aminoA)]3. Nucleic Acids Res. 1982 Jul 24;10(14):4351–4361. doi: 10.1093/nar/10.14.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S. C. DNA structural dynamics: longitudinal breathing as a possible mechanism for the B in equilibrium Z transition. Nucleic Acids Res. 1983 Jul 25;11(14):4867–4878. doi: 10.1093/nar/11.14.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holak T. A., Borer P. N., Levy G. C., van Boom J. H., Wang A. H. 31P-NMR analysis of the B to Z transition in double-stranded (dC-dG)3 and (dC-dG)4 in high salt solution. Nucleic Acids Res. 1984 Jun 11;12(11):4625–4635. doi: 10.1093/nar/12.11.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M., van de Sande J. H., Zarling D. A., Arndt-Jovin D. J., Eckstein F., Füldner H. H., Greider C., Grieger I., Hamori E., Kalisch B. Generation of left-handed Z-DNA in solution and visualization in polytene chromosomes by immunofluorescence. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):143–154. doi: 10.1101/sqb.1983.047.01.019. [DOI] [PubMed] [Google Scholar]
- Kang D. S., Harvey S. C., Wells R. D. Diepoxybutane forms a monoadduct with B-form (dG-dC)n.(dG-dC)n and a crosslinked diadduct with the left-handed Z-form. Nucleic Acids Res. 1985 Aug 12;13(15):5645–5656. doi: 10.1093/nar/13.15.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmich S., Marky L. A., Jones R. A. Specifically alkylated DNA fragments. Synthesis and physical characterization of d[CGC(O6Me)GCG] and d[CGT(O6Me)GCG]. Nucleic Acids Res. 1983 May 25;11(10):3393–3403. doi: 10.1093/nar/11.10.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Agris C. H., Murakami A., Reddy P. M., Spitz S. A., Ts'o P. O. Preparation of oligodeoxyribonucleoside methylphosphonates on a polystyrene support. Nucleic Acids Res. 1983 Sep 24;11(18):6225–6242. doi: 10.1093/nar/11.18.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Yano J., Yano E., Carroll C., Jayaraman K., Ts'o P. O. Nonionic nucleic acid analogues. Synthesis and characterization of dideoxyribonucleoside methylphosphonates. Biochemistry. 1979 Nov 13;18(23):5134–5143. doi: 10.1021/bi00590a017. [DOI] [PubMed] [Google Scholar]
- Olson W. K., Srinivasan A. R., Marky N. L., Balaji V. N. Theoretical probes of DNA conformation examining the B leads to Z conformational transition. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):229–241. doi: 10.1101/sqb.1983.047.01.028. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Reese C. B., Zard L. Some observations relating to the oximate ion promoted unblocking of oligonucleotide aryl esters. Nucleic Acids Res. 1981 Sep 25;9(18):4611–4626. doi: 10.1093/nar/9.18.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma M. H., Gupta G., Dhingra M. M., Sarma R. H. During B-Z transition there is no large scale breakage of Watson-Crick base pairs. A direct demonstration using 500 MHz 1H NMR spectroscopy. J Biomol Struct Dyn. 1983 Oct;1(1):59–81. doi: 10.1080/07391102.1983.10507426. [DOI] [PubMed] [Google Scholar]
- Vorlíckovă M., Kypr J., Stokrová S., Sponar J. A Z-like form of poly(dA-dC).poly(dG-dT) in solution? Nucleic Acids Res. 1982 Feb 11;10(3):1071–1080. doi: 10.1093/nar/10.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wing R., Drew H., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R. E. Crystal structure analysis of a complete turn of B-DNA. Nature. 1980 Oct 23;287(5784):755–758. doi: 10.1038/287755a0. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Jovin T. M. Z* DNA, the left-handed helical form of poly[d(G-C)] in MgCl2-ethanol, is biologically active. EMBO J. 1982;1(1):115–120. doi: 10.1002/j.1460-2075.1982.tb01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]