Abstract
In this study, the urea-enzymatic field effect transistors (EnFETs) were investigated based on pH-ion sensitive field effect transistors (ISFETs) with tantalum pentoxide (Ta2O5) sensing membranes. In addition, a post N2 annealing was used to improve the sensing properties. At first, the pH sensitivity, hysteresis, drift, and light induced drift of the ISFETs were evaluated. After the covalent bonding process and urease immobilization, the urea sensitivity of the EnFETs were also investigated and compared with the conventional Si3N4 sensing layer. The ISFETs and EnFETs with annealed Ta2O5 sensing membranes showed the best responses, including the highest pH sensitivity (56.9 mV/pH, from pH 2 to pH 12) and also corresponded to the highest urea sensitivity (61 mV/pCurea, from 1 mM to 7.5 mM). Besides, the non-ideal factors of pH hysteresis, time drift, and light induced drift of the annealed samples were also lower than the controlled Ta2O5 and Si3N4 sensing membranes.
Keywords: urea, enzymatic field effect transistor (EnFET), ion sensitive field effect transistor (ISFET), tantalum pentoxide (Ta2O5), post N2 annealing
1. Introduction
Variation of human body fluid in even tiny concentrations can be critical for clinical diagnosis. To monitor the small changes in the early stage, an accurate and stable sensor is needed. Enzymatic field effect transistor (EnFETs) is one of the sensor platforms. The earliest report of EnFET was proposed by Janata and Moss in 1980 [1]. Subsequently, many biomarkers have been detected by EnFET, such as penicillin [1], urea [2], glucose [3], creatinine [4], etc. To fabricate the EnFET, specific enzyme is immobilized on the surface of the sensing membrane of an ion sensitive field effect transistor (ISFET). Therefore, the sensing properties are not only dependent on the enzymatic layer but also dominated by the sensing membrane of ISFETs. For EnFET applications, Si3N4 layers deposited by low pressure chemical vapor deposition (LPCVD) are the most common sensing membranes of ISFETs because of the high sensitivity and good stability.
Until now, plenty of materials have been applied for the sensing membranes of pH ISFETs, including: SiO2, Si3N4 [5,6], Ta2O5 [7–9], Al2O3 [10], TiO2 [11,12], HfO2 [13,14], SnO2 [15], etc. In these materials, Ta2O5 layer has the largest surface buffer capacity (β), which gives the sensor better immunity to interference ions; wide applicable pH range, and high chemical resistance for the cleaning-in-place (CIP) test [14,16,17]. Moreover, the Ta2O5-ISFETs were also used as the platform of EnFETs [18,19]. However, the serious effect of light induced drift was observed on the ISFETs with Ta2O5 layers [20,21]. This drawback limited the applicable range of Ta2O5 sensing membrane, and many research groups are using different fabrication process and post annealing treatment to solve this problem.
In this paper, urea concentration was monitored by a pH ISFET based EnFET. The urea hydrolysis reaction by urease is as follows:
| (1) |
The sensing membrane of the pH ISFET was the Ta2O5 layer deposited by reactive radio frequency (rf) sputter system. In order to improve the sensing properties, the Ta2O5 layer was treated by post N2 annealing at 900 °C for 30 min. In addition, the sensing properties were also compared with the urea-EnFET with conventional Si3N4 sensing membrane.
2. Experimental
2.1. Chemicals
The pH sensing properties were measured to evaluate the pH-ISFETs. The phosphate buffer solutions were prepared with 5 mM NaH2PO4, 0.1 M NaCl, and deionized (DI) water as the background solution. The initial pH value was 5.7. Then, the pH value of the buffer solutions were adjusted by adding 0.1 M NaOH and 0.1 M HCl solutions with autoburettes (Mettler-Toledo) and monitored by a combined pH glass electrode.
For the urea detection, all solutions were purchased from Sigma, including urease (E.C. 3.5.1.5, type IX, at activity 20 kU), glutaraldehyde (GA), 3-aminopropyl-triethoxysilane (APTS), and urea (U5378, 98+%). Urea and urease solutions were diluted with 5 mM phosphate buffer, which has been adjusted to pH 6 as background because of the high sensitivity for urea detection [2].
2.2. Device Fabrication
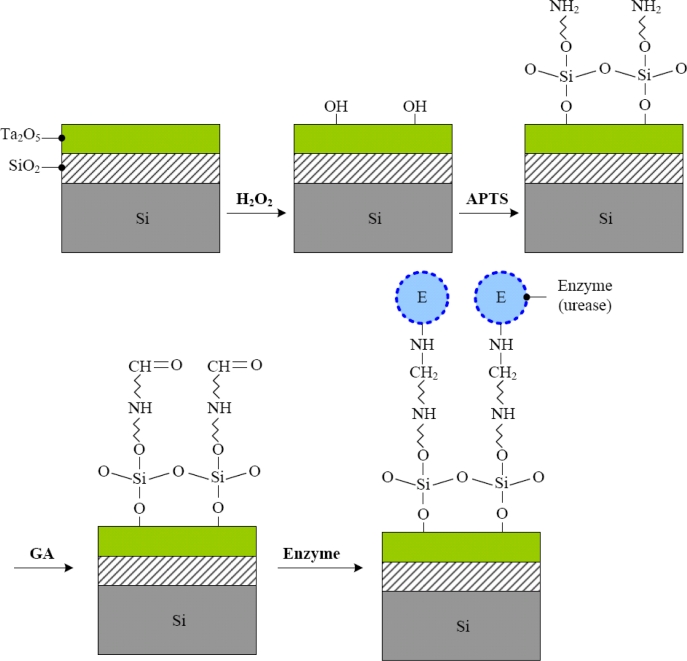
The ISFETs were fabricated by Institute of Electron Technology (ITE, the polish abbreviation), Poland. The structure and cross section are shown in Figure 1. The ISFETs are designed with p-well arrangement on 3 inch (100) n-type wafers. The ratio of channel width and length was W/L = 600/16 (in μm). The 50 nm-thick buffering oxide layers between silicon and sensing membrane were deposited by dry oxidation. The sensing membranes, Ta2O5 layers, were deposited by rf sputtering in Chang-Gung University (CGU), Taiwan. For the rf sputtering of Ta2O5 layers, a pure Ta target was used. The rf power was fixed at 150 W, and the process pressure was 10 mTorr. The Ar/O2 gas mixture ratio was controlled by mass flow controllers (MFC) as 22.5/2.5 in sccm. To improve the sensing membrane, a post deposition annealing (PDA) in N2 ambient at 900 °C for 30 min was used. For the control group, the ISFETs with Si3N4 sensing membrane were prepared. The Si3N4 layers were deposited by LPCVD using the standard process parameters of ITE [2,22,23].
Figure 1.
The structure and cross section of Ta2O5-ISFET.
Finally, all ISFETs were assembled on printed circuit boards (PCB) with a silver paste (TED PELLA, Inc.) and then encapsulated with epoxy resin type adhesive JU-100 (KOKI Company Ltd.) with open windows of 3 × 3 mm2.
2.3. Enzyme Immobilization
The covalent bonding steps are shown in Figure 2. At first, to generate hydroxyl bond, the encapsulated Ta2O5-ISFETs were immersed into 10% hydrogen peroxide (H2O2) solution for 24 hours. Afterwards, the samples were put into 40 °C, 9% APTS solution for 4 hours for silylation. Then, the samples were immersed into 10% of glutaraldehyde for 1 hour, which is a dual function group to crosslink amine bond on urease and APTS. Finally, the urease powder was mixed with the phosphate buffer as the concentration of 1.5 μg/mL, and then 20 μL-urease was dripped on the open window of ISFET before storing the sample at 4 °C (fridge) overnight. After rinsing non-immobilized enzyme by phosphate buffer, the EnFETs were ready for measurement.
Figure 2.
Schematic of covalent bonding process flow of urea-EnFET based on Ta2O5 sensing membrane.
2.4. Measurement Setup
To evaluate the performance of ISFETs and EnFETs, the drain-to-source current vs. gate-to-source voltage (IDS–VGS) curves were measured by HP 4156, firstly. Afterwards, a constant drain-source voltage and constant drain-source current (CVCC) circuit was used in measurements with IDS = 100 μA and VDS = +0.5 V. To evaluate the pH sensing properties, the commercial pH buffer solutions from pH 2 to pH 12 were used. For the urea sensitivity, different urea concentrations from 1 mM to 10 mM were prepared by diluting 5 mM phosphate buffer at pH 6. All of the measurements were done at 25 °C.
In addition, for all the measurement, a commercial Ag/AgCl electrode was used to provide the sensing system a constant and stable reference potential.
3. Results and Discussion
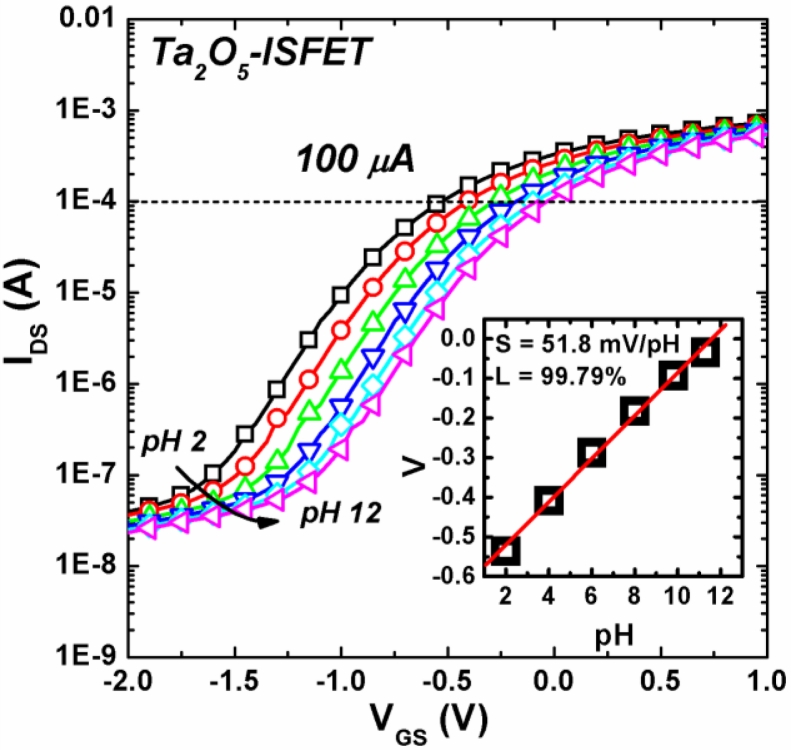
The IDS–VGS curves of Ta2O5-ISFET measured in the buffer solutions from pH 2 to pH 12 are shown in Figure 3. The curves shifted to the right-side when the ISFETs were measured in buffer solutions of high pH values. The output voltage of different pH buffer solution was calculated from the corresponding point at IDS = 100 μA. The output voltage and calibration curve are shown in the insert of Figure 3. For this Ta2O5-ISFET without post treatment, the pH sensitivity was 51.8 mV/pH with a high linearity (99.79%) of the calibration curve.
Figure 3.
IDS–VGS curves of Ta2O5-ISFET measured in the buffer solutions with different pH value (from pH 2 to pH 12).
Afterwards, the sensing properties including sensitivity, hysteresis, and drift were measured by the CVCC circuit. The results of Ta2O5-ISFETs without and with post annealing are listed in Table 1. Besides, the results of standard Si3N4-ISFET are also included. In this table, both Ta2O5-ISFETs without and with post annealing show higher sensitivity than Si3N4-ISFET. Especially for the ISFETs with annealed Ta2O5 sensing membrane, a near Nernstian response of 56.9 mV/pH was performed. In addition, better reliability was observed from the low hysteresis (with the measurement loop of pH 6-2-6-12-6, and 6-12-6-2-6) and drift (measured in pH 4 for 12 hours). The improved sensing properties of ISFETs with Ta2O5 layer could be caused by the formation of polycrystalline, the increase of surface area, and the increasing of oxygen density [13,24,25].
Table 1.
The sensing properties of ISFETs with Si3N4, Ta2O5, and annealed Ta2O5 sensing membranes.
| pH sensitivity (mV/pH) | Hysteresis (6-2-6-12-6) (mV) | Hysteresis (6-12-6-2-6) (mV) | Drift coefficient (mV/h) | |
|---|---|---|---|---|
| Si3N4 | 51.1 | — | 5.1 | <1 mV/h |
| Ta2O5 | 51.8 | 15.2 | 6.3 | <1 mV/h |
| Ta2O5 (anneal) | 56.9 | 0.3 | 0.7 | <1 mV/h |
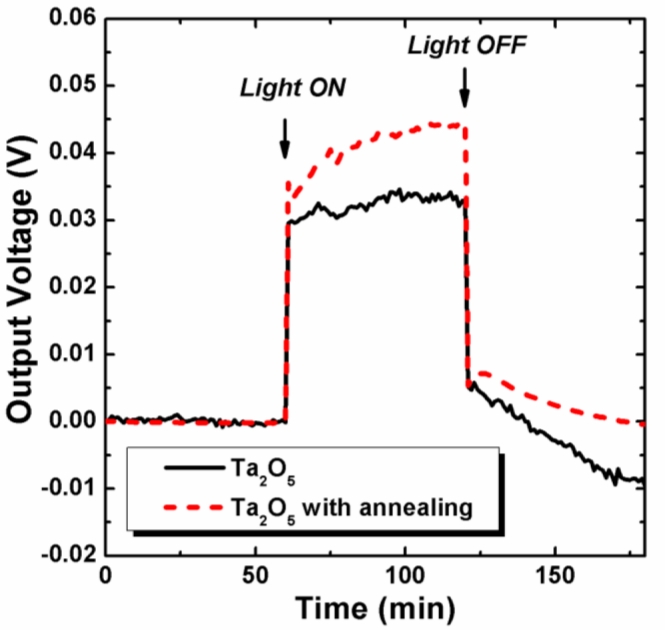
In addition, the light induced drift of the Ta2O5 ISFETs without and with post annealing was measured with a light source of optical microscope. The light intensity was 4,500 lux measured by a light meter. The ISFETs were stored in a beaker covered with aluminum foil for the uniform light illumination for all ISFETs. The VGS of ISFETs were firstly measured under dark condition for 1 hour, then with the light turned on for 1 hour, and then back under the dark condition for 1 hour. The results are shown in Figure 4. During the illumination, both curves shifted to the higher potential, similar to drift phenomena, which could be resulted by the light generated electrons and holes in the dielectric layer and silicon substrate [9]. The curves of Ta2O5-ISFETs with post annealing showed better stability. Besides, after illuminating and back to the dark condition, the output voltage returned to the initial output voltage.
Figure 4.
Light induced drift of the Ta2O5 ISFETs without and with post annealing.
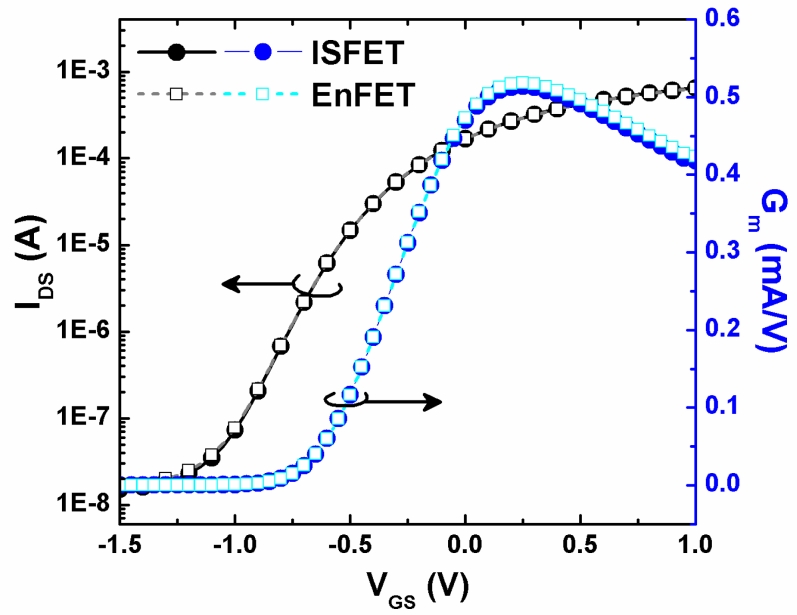
After the evaluation of the pH sensing properties of the test ISFETs, other ISFETs were processed for the covalent bonding and urease immobilization. For monitoring the urea concentration by EnFET, at first, the IDS–VGS curves and transconductance (Gm) of Ta2O5-ISFET and EnFET were measured by HP 4156, as shown in Figure 5. The similar response of ISFET and EnFET means the surface capacitance had almost no change before and after the chemical reaction of covalent bonding and urease immobilization. The good electrical compatibility between ISFET and EnFET are very suitable for the further application of enzymatic EnFET/ISFET (REFET) pair [26].
Figure 5.
IDS–VGS curves and transconductance of ISFET and EnFET.
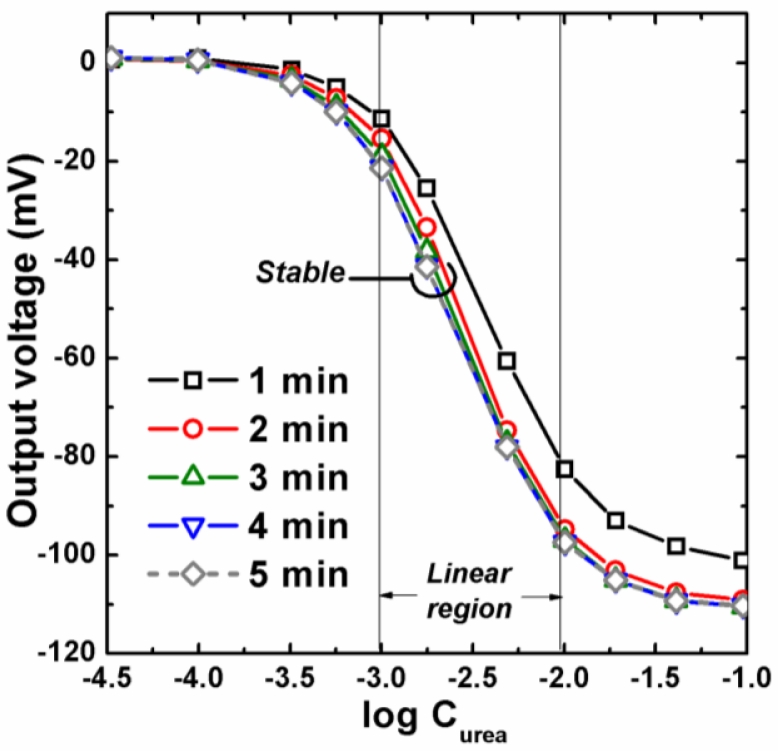
The time response to output voltage measured in 0–30 mM urea are shown in Figure 6. In this figure, the output voltages were stable in about 2 min. In addition, the suitable operation region of urea concentration could also be observed in this figure. The response could be divided to three regions: (I) Low response (lower than 1 mM), which could be due to the limitation of the lowest urea concentration, (II) High response (between 1 mM to 15 mM), which showed the higher voltage shift and could be the suitable region for urea detection, (III) Saturation region (higher than 15 mM), where very low voltage shift was observed even though the urea concentration increased.
Figure 6.
Time response to output voltage measured in 0–30 mM urea.
Based on Figure 6, the calibration curves of output voltage with different sampling time were also replotted and shown in Figure 7. In this figure, the calibration curves sampled from different time were checked again, which showed only slight shift between 2 and 5 min. Concerning time and accurate response, 5 min sampling time was chosen for further evaluation. In addition, the suitable operating region for urea detection by EnFET was redefined as logCurea from −3.00 (1 mM) to −2.12 (7.5 mM), which was caused by the change of the unit of urea concentration from linear scale to log scale.
Figure 7.
Calibration curves of output voltage with 1–5 min sampling time.
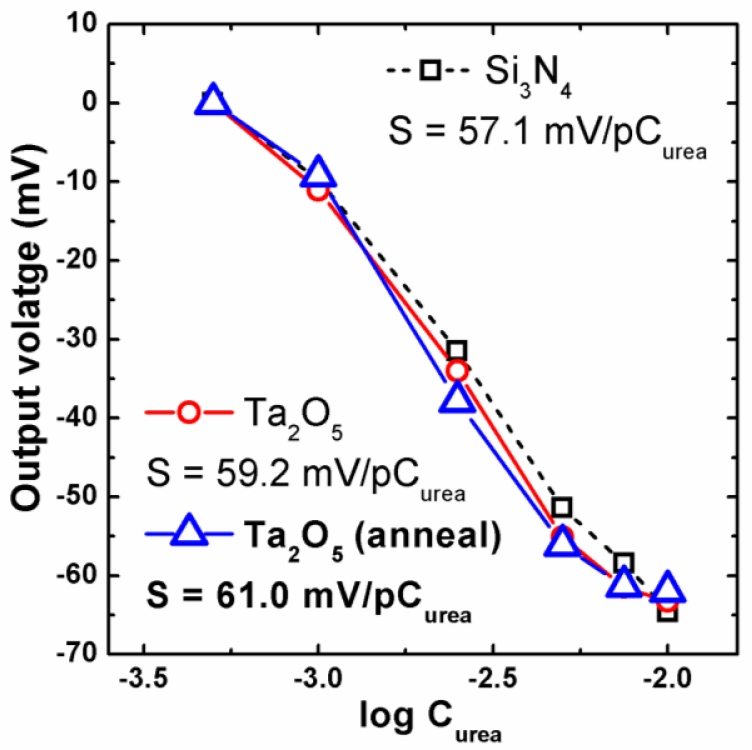
Finally, the calibration curves of the urea-EnFETs with Si3N4, Ta2O5, and annealed Ta2O5 sensing membranes were compared in Figure 8. For all samples, the linearity is higher than 99% between 1 mM to 7.5 mM of urea. The sensitivity of EnFET with Si3N4 sensing membrane is 57.1 mV/pCurea, and the sensitivity for the EnFETs with Ta2O5 and annealed Ta2O5 sensing membranes are 59.2 mV/pCurea and 61.0 mV/pCurea, respectively. Since the urea detection is based on the increase of the byproduct of hydrogen ions, the sensitivity of EnFETs for urea concentration is also dependent on the pH sensitivity of ISFETs. In this case, the ratio of pH sensitivity and urea sensitivity is about 1.1 ± 0.43.
Figure 8.
Calibration curves of the urea-EnFETs with Si3N4, Ta2O5, and annealed Ta2O5 sensing membranes.
4. Conclusions
The urea-EnFET with covalent bonding process was investigated based on the pH-ISFETs with Ta2O5 sensing membranes. In addition, the sensing properties of Ta2O5-ISFETs were also improved from the control samples by the post N2 annealing, including higher pH sensitivity, lower hysteresis, lower drift coefficient, and lower light induced drift. The improved sensing properties were also corresponding to the EnFETs. In this research, the output responses of EnFETs were stabilized in 2 min, and the applicable and linear measurement range for urea concentration was from 1 mM to 7.5 mM. For the EnFET with annealed Ta2O5 sensing membrane, the sensitivity was 61 mV/pCurea. Based on the high sensitivity and minimized non-ideal factors, the Ta2O5 layer with post annealing could be used to replace the conventional Si3N4 layer as the basic substrate platform for biomedical applications, such as chemically modified field effect transistors (ChemFETs), EnFETs with other enzyme, and DNA-FETs.
Acknowledgments
This work was supported by the National Science Council under the contract of NSC-99-2221-E-182-056-MY3, NSC-99-2811-E-182-006 and NSC-98-2221-E-182-057-MY3.
References
- 1.Caras S, Janata J. Field effect transistor sensitive to penicillin. Anal. Chem. 1980;52:1935–1937. [Google Scholar]
- 2.Pijanowska DG, Torbicz W. pH-ISFET based urea biosensor. Sens. Actuat. B. 1997;44:370–376. [Google Scholar]
- 3.Dzyadevich SV, Korpan YI, Arkhipova VN. Application of enzyme field-effect transistors for determination of glucose concentrations in blood serum. Biosens. Bioelectron. 1999;14:283–287. doi: 10.1016/s0956-5663(99)00007-x. [DOI] [PubMed] [Google Scholar]
- 4.Soldatkin AP, Montoriol J, Sant W, Martelet C, Jaffrezic-Renault N. Creatinine sensitive biosensor based on ISFETs and creatinine deiminase immobilised in BSA membrane. Talanta. 2002;58:351. doi: 10.1016/s0039-9140(02)00283-7. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo T, Wise KD. An integrated field-effect electrode for biopotential recording. IEEE Trans. Biomed. Eng. 1974;21:485. [Google Scholar]
- 6.Bousse L, Mostarshed S, van der Schoot B, de Rooij NF. Comparison of the hysteresis of Ta2O5 and Si3N4 pH-sensing insulators. Sens. Actuat. B. 1994;17:157–164. [Google Scholar]
- 7.Bobrov PV, Tarantov YA, Krause S, Moritz W. Chemical sensitivity of an ISFET with Ta2O5 membrane in strong acid and alkaline solutions. Sens. Actuat. B. 1991;3:75–81. [Google Scholar]
- 8.Kwon D-H, Cho B-W, Kim C-S, Sohn B-K. Effects of heat treatment on Ta2O5 sensing membrane for low drift and high sensitivity pH-ISFET. Sens. Actuat. B. 1996;34:441–445. [Google Scholar]
- 9.Ito Y. Long-term drift mechanism of Ta2O5 gate pH-ISFETs. Sens. Actuat. B. 2000;64:152–155. [Google Scholar]
- 10.Chou J-C, Weng C-Y. Sensitivity and hysteresis effect in Al2O3 gate pH-ISFET. Mater. Chem. Phys. 2001;71:120. [Google Scholar]
- 11.Shin P-K. The pH-sensing and light-induced drift properties of titanium dioxide thin films deposited by MOCVD. Appl. Surf. Sci. 2003;214:214–221. [Google Scholar]
- 12.Chou J-C, Liao LP. Study on pH at the point of zero charge of TiO2 pH ion-sensitive field effect transistor made by the sputtering method. Thin Solid Film. 2005;476:157–161. [Google Scholar]
- 13.Lai C-S, Yang C-M, Lu T-F. pH sensitivity improvement on 8 nm thick hafnium oxide by post deposition annealing. Electrochem. Solid-State Lett. 2006;9:G90–G92. [Google Scholar]
- 14.van der Wal PD, Briand D, Mondin G, Jenny S, Jeanneret S, Millon C, Roussel H, Dubourdieu C, de Rooij NF. High-k dielectrics for use as ISFET gate oxides. Proceedings of IEEE Sensors; Vienna, Austria. 24–27 October 2004; pp. 677–680. [Google Scholar]
- 15.Chou JC, Wang YF. Preparation and study on the drift and hysteresis properties of the tin oxide gate ISFET by the sol-gel method. Sens. Actuat. B. 2002;86:58. [Google Scholar]
- 16.Bobrov PV, Tarantov YA, Krause S, Moritz W. Chemical sensitivity of an ISFET with Ta2O5 membrane. Sens. Actuat. B. 1991;3:75–81. [Google Scholar]
- 17.Schöning MJ, Brinkmann D, Rolka D, Demuth C, Poghssian A. CIP (cleaning-in-place) suitable “non-glass” pH sensor based on a Ta2O5-gate EIS structure. Sens Actuat B. 2005;111–112:423–429. [Google Scholar]
- 18.Braeken D, Rand DR, Andrei A, Huys R, Spira ME, Yitzchaik S, Shappir J, Borghs G, Callewaert G, Bartic C. Glutamate sensing with enzyme-modified floating-gate field effect transistors. Biosens. Bioelectron. 2009;24:2384. doi: 10.1016/j.bios.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Migita S, Ozasa K, Ikeno S, Tanaka T, Haruyama T. Molecular commonality sensing of phosphoric anhydride substances using an ion-sensitive field-effect transistor covered with an artificial enzyme membrane. Jpn. J. Appl. Phys, Part 1. 2007;46:7539. [Google Scholar]
- 20.Voorthuyzen JA, Bergveld P. Photoelectric effects in Ta2O5-SiO2-Si structures. Sens. Actuat. 1990;B1:350–353. [Google Scholar]
- 21.Mikolajick T, Kühnhold R, Ryssel H. The pH-sensing properties of tantalum pentoxide filmes fabricated by metal organic low pressure chemical vapor depostition. Sens. Actuat. B. 1997;44:262–267. [Google Scholar]
- 22.Dawgul M, Pijanowska DG, Krzyskow A, Kruk J, Torbicz W. An influence of polyHEMA gate layer on properties of ChemFETs. Sensors. 2003;3:146–159. [Google Scholar]
- 23.Lai C-S, Lue C-E, Yang C-M, Dawgul M, Pijanowska DG. Optimization of a PVC Membrane for Reference Field Effect Transistors. Sensors. 2009;9:2076–2087. doi: 10.3390/s90302076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai C-S, Lue C-E, Yang C-M, Jao J-H, Tai C-C. New pH-sensitive TaOxNy membranes prepared by NH3 plasma surface treatment and nitrogen incorporated reactive sputtering. Sens. Actuat. B. 2008;130:77–81. [Google Scholar]
- 25.Lai C-S, Lue C-E, Yang C-M, Jao J-H. Modifications on pH Sensitivitiy of Si3N4 Membrane by CF4 Plasma and Rapid Thermal Annealing for ISFET/REFET Applications. International Conference on Solid State Devices and Materials; Yokohama, Japan. 12–15 September 2006; pp. 790–791. [Google Scholar]
- 26.Sant W, Pourciel ML, Launay J, Conto TD, Martinez A, Temple-Boyer P. Development of chemical field effect transistors for detection of urea. Sens. Actuat. B. 2004;95:309–314. [Google Scholar]