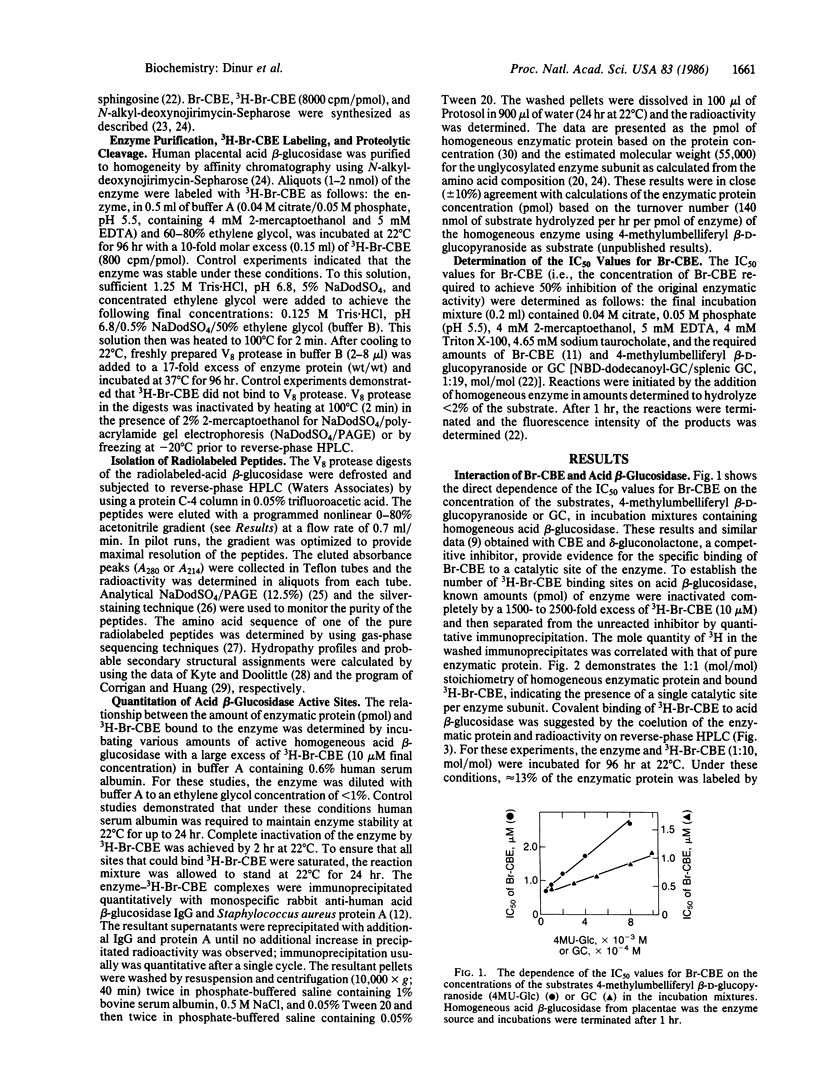
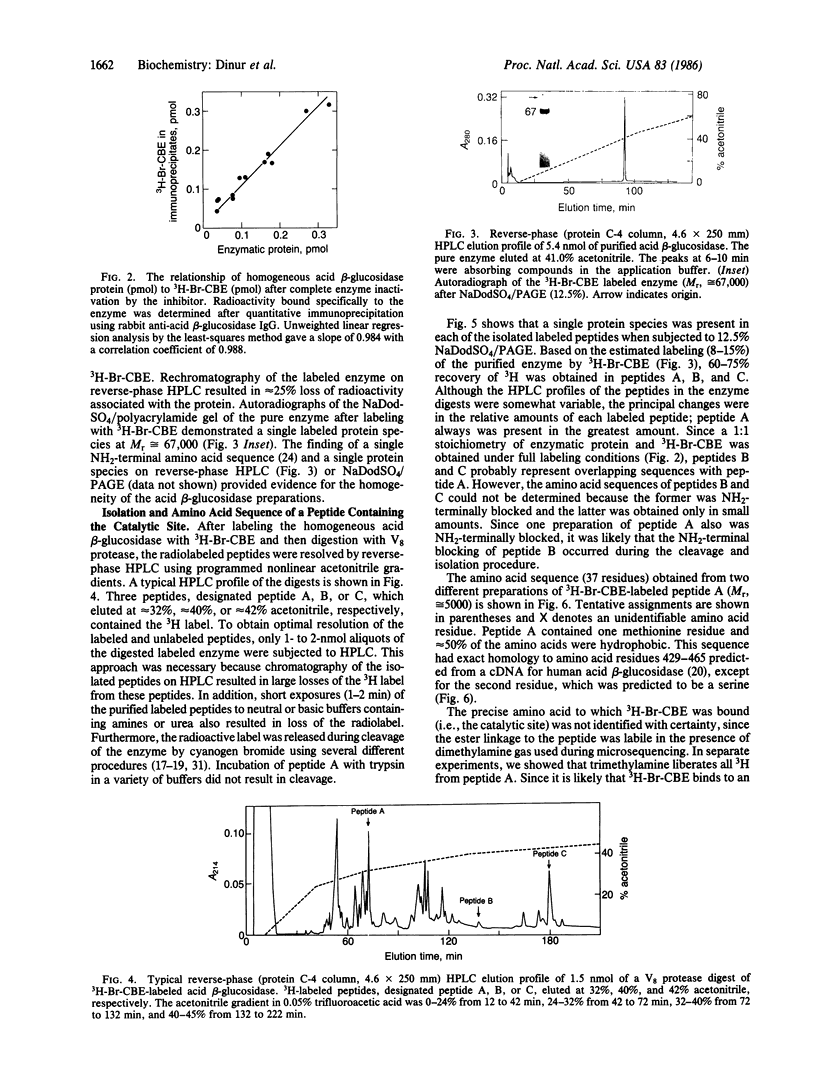
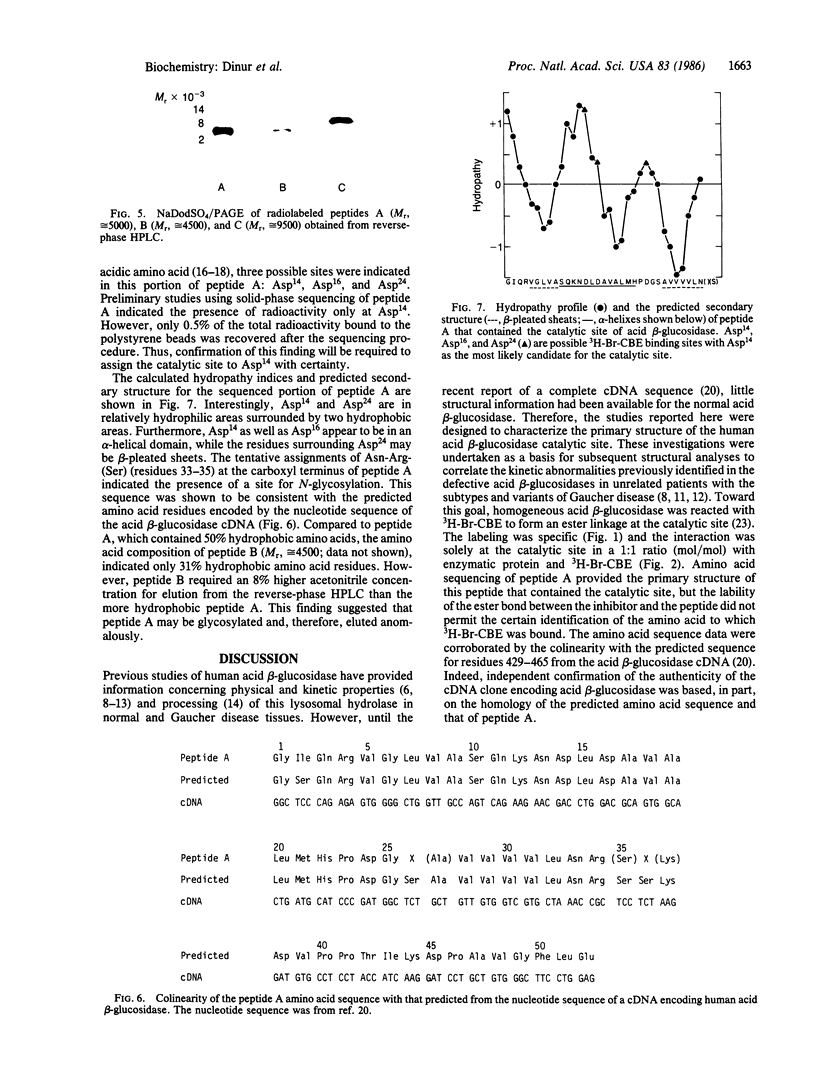
Abstract
Human acid beta-glucosidase (D-glucosyl-N-acylsphingosine glucohydrolase, EC 3.2.1.45) cleaves the glucosidic bonds of glucosylceramide and synthetic beta-glucosides. The deficient activity of this hydrolase is the enzymatic defect in the subtypes and variants of Gaucher disease, the most prevalent lysosomal storage disease. To isolate and characterize the catalytic site of the normal enzyme, brominated 3H-labeled conduritol B epoxide (3H-Br-CBE), which inhibits the enzyme by binding covalently to this site, was used as an affinity label. Under optimal conditions 1 mol of 3H-Br-CBE bound to 1 mol of pure enzyme protein, indicating the presence of a single catalytic site per enzyme subunit. After V8 protease digestion of the 3H-Br-CBE-labeled homogeneous enzyme, three radiolabeled peptides, designated peptide A, B, or C, were resolved by reverse-phase HPLC. The partial amino acid sequence (37 residues) of peptide A (Mr, 5000) was determined. The sequence of this peptide, which contained the catalytic site, had exact homology to the sequence near the carboxyl terminus of the protein, as predicted from the nucleotide sequence of the full-length cDNA encoding acid beta-glucosidase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADY R. O., KANFER J., SHAPIRO D. THE METABOLISM OF GLUCOCEREBROSIDES. I. PURIFICATION AND PROPERTIES OF A GLUCOCEREBROSIDE-CLEAVING ENZYME FROM SPLEEN TISSUE. J Biol Chem. 1965 Jan;240:39–43. [PubMed] [Google Scholar]
- Bause E., Legler G. Isolation and amino acid sequence of a hexadecapeptide from the active site of beta-glucosidase A3 from Aspergillus wentii. Hoppe Seylers Z Physiol Chem. 1974 Apr;355(4):438–442. doi: 10.1515/bchm2.1974.355.1.438. [DOI] [PubMed] [Google Scholar]
- Bause E., Legler G. Isolation and structure of a tryptic glycopeptide from the active site of beta-glucosidase A3 from Aspergillus wentii. Biochim Biophys Acta. 1980 Dec 16;626(2):459–465. doi: 10.1016/0005-2795(80)90142-7. [DOI] [PubMed] [Google Scholar]
- Berent S. L., Radin N. S. Mechanism of activation of glucocerebrosidase by co-beta-glucosidase (glucosidase activator protein). Biochim Biophys Acta. 1981 Jun 23;664(3):572–582. doi: 10.1016/0005-2760(81)90134-x. [DOI] [PubMed] [Google Scholar]
- Corrigan A. J., Huang P. C. A BASIC microcomputer program for plotting the secondary structure of proteins. Comput Programs Biomed. 1982 Dec;15(3):163–168. doi: 10.1016/0010-468x(82)90001-0. [DOI] [PubMed] [Google Scholar]
- Dale G. L., Villacorte D. G., Beutler E. Solubilization of glucocerebrosidase from human placenta and demonstration of a phospholipid requirement for its catalytic activity. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1048–1053. doi: 10.1016/0006-291x(76)90760-9. [DOI] [PubMed] [Google Scholar]
- Dinur T., Grabowski G. A., Desnick R. J., Gatt S. Synthesis of a fluorescent derivative of glucosyl ceramide for the sensitive determination of glucocerebrosidase activity. Anal Biochem. 1984 Jan;136(1):223–234. doi: 10.1016/0003-2697(84)90329-4. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R. The primary structure of staphylococcal protease. Can J Biochem. 1978 Jun;56(6):534–544. doi: 10.1139/o78-082. [DOI] [PubMed] [Google Scholar]
- Erickson J. S., Radin N. S. N-hexyl-O-glucosyl sphingosine, an inhibitor of glucosyl ceramide -glucosidase. J Lipid Res. 1973 Mar;14(2):133–137. [PubMed] [Google Scholar]
- Furbish F. S., Blair H. E., Shiloach J., Pentchev P. G., Brady R. O. Enzyme replacement therapy in Gaucher's disease: large-scale purification of glucocerebrosidase suitable for human administration. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3560–3563. doi: 10.1073/pnas.74.8.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt S., Dinur T., Osiecki K., Desnick R. J., Grabowski G. A. Use of activators and inhibitors to define the properties of the active site of normal and Gaucher disease lysosomal beta-glucosidase. Enzyme. 1985;33(2):109–119. doi: 10.1159/000469416. [DOI] [PubMed] [Google Scholar]
- Gatt S., Rapport M. M. Isolation of beta-galactosidase and beta-glucosidase from brain. Biochim Biophys Acta. 1966 Mar 7;113(3):567–576. doi: 10.1016/s0926-6593(66)80014-0. [DOI] [PubMed] [Google Scholar]
- Ginns E. I., Brady R. O., Pirruccello S., Moore C., Sorrell S., Furbish F. S., Murray G. J., Tager J., Barranger J. A. Mutations of glucocerebrosidase: discrimination of neurologic and non-neurologic phenotypes of Gaucher disease. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5607–5610. doi: 10.1073/pnas.79.18.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew R. H., Daniels L. B., Clark L. S., Hoyer S. W. Enzymic differentiation of neurologic and nonneurologic forms of Gaucher's disease. J Neuropathol Exp Neurol. 1982 Nov;41(6):630–641. doi: 10.1097/00005072-198211000-00006. [DOI] [PubMed] [Google Scholar]
- Grabowski G. A., Dagan A. Human lysosomal beta-glucosidase: purification by affinity chromatography. Anal Biochem. 1984 Aug 15;141(1):267–279. doi: 10.1016/0003-2697(84)90456-1. [DOI] [PubMed] [Google Scholar]
- Grabowski G. A., Dinur T., Osiecki K. M., Kruse J. R., Legler G., Gatt S. Gaucher disease types 1, 2, and 3: differential mutations of the acid beta-glucosidase active site identified with conduritol B epoxide derivatives and sphingosine. Am J Hum Genet. 1985 May;37(3):499–510. [PMC free article] [PubMed] [Google Scholar]
- Grabowski G. A., Gatt S., Kruse J., Desnick R. J. Human lysosomal beta-glucosidase: kinetic characterization of the catalytic, aglycon, and hydrophobic binding sites. Arch Biochem Biophys. 1984 May 15;231(1):144–157. doi: 10.1016/0003-9861(84)90371-0. [DOI] [PubMed] [Google Scholar]
- Grabowski G. A., Goldblatt J., Dinur T., Kruse J., Svennerholm L., Gatt S., Desnick R. J. Genetic heterogeneity in Gaucher disease: physicokinetic and immunologic studies of the residual enzyme in cultured fibroblasts from non-neuronopathic and neuronopathic patients. Am J Med Genet. 1985 Jul;21(3):529–549. doi: 10.1002/ajmg.1320210316. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legler G. Glucosidases. Methods Enzymol. 1977;46:368–381. doi: 10.1016/s0076-6879(77)46044-0. [DOI] [PubMed] [Google Scholar]
- Legler G., Harder A. Amino acid sequence at the active site of beta-glucosidase A from bitter almonds. Biochim Biophys Acta. 1978 May 11;524(1):102–108. doi: 10.1016/0005-2744(78)90108-0. [DOI] [PubMed] [Google Scholar]
- Quaroni A., Gershon E., Semenza G. Affinity labeling of the active sites in the sucrase-isomaltase complex from small intestine. J Biol Chem. 1974 Oct 25;249(20):6424–6433. [PubMed] [Google Scholar]
- Quaroni A., Semenza G. Partial amino acid sequences around the essential carboxylate in the active sites of the intestinal sucrase-isomaltase complex. J Biol Chem. 1976 Jun 10;251(11):3250–3253. [PubMed] [Google Scholar]
- Radin N. S. Preparation of psychosines (1-O-hexosyl sphingosine) from cerebrosides. Lipids. 1974 May;9(5):358–360. doi: 10.1007/BF02533114. [DOI] [PubMed] [Google Scholar]
- Sorge J., West C., Westwood B., Beutler E. Molecular cloning and nucleotide sequence of human glucocerebrosidase cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7289–7293. doi: 10.1073/pnas.82.21.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasberg P. M., Lowden J. A., Mahuran D. Purification of glucosylceramidase by affinity chromatography. Can J Biochem. 1982 Nov;60(11):1025–1031. doi: 10.1139/o82-132. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]





