Abstract
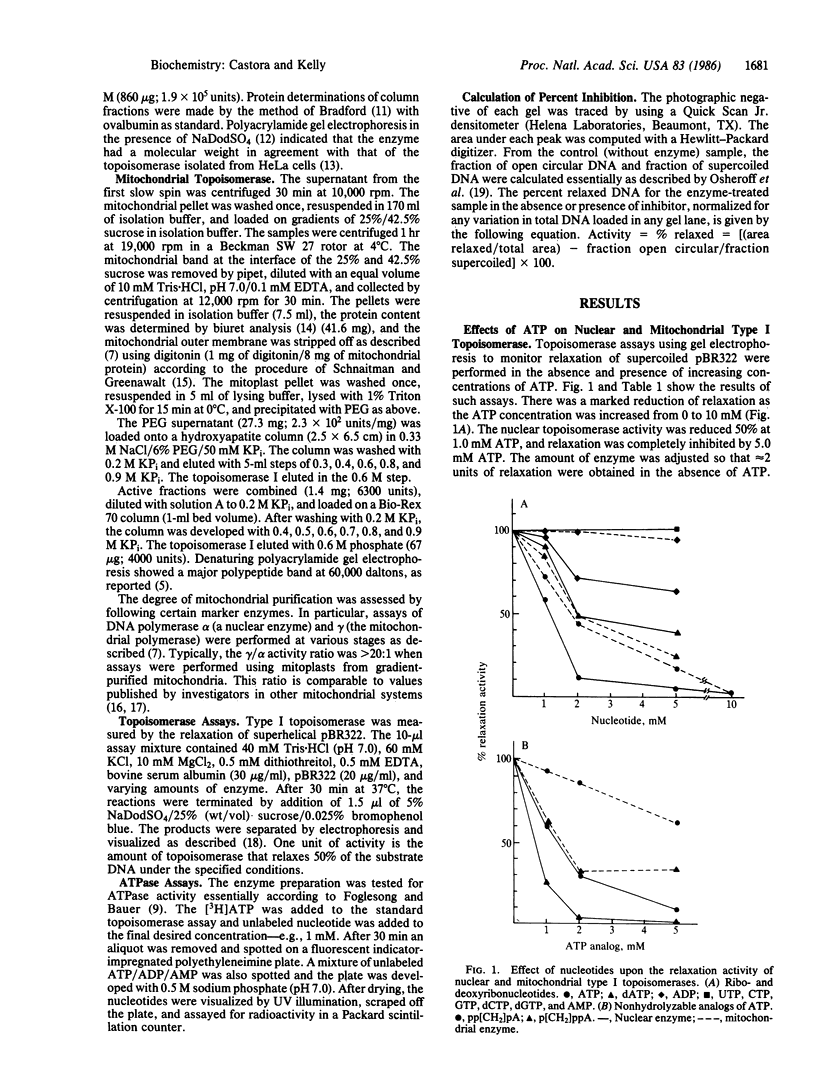
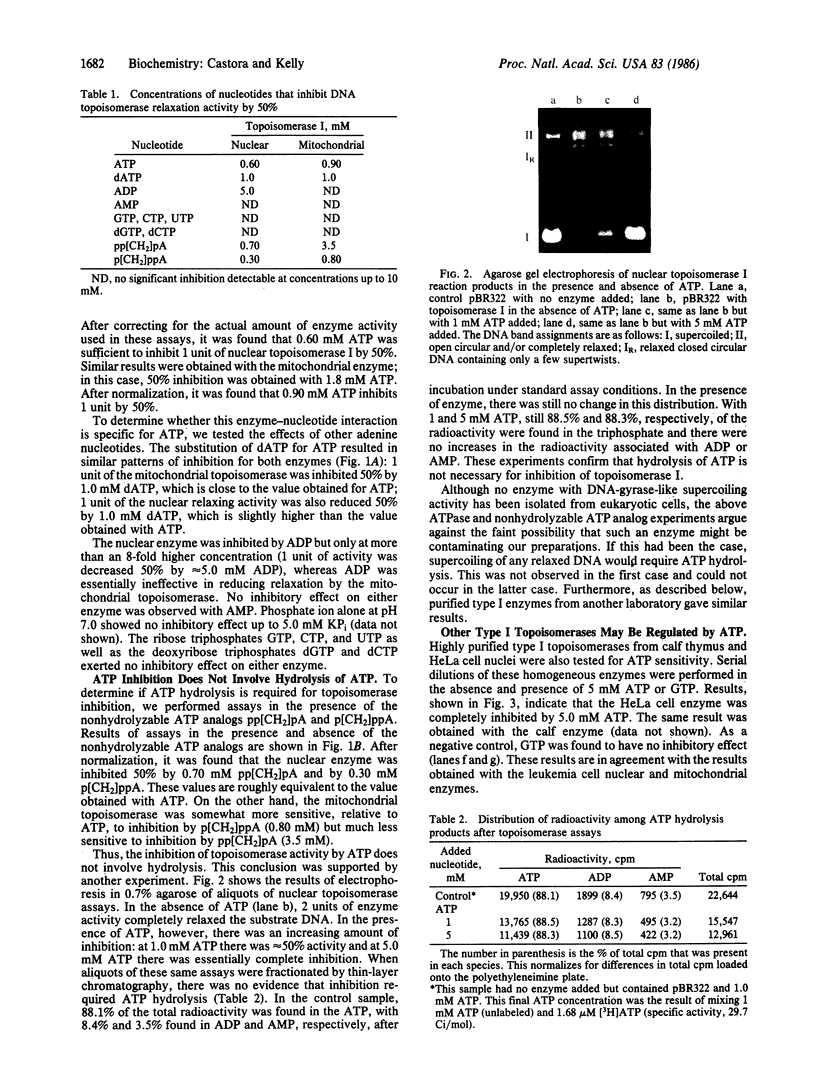
Type I topoisomerases have been purified from nuclei and mitochondria of human acute lymphoblastic leukemia cells. Both of these ATP-independent enzymes are actually found to be inhibited by ATP at physiologically significant concentrations. Other adenine nucleotides showed varying effects: ADP inhibited only at high concentrations; AMP had no effect on either topoisomerase. Both enzymes were also inhibited by dATP. The importance of the adenine ring structure was confirmed by the lack of an inhibitory effect observed with equivalent levels of GTP, UTP, CTP, or their deoxy counterparts. Assays performed in the presence of nonhydrolyzable analogs of ATP suggest that hydrolysis of ATP does not accompany this enzyme inhibition. This was supported by direct determination of the ATPase activity of the purified enzymes. Type I topoisomerase from calf thymus and HeLa cells were also found to be sensitive to ATP. These results suggest that mammalian type I topoisomerases in general may possess a nucleotide-binding site that may be involved in regulation of enzyme activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brun G., Vannier P., Scovassi I., Callen J. C. DNA topoisomerase I from mitochondria of Xenopus laevis oocytes. Eur J Biochem. 1981 Aug;118(2):407–415. doi: 10.1111/j.1432-1033.1981.tb06417.x. [DOI] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- Castora F. J., Arnheim N., Simpson M. V. Mitochondrial DNA polymorphism: evidence that variants detected by restriction enzymes differ in nucleotide sequence rather than in methylation. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6415–6419. doi: 10.1073/pnas.77.11.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castora F. J., Lazarus G. M. Isolation of a mitochondrial DNA topoisomerase from human leukemia cells. Biochem Biophys Res Commun. 1984 May 31;121(1):77–86. doi: 10.1016/0006-291x(84)90690-9. [DOI] [PubMed] [Google Scholar]
- Castora F. J., Lazarus G. M., Kunes D. The presence of two mitochondrial DNA topoisomerases in human acute leukemia cells. Biochem Biophys Res Commun. 1985 Jul 31;130(2):854–866. doi: 10.1016/0006-291x(85)90495-4. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA gyrase and the supercoiling of DNA. Science. 1980 Feb 29;207(4434):953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Veech R. L., Wilson D. F. Thermodynamic relationships between the oxidation-reduction reactions and the ATP synthesis in suspensions of isolated pigeon heart mitochondria. Arch Biochem Biophys. 1974 Feb;160(2):412–421. doi: 10.1016/0003-9861(74)90415-9. [DOI] [PubMed] [Google Scholar]
- Foglesong P. D., Bauer W. R. Effects of ATP and inhibitory factors on the activity of vaccinia virus type I topoisomerase. J Virol. 1984 Jan;49(1):1–8. doi: 10.1128/jvi.49.1.1-8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Goto T., Laipis P., Wang J. C. The purification and characterization of DNA topoisomerases I and II of the yeast Saccharomyces cerevisiae. J Biol Chem. 1984 Aug 25;259(16):10422–10429. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerases--enzymes that catalyse the breaking and rejoining of DNA. CRC Crit Rev Biochem. 1983;15(1):1–24. doi: 10.3109/10409238309102799. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Miller K. G. Eukaryotic DNA topoisomerases: two forms of type I DNA topoisomerases from HeLa cell nuclei. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3487–3491. doi: 10.1073/pnas.78.6.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Liu L. F., Englund P. T. A homogeneous type II DNA topoisomerase from HeLa cell nuclei. J Biol Chem. 1981 Sep 10;256(17):9334–9339. [PubMed] [Google Scholar]
- Osheroff N., Shelton E. R., Brutlag D. L. DNA topoisomerase II from Drosophila melanogaster. Relaxation of supercoiled DNA. J Biol Chem. 1983 Aug 10;258(15):9536–9543. [PubMed] [Google Scholar]
- Rowe T. C., Rusche J. R., Brougham M. J., Holloman W. K. Purification and properties of a topoisomerase from Ustilago maydis. J Biol Chem. 1981 Oct 25;256(20):10354–10361. [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scovassi A. I., Wicker R., Bertazzoni U. A phylogenetic study on vertebrate mitochondrial DNA polymerase. Eur J Biochem. 1979 Oct 15;100(2):491–496. doi: 10.1111/j.1432-1033.1979.tb04193.x. [DOI] [PubMed] [Google Scholar]
- Trask D. K., DiDonato J. A., Muller M. T. Rapid detection and isolation of covalent DNA/protein complexes: application to topoisomerase I and II. EMBO J. 1984 Mar;3(3):671–676. doi: 10.1002/j.1460-2075.1984.tb01865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Sci Am. 1982 Jul;247(1):94-7, 100-9. doi: 10.1038/scientificamerican0782-94. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]