Abstract
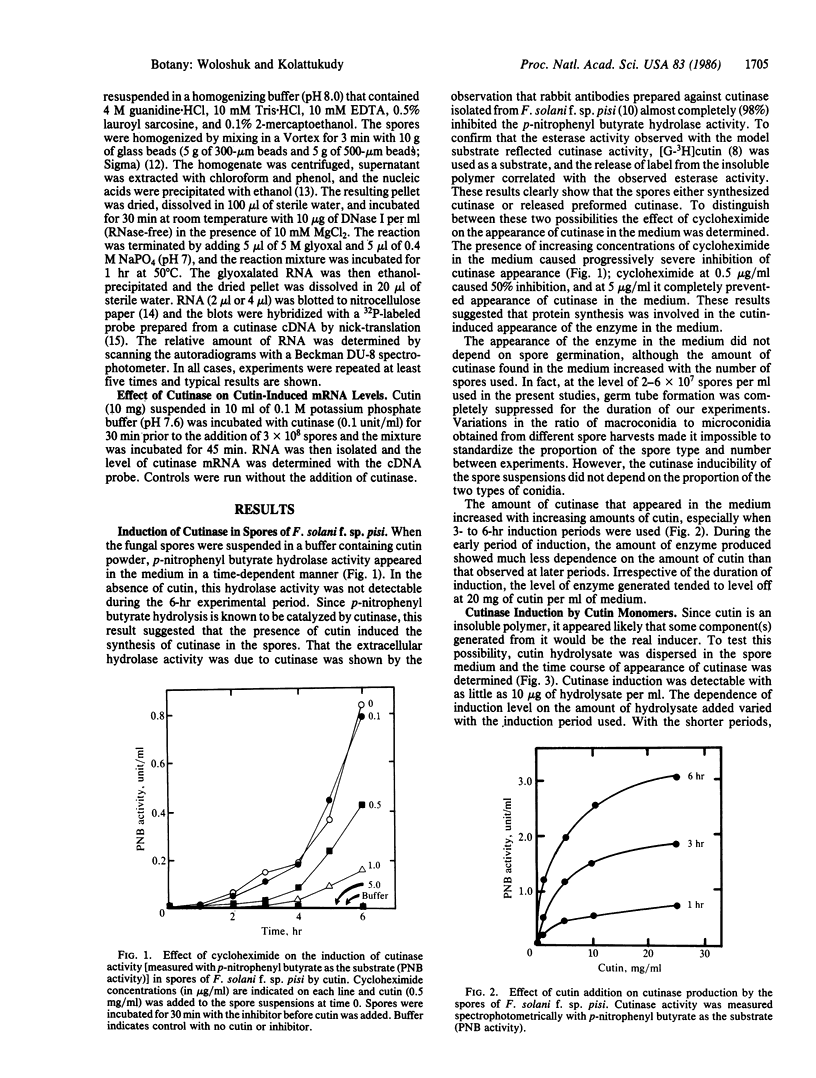
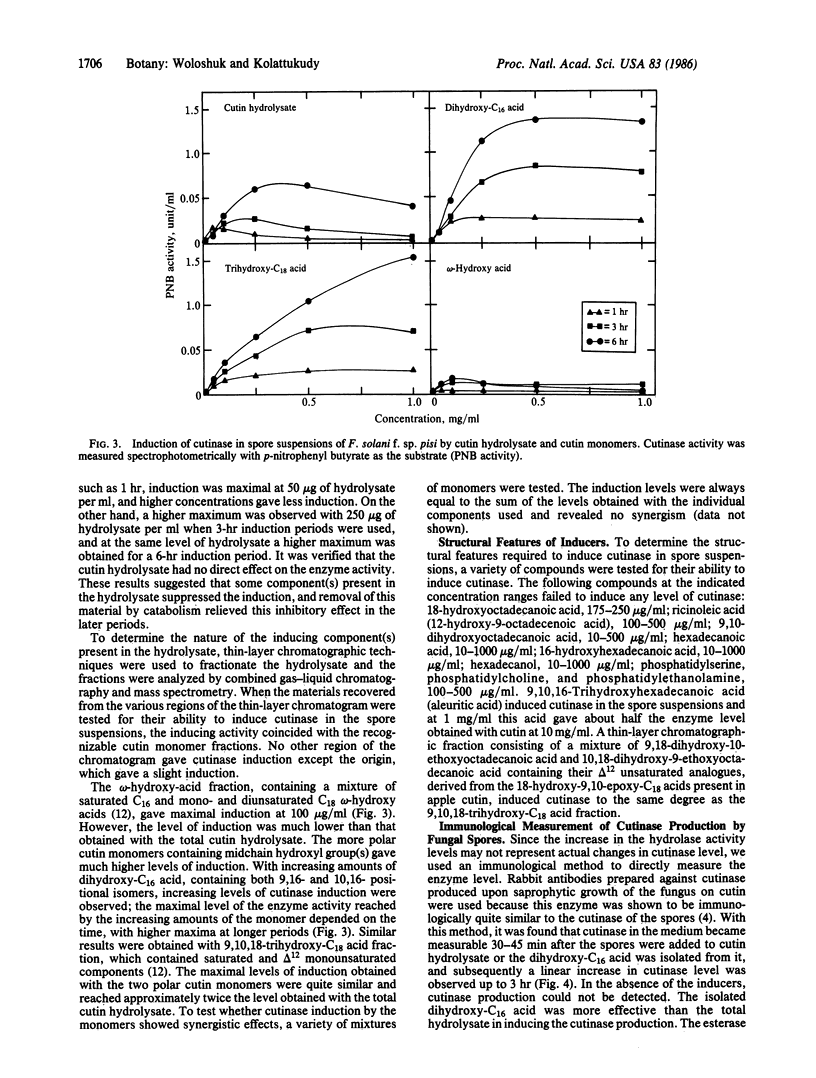
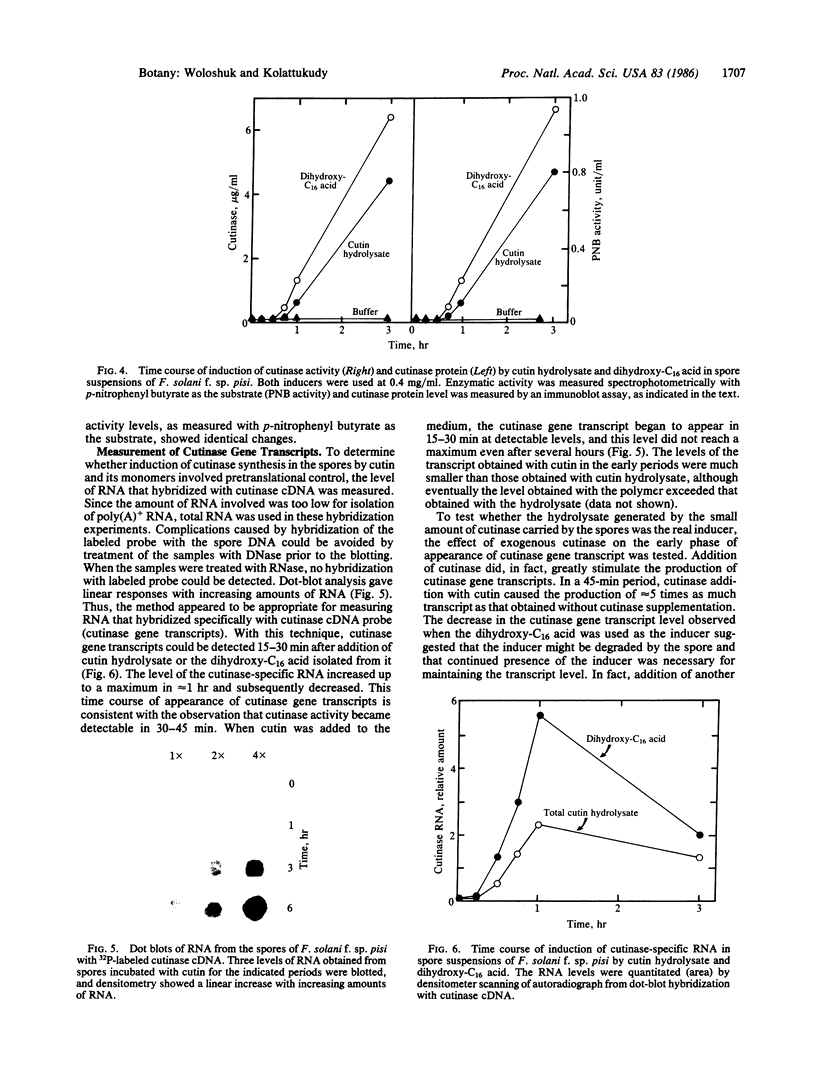
Spores of the phytopathogenic fungus Fusarium solani f. sp. pisi were shown to produce the extracellular enzyme, cutinase, only when cutin or cutin hydrolysate was added to the spore suspension. Dihydroxy-C16 acid and trihydroxy-C18 acid, which are unique cutin monomers, showed the greatest cutinase-inducing activity. Experiments with several compounds structurally related to these fatty acids suggested that both a ω-hydroxyl and a midchain hydroxyl are required for cutinase-inducing activity. Cutinase appeared in the medium 30-45 min after the addition of the inducers to the spore suspension, and the activity level increased for 6 hr. Addition of cycloheximide (5 μg/ml) completely inhibited cutinase production, suggesting that protein synthesis was involved in the increase of cutinase activity. Immunoblot analysis with rabbit antibodies prepared against cutinase showed that cutinase protein increased in parallel with the increase in enzyme activity. Measurement of cutinase-specific RNA levels by dot-blot hybridization with 32P-labeled cutinase cDNA showed that the cutinase gene transcripts could be detected within 15 min after addition of the inducers. Addition of exogenous cutinase greatly enhanced the level of cutinase gene transcripts induced by cutin. These results strongly suggest that the fungal spore senses that it is in contact with the plant by the production of small amounts of cutin monomers catalyzed by the low level of cutinase carried by the spore and that these monomers induce the synthesis of cutinase needed for penetration of the fungus into the plant.
Keywords: cutin, fungal penetration of cuticle, cutinase mRNA, hydroxy fatty acids
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Jahn R., Schiebler W., Greengard P. A quantitative dot-immunobinding assay for proteins using nitrocellulose membrane filters. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1684–1687. doi: 10.1073/pnas.81.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy P. E. Biopolyester membranes of plants: cutin and suberin. Science. 1980 May 30;208(4447):990–1000. doi: 10.1126/science.208.4447.990. [DOI] [PubMed] [Google Scholar]
- Lin T. S., Kolattukudy P. E. Induction of a biopolyester hydrolase (cutinase) by low levels of cutin monomers in Fusarium solani f.sp. pisi. J Bacteriol. 1978 Feb;133(2):942–951. doi: 10.1128/jb.133.2.942-951.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy R. E., Kolattukudy P. E. Hydrolysis of plant cuticle by plant pathogens. Purification, amino acid composition, and molecular weight of two isozymes of cutinase and a nonspecific esterase from Fusarium solani f. pisi. Biochemistry. 1975 Jul;14(13):2824–2831. doi: 10.1021/bi00684a006. [DOI] [PubMed] [Google Scholar]
- Soliday C. L., Flurkey W. H., Okita T. W., Kolattukudy P. E. Cloning and structure determination of cDNA for cutinase, an enzyme involved in fungal penetration of plants. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3939–3943. doi: 10.1073/pnas.81.13.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliday C. L., Kolattukudy P. E. Isolation and characterization of a cutinase from Fusarium roseum culmorum and its immunological comparison with cutinases from F. solani pisi. Arch Biochem Biophys. 1976 Sep;176(1):334–343. doi: 10.1016/0003-9861(76)90172-7. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Freer S. N. Simple procedure for disruption of fungal spores. Appl Environ Microbiol. 1978 Mar;35(3):622–623. doi: 10.1128/aem.35.3.622-623.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]