Abstract
We prospectively studied the correlations between plasma levels of von Willebrand factor and its cleaving protease—a disintegrin and metalloproteinase with thrombospondin type I motif, member 13 (ADAMTS13)—in 126 patients who did or did not develop no-reflow phenomenon after primary percutaneous intervention for acute ST-segment–elevation myocardial infarction. Quantitative plasma levels of von Willebrand factor and ADAMTS13 were measured by immunoturbidometric assay.
Angiographic no-reflow was observed in 46 (37%) of the 126 patients. At admission, plasma levels of von Willebrand factor were significantly higher in the no-reflow group (P < 0.001), but levels of ADAMTS13 at admission were similar in the 2 groups (P = 0.143). At logistic regression, after adjustment for serum creatinine, left ventricular ejection fraction, high-sensitivity C-reactive protein, and N-terminal pro B-type natriuretic peptide, plasma von Willebrand factor level at admission (≥5,531 mU/mL) was still the predictive factor for the no-reflow phenomenon. The area under the receiver operating characteristics curve was 0.785.
Our results suggest that high von Willebrand factor level is related to the no-reflow phenomenon in such a way that it might be a predictor of the phenomenon.
Key words: ADAMTS13 protein, human; angioplasty, balloon, coronary/adverse effects; microcirculation; myocardial ischemia; myocardial reperfusion; no-reflow phenomenon/drug therapy; predictive value of tests; prospective studies; von Willebrand factor
In approximately 10% to 40% of cases in which primary percutaneous coronary intervention (PCI) is performed as intervention for ST-elevation myocardial infarction (STEMI), there is a subsequent angiographic “no-reflow” phenomenon, which is associated with a poor clinical prognosis in both the short and long terms.1–3 The mechanisms that underlie no-reflow are complex and are only partially understood. Neutrophil and platelet plugging, intense vasoconstriction, and external microvascular compression caused by myocardial edema are potential causes of the microvascular obstruction associated with no-reflow.4–7 Furthermore, in patients treated by primary PCI, distal thrombosis is likely to play a contributory role.
A central molecule in hemostasis, von Willebrand factor (vWF), plays an important role in the microcirculation.8–11 Meta-analyses of prospective studies have suggested that high levels of circulating vWF are associated with increased risk of coronary heart disease.12 A disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13 (ADAMTS13) cleaves the vWF A2 domain, reducing its molecular weight and consequently also its platelet-tethering function.8 Deficiency of ADAMTS13 promotes vWF-induced platelet aggregation, which can result in thrombotic thrombocytopenic purpura. In mouse models of thrombosis, ADAMTS13 downregulates both platelet adhesion to the exposed subendothelial matrix and thrombus formation in injured arterioles.13 Consequently, it has been suggested that the circulating levels of ADAMTS13 can influence either the circulating levels of vWF or the function of vWF (or both) and can therefore influence the risk of thrombotic events, such as myocardial infarction, in the general population.
von Willebrand Factor participates in endothelial activation, which is known to play a fundamental role in the no-reflow phenomenon.3,14,15 Previous study has shown elevated plasma vWF levels in patients with no-reflow.16 However, we lack data that compare vWF and ADAMTS13 levels with various risk scores—especially in the setting of no-reflow phenomenon after primary PCI. To examine this issue, we prospectively evaluated the relationship between baseline vWF and ADAMTS13 plasma levels and no-reflow in a population of consecutive patients who underwent primary PCI within 12 hours of the onset of chest pain because of STEMI.
Patients and Methods
Study Population
Between August 2008 and December 2009, we studied 126 consecutive patients who underwent PCI as an intervention for STEMI. The inclusion criteria were prolonged chest pain (>30 min), ST-segment elevation greater than 2 mm in at least 2 continuous electrocardiographic leads, and a more than 2-fold increase in serum creatine kinase. We excluded patients with severe chronic heart failure, heart failure of Killip class II or higher on admission, severe valvular heart disease, or a calculated creatinine clearance of less than 80 mL/min/m2.
The study protocol was approved by the hospital's ethics committee, and patients gave written, informed consent. All clinical data were collected prospectively.
We enrolled 126 patients, 46 (37%) of whom exhibited a Thrombolysis in Myocardial Infarction (TIMI) flow grade of less than 3 within 12 hours after stent deployment. The clinical characteristics in each group are shown in Table I. At baseline, the no-reflow group showed higher levels of serum creatinine (79.26 ± 19.02 vs 64.98 ± 21.74 μmol/L, P = 0.002), N-terminal pro B-type natriuretic peptide (NT-pro-BNP) (1,261.77 ± 1,666 vs 354.28 ± 652.9 pg/mL, P = 0.001), and high-sensitivity C-reactive protein (hs-CRP) (6.17 ± 3.46 vs 3.14 ± 3.25 mg/L, P < 0.001); the no-reflow group also exhibited a lower left ventricular ejection fraction (LVEF) than did the optimal perfusion group (0.58 ± 0.09 vs 0.64 ± 0.93, P = 0.002). There were no significant differences between the 2 groups in age, sex, diabetes mellitus, hypertension, total cholesterol, triglycerides, low-density-lipoprotein cholesterol (LDL-C), high-density-lipoprotein cholesterol (HDL-C), Gensini score, pre-PCI time, and drugs administered before admission.
TABLE I. Baseline Characteristics of the Patients Enrolled in the Study
Procedures and Medical Treatment
All patients received oral aspirin (300 mg) and clopidogrel (300 mg) immediately after admission, and they received intravenous heparin (5,000 U) before PCI. Low-molecular-weight heparin was continued for the subsequent 5 to 7 days. All patients underwent coronary angiography via the same standard technique. We repeated balloon angioplasty or we implanted stents as needed to reduce the stenosis diameter to under 50%. All stents were deployed at 10 to 16 atm. Angiograms were analyzed by means of a validated quantitative coronary angiographic system (Allura DCI program, Philips Medical Systems; Best, The Netherlands). Angiographic no-reflow after PCI was defined as a TIMI flow of less than 3 immediately after stent implantation, without evidence of dissection or vasospasm.
Blood Sampling
Venous blood samples were obtained at 3 time points from all patients: on admission, at the end of the procedure, and a week after PCI. At the time of sampling, the first 3 mL of blood was obtained for biochemical assessment, and the subsequent 4.5 mL of venous blood was collected in a sequential manner into an evacuated tube containing 0.5 mL of sodium citrate solution (0.13 mol/L, pH 7.5). All blood samples were immediately centrifuged at 3,000 revolutions/min for 10 min at 4 °C, and aliquots of samples were stored at −80 °C until analysis.
Measurement of Plasma von Willebrand Factor and ADAMTS13 Levels
Plasma vWF and ADAMTS13 levels were measured using IMUBIND® vWF ELISA kits (Product Nos. 828 and 813, American Diagnostica Inc.; Stamford, Conn), according to the manufacturer's instructions. The intra- and interassay coefficients of variations for the ELISA kit have been found to be 4% and 7.3%, respectively.
Follow-Up Study
After laboratory samples and angiographic data had been obtained, all patients were monitored for 6 months. The recorded events included heart failure, recurrence of myocardial infarction or unstable angina requiring hospitalization, life-threatening arrhythmia, and death from any cause.
Statistical Analysis
Patients were divided into 2 groups according to the angiographic results after primary PCI: optimal perfusion group (TIMI = 3) and no-reflow group (TIMI ≤2). Comparisons between the 2 groups were carried out using the Student t test or Mann-Whitney U test (as indicated) for continuous variables, whereas proportions were compared by use of the Fisher exact test. Data are expressed as mean ± SD (X ± SD), unless otherwise indicated.
All analyses were performed with SPSS statistical software version 15.0 (SPSS Inc., an IBM company; Chicago, Ill). Exploratory analysis was performed in accordance with standard tests, in order to determine differences in vWF and ADAMTS13 plasma levels between patients with no-reflow and those with optimal myocardial perfusion. Receiver operating characteristics (ROC) curve analysis of plasma vWF levels was performed to identify the optimal cutoff value for predicting the no-reflow phenomenon. Multivariate logistic regression analysis was used to identify independent predictors for the development of the no-reflow phenomenon. Variables included in the multivariable model were those showing a significant association with no-reflow at univariate analysis. Correlations between 2 continuous variables were evaluated using linear regression analysis. P < 0.05 was always required for statistical significance.
As a further characterization of the relationship between vWF plasma levels and no-reflow, patients were divided into 3 groups according to tertiles of vWF levels, and the presence or absence of no-reflow was compared across groups.
Results
All patients with a TIMI flow grade of less than 2 were treated with 100 to 200 μg nitroglycerin via intracoronary administration; if perfusion did not improve, we then administered intravenous tirofiban (a loading dose of 0.5 mg, followed by an infusion at 0.01 mg/kg/hr). At the end of the procedure, the last angiograms showed a TIMI flow grade of 2 or greater in all patients.
Plasma von Willebrand Factor and ADAMTS13 Levels
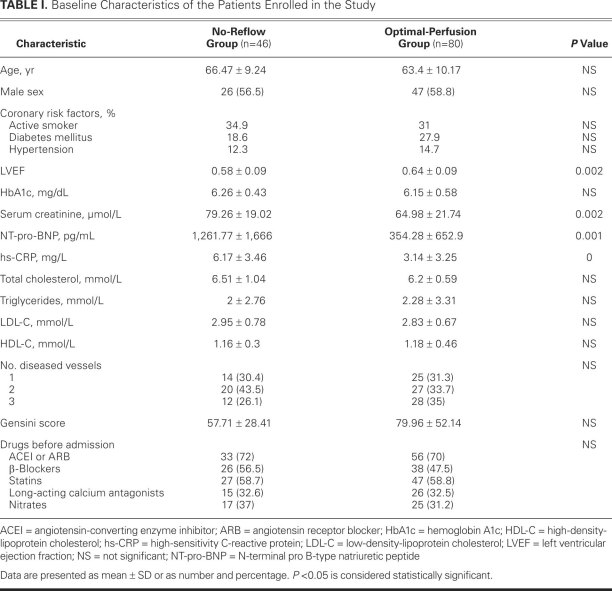
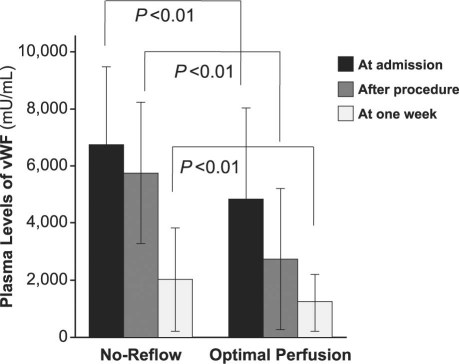
Elevated vWF concentrations were found in patients with no-reflow phenomenon, regardless of whether the samples had been collected on admission, just after stent deployment, or a week after PCI (P < 0.01) (Fig. 1). However, there were no significant differences in the plasma levels of ADAMTS13 between the 2 groups except for the samples collected a week after PCI, which showed a clear elevation in the no-reflow group (Fig. 2).
Fig. 1 The mean plasma levels of von Willebrand factor (vWF), at the same point of time, were higher in the no-reflow group than in the optimal-perfusion group. At a P value < 0.01, the differences were statistically significant.
Fig. 2 The mean plasma levels of ADAMTS13 in the 2 groups are shown at different points of time. At 1 week after PCI, the level in the no-reflow group increased markedly over that in the optimal-perfusion group, for a significant difference (P < 0.01).
ADAMTS13 = a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13
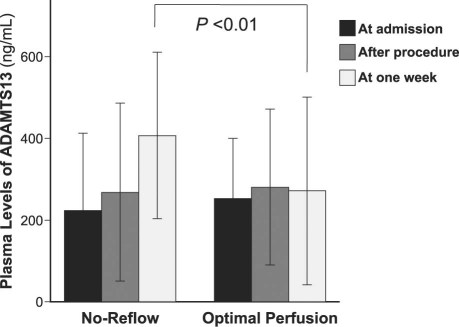
As a further characterization of the relationship between vWF and no-reflow, all 126 patients were divided into 3 groups according to tertiles of vWF plasma levels on admission, and the presence or absence of no-reflow was compared across groups. Figure 3 shows the percentage of patients with no-reflow according to tertiles of vWF plasma levels (1st vs 2nd tertile, P < 0.01; 2nd vs 3rd tertile, P < 0.01; and 3rd vs 1st tertile, P < 0.01).
Fig. 3 Percentages of patients with angiographic no-reflow, by tertiles of von Willebrand factor (vWF) plasma levels on admission. Patients were divided into 3 groups according to tertiles of vWF plasma levels on admission, and the presence or absence of no-reflow was compared across groups (1st vs 2nd tertile, P < 0.01; 2nd vs 3rd tertile, P < 0.01; and 3rd vs 1st tertile, P < 0.01).
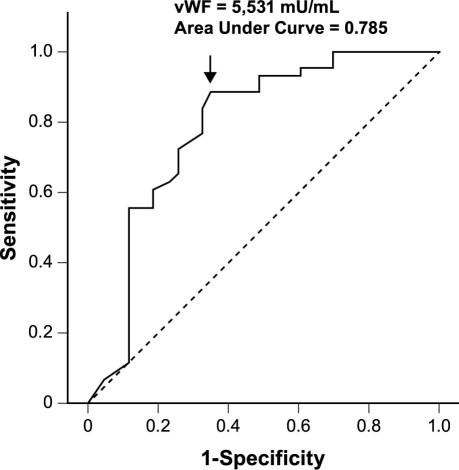
An ROC curve analysis was performed to evaluate the predictive power of vWF for the no-reflow phenomenon. The area under the ROC curve for vWF was 0.785 (Fig. 4). A vWF concentration ≥5,531 mU/mL showed a sensitivity of 88.4% and a specificity of 65.1%.
Fig. 4 Receiver operating characteristic curve analysis of von Willebrand factor (vWF) for predicting the angiographic no-reflow phenomenon. The number on the curve indicates the cutoff value of the point.
Predictors of the No-Reflow Phenomenon
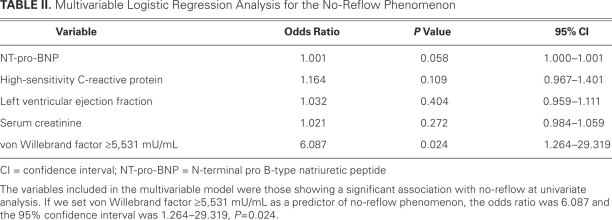
A multivariable logistic regression analysis (Table II) was performed to determine the independent factors related to the development of the no-reflow phenomenon. Along with the plasma levels of vWF on admission, the variables included in the multivariable model were those that showed a significant association with no-reflow at univariate analysis: NT-pro-BNP, LVEF, serum creatinine, and hs-CRP. A vWF of 5,531 mU/mL or greater was the potent predictor of no-reflow at multivariable analysis (odds ratio; 6.087; 95% confidence interval, 1.264–29.319; P = 0.024), when adjusted by hs-CRP, NT-pro-BNP, serum creatinine, and LVEF.
TABLE II. Multivariable Logistic Regression Analysis for the No-Reflow Phenomenon
Correlation on Admission between von Willebrand Factor and ADAMTS13 Levels (and Other Variables)
At the time of admission, the vWF levels correlated negatively with ADAMTS13 levels (r = −0.433; P < 0.001) and with LVEF (r = −0.411; P < 0.001), but correlated positively with hs-CRP (r = 0.39; P < 0.001), serum creatinine (r = 0.254; P = 0.018), and NT-pro-BNP (r = 0.235; P = 0.029). On admission, the ADAMTS13 levels correlated negatively with hs-CRP (r = −0.282; P = 0.009), LVEF (r = 0.014; P = 0.898), serum creatinine (r = −0.210; P = 0.052), and NT-pro-BNP (r = 0.072; P = 0.511).
Six-Month Clinical Outcomes
Short-term adverse sequelae occurred in 5 members of the no-reflow group and in 8 members of the optimal perfusion group (10.9% vs 10%, P >0.05); these included hematoma and minor bleeding at the puncture site and limitations on lower-extremity activity, but all patients recovered within 1 month.
During 6 months after primary PCI, there were no significant differences between the no-reflow group and the optimal perfusion group in terms of mortality rate (1 [2.2%] vs 0, P = 0.365), recurrent acute myocardial infarction or unstable angina pectoris (4 [8.7%] vs 1 [1.3%], P = 0.059), life-threatening arrhythmia (3 [6.5%] vs 1 [1.3%], P = 0.138), and heart failure requiring hospitalization (4 [8.7%] vs 3 [3.8%], P = 0.257). In the combined analysis, the incidence of major adverse cardiovascular events was significantly higher in the no-reflow group than in the optimal-perfusion group (12 [26.1%] vs 5 [6.3%], P = 0.003).
Discussion
After the onset of STEMI, primary PCI is a life-saving emergency procedure. The benefit comes from myocardial reperfusion in a short time. However, this benefit can be offset by the no-reflow phenomenon, which is a pathologic process arising from multiple factors, such as diabetes mellitus, hyperlipidemia, ischemic preconditioning, long ischemic duration, and thrombosis. Before performing primary PCI, one should evaluate the patient for the risk of no-reflow phenomenon. Although no direct pathophysiologic role has been verified, there have been suggestions that white blood cell count and levels of glucose, cholesterol, and inflammatory markers (such as hs-CRP) independently predict no-reflow.17–19
In our study, the incidence rate of no-reflow after primary PCI was 37%, which is similar to the incidence reported by others.15 There were no significant differences between the 2 groups in the presence of active smoking and diabetes, but there were clear differences in LVEF and in the levels of serum creatinine, HDL-C, and hs-CRP.
Our study showed not only that there is a correlation between plasma vWF levels on admission and the no-reflow phenomenon after primary PCI for STEMI, but that this correlation is sufficient to be predictive of no-reflow. In the ROC curve analysis (using a plasma vWF level ≥5,531 mU/mL), the sensitivity of 88.4% was excellent, but the specificity was only 65%. This relatively low specificity can be attributed to the wide variety of stimuli that cause endothelial cells to produce and release vWF: these include hypoxia, inflammatory cytokines, thrombin, leukocyte elastase, histamine, endotoxin, adrenaline,20 and other conditions associated with vascular damage, including primary pulmonary arterial hypertension and diabetic angiopathy.21 In the multivariable logistic regression analysis, we found that patients with high vWF levels on admission (≥5,531 mU/mL) had a 6.087-fold higher rate of no-reflow after PCI than those with lower vWF values (<5,531 mU/mL), after adjusting for LVEF and levels of serum creatinine, HDL-C, and hs-CRP. This finding implies that vWF levels on admission correspond with TIMI flow grade after primary PCI.
At admission, we did not find significant differences between the groups in plasma ADAMTS13 levels, but at a week after PCI the plasma levels of ADAMTS13 in the no-reflow group increased significantly. This suggested that abnormal increase of vWF, rather than lack of ADAMTS13, is the direct reason for no-reflow.
The no-reflow phenomenon is important not only because it correlates with infarct size but also because it provides additional prognostic information. Patients with the no-reflow phenomenon are in the highest risk subgroup of patients who undergo reperfusion, with increased associated risks of early and more protracted congestive heart failure and of death.1–3 As a result, the focus of reperfusion therapy is shifting toward improving the patency of the wider vasculature in the affected area rather than the patency merely of the target vessel. Although the preventive effects of embolic protection and some mechanical thrombectomy devices have been disappointing in the setting of STEMI,22–24 the preventive administration of some drugs before PCI may be helpful. For example, the use of GPIIb/IIIa inhibitor reduced the rate of no-reflow,25,26 and intracoronary thrombolytics or adenosine infusion before PCI was beneficial in the prevention of no-reflow in small studies.27,28
Limitations. Our study has some limitations. First, the techniques used to evaluate no-reflow may be somewhat inadequate in comparison with contrast echocardiography of the myocardium or magnetic resonance perfusion imaging, which are now considered the gold standard for that purpose. Second, we cannot exclude the possibility that the small sample size underpowered the study. Third, the number of diseased vessels does not precisely indicate the extent of the myocardial necrosis. The present study did not include objective data regarding the size of the infarct-related necrosis.
Conclusion
In clinical practice, the identification of no-reflow predictors will assist in determining which patients are at high risk. In this study, all patients with a TIMI flow grade of less than 2 were treated with intracoronary injection of nitroglycerin or tirofiban (or both), after which a TIMI flow grade or 2 or greater was restored in every case. Although in the combined analysis, the incidence of major adverse cardiovascular events was significantly higher in the no-reflow group than in the optimal-perfusion group, there were no significant differences between the groups in the prevalence of heart failure, recurrence of myocardial infarction or angina, life-threatening arrhythmia, or death for any reason during the 6-month follow-up period. Our result was much better than the outcomes observed in other studies,29,30 and it implies that a vWF-guided approach can be useful in identifying patients at high risk of no-reflow after primary stent implantation for STEMI. Consequently, we might be able to give more intensive treatment to patients at higher probable risk, in order to improve their clinical outcomes.
Footnotes
Address for reprints: Jian Li, MD, Department of Cardiology, Huashan Hospital, Fudan University, 12 Wulumuqi Zhong Rd., Shanghai 200040, PRC
E-mail: doctorlijian@yahoo.com.cn
This study, carried out in Huashan Hospital of Fudan University (Shanghai, PRC), was supported by a scientific grant (No. 2008-126) from Huashan Hospital.
References
- 1.Reffelmann T, Kloner RA. The no-reflow phenomenon: a basic mechanism of myocardial ischemia and reperfusion. Basic Res Cardiol 2006;101(5):359–72. [DOI] [PubMed]
- 2.Ito H. No-reflow phenomenon in patients with acute myocardial infarction: its pathophysiology and clinical implications. Acta Med Okayama 2009;63(4):161–8. [DOI] [PubMed]
- 3.Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol 2009;54(4):281–92. [DOI] [PubMed]
- 4.Ndrepepa G, Tiroch K, Keta D, Fusaro M, Seyfarth M, Pache J, et al. Predictive factors and impact of no reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ Cardiovasc Interv 2010;3(1):27–33. [DOI] [PubMed]
- 5.Kiernan TJ, Ruggiero NJ 2nd, Bernal JM, Don CW, Witzke C, Kiernan GD, et al. The no-reflow phenomenon in the coronary circulation. Cardiovasc Hematol Agents Med Chem 2009;7(3):181–92. [DOI] [PubMed]
- 6.Kojima S, Sakamoto T, Ishihara M, Kimura K, Miyazaki S, Tei C, et al. The white blood cell count is an independent predictor of no-reflow and mortality following acute myocardial infarction in the coronary interventional era. Ann Med 2004; 36(2):153–60. [DOI] [PubMed]
- 7.Haeck JD, Verouden NJ, Henriques JP, Koch KT. Current status of distal embolization in percutaneous coronary intervention: mechanical and pharmacological strategies. Future Cardiol 2009;5(4):385–402. [DOI] [PubMed]
- 8.Siedlecki CA, Lestini BJ, Kottke-Marchant KK, Eppell SJ, Wilson DL, Marchant RE. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood 1996;88(8):2939–50. [PubMed]
- 9.Miura S, Li CQ, Cao Z, Wang H, Wardell MR, Sadler JE. Interaction of von Willebrand factor domain A1 with platelet glycoprotein Ibalpha-(1–289). Slow intrinsic binding kinetics mediate rapid platelet adhesion. J Biol Chem 2000;275(11):7539–46. [DOI] [PubMed]
- 10.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 1996;84(2):289–97. [DOI] [PubMed]
- 11.Fredrickson BJ, Dong JF, McIntire LV, Lopez JA. Shear-dependent rolling on von Willebrand factor of mammalian cells expressing the platelet glycoprotein Ib-IX-V complex. Blood 1998;92(10):3684–93. [PubMed]
- 12.Mendolicchio GL, Ruggeri ZM. New perspectives on von Willebrand factor functions in hemostasis and thrombosis. Semin Hematol 2005;42(1):5–14. [DOI] [PubMed]
- 13.Tsai HM. ADAMTS13 and microvascular thrombosis. Expert Rev Cardiovasc Ther 2006;4(6):813–25. [DOI] [PMC free article] [PubMed]
- 14.Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation 2002;105(5):656–62. [DOI] [PubMed]
- 15.Reffelmann T, Kloner RA. The “no-reflow” phenomenon: basic science and clinical correlates. Heart 2002;87(2):162–8. [DOI] [PMC free article] [PubMed]
- 16.Sgueglia GA, Niccoli G, Spaziani C, Cosentino N, Russo E, Andreotti F, et al. Baseline von Willebrand factor plasma levels and no-reflow phenomenon after primary percutaneous coronary intervention for ST segment elevation myocardial infarction. Int J Cardiol 2010;145(2):230–2. [DOI] [PubMed]
- 17.Iwakura K, Ito H, Ikushima M, Kawano S, Okamura A, Asano K, et al. Association between hyperglycemia and the no-reflow phenomenon in patients with acute myocardial infarction. J Am Coll Cardiol 2003;41(1):1–7. [DOI] [PubMed]
- 18.Genda S, Miura T, Miki T, Ichikawa Y, Shimamoto K. K(ATP) channel opening is an endogenous mechanism of protection against the no-reflow phenomenon but its function is compromised by hypercholesterolemia. J Am Coll Cardiol 2002;40(7):1339–46. [DOI] [PubMed]
- 19.Foussas SG, Zairis MN, Lyras AG, Patsourakos NG, Tsirimpis VG, Katsaros K, et al. Early prognostic usefulness of C-reactive protein added to the Thrombolysis In Myocardial Infarction risk score in acute coronary syndromes. Am J Cardiol 2005;96(4):533–7. [DOI] [PubMed]
- 20.Spiel AO, Gilbert JC, Jilma B. von Willebrand factor in cardiovascular disease: focus on acute coronary syndromes. Circulation 2008;117(11):1449–59. [DOI] [PubMed]
- 21.Rubin DB, Wiener-Kronish JP, Murray JF, Green DR, Turner J, Luce JM, et al. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest 1990;86(2):474–80. [DOI] [PMC free article] [PubMed]
- 22.Stone GW, Webb J, Cox DA, Brodie BR, Qureshi M, Kalynych A, et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA 2005;293(9):1063–72. [DOI] [PubMed]
- 23.Gick M, Jander N, Bestehorn HP, Kienzle RP, Ferenc M, Werner K, et al. Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation. Circulation 2005;112(10):1462–9. [DOI] [PubMed]
- 24.Ali A, Cox D, Dib N, Brodie B, Berman D, Gupta N, et al. Rheolytic thrombectomy with percutaneous coronary intervention for infarct size reduction in acute myocardial infarction: 30-day results from a multicenter randomized study. J Am Coll Cardiol 2006;48(2):244–52. [DOI] [PubMed]
- 25.de Lemos JA, Antman EM, Gibson CM, McCabe CH, Giugliano RP, Murphy SA, et al. Abciximab improves both epicardial flow and myocardial reperfusion in ST-elevation myocardial infarction. Observations from the TIMI 14 trial. Circulation 2000;101(3):239–43. [DOI] [PubMed]
- 26.Giri S, Mitchel JF, Hirst JA, McKay RG, Azar RR, Mennett R, et al. Synergy between intracoronary stenting and abciximab in improving angiographic and clinical outcomes of primary angioplasty in acute myocardial infarction. Am J Cardiol 2000;86(3):269–74. [DOI] [PubMed]
- 27.Claeys MJ, Bosmans J, De Ceuninck M, Beunis A, Vergauwen W, Vorlat A, Vrints CJ. Effect of intracoronary adenosine infusion during coronary intervention on myocardial reperfusion injury in patients with acute myocardial infarction. Am J Cardiol 2004;94(1):9–13. [DOI] [PubMed]
- 28.Ishibashi F, Saito T, Hokimoto S, Noda K, Moriyama Y, Oshima S. Combined revascularization strategy for acute myocardial infarction in patients with intracoronary thrombus: preceding intracoronary thrombolysis and subsequent mechanical angioplasty. Jpn Circ J 2001;65(4):251–6. [DOI] [PubMed]
- 29.Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol 2000;36(4):1202–9. [DOI] [PubMed]
- 30.Jeong YH, Kim WJ, Park DW, Choi BR, Lee SW, Kim YH, et al. Serum B-type natriuretic peptide on admission can predict the ‘no-reflow’ phenomenon after primary drug-eluting stent implantation for ST-segment elevation myocardial infarction. Int J Cardiol 2010;141(2):175–81. [DOI] [PubMed]