Abstract
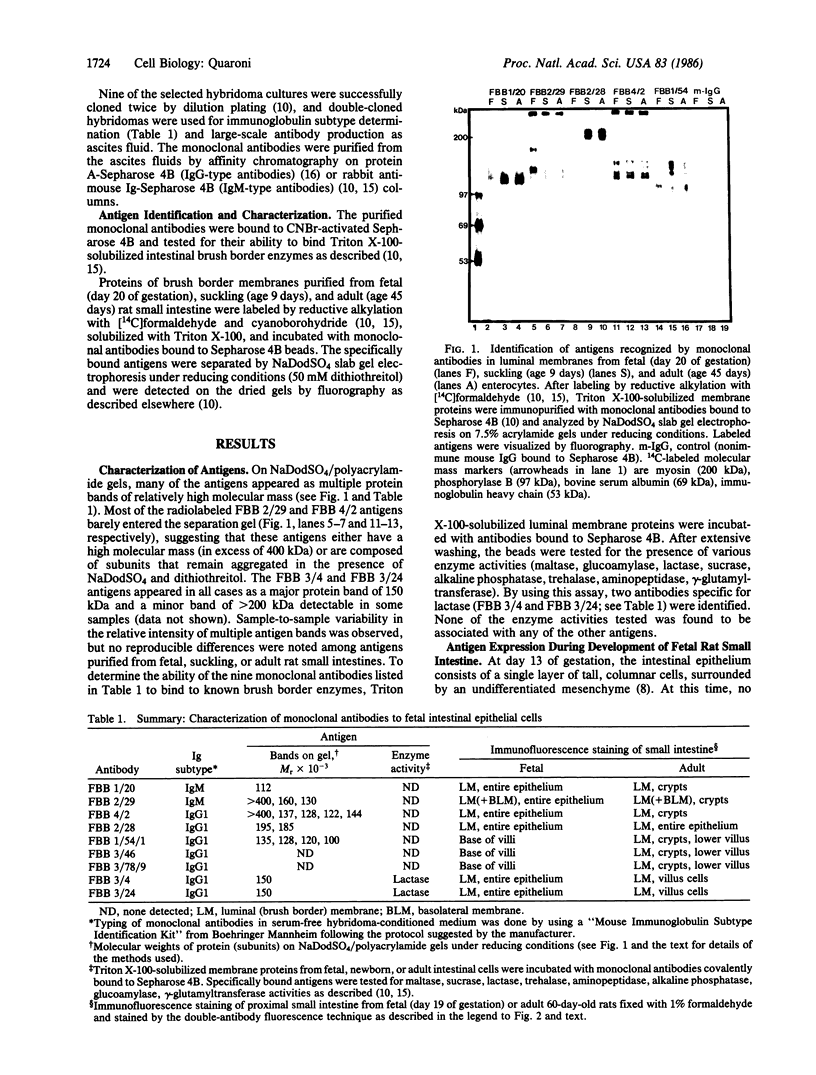
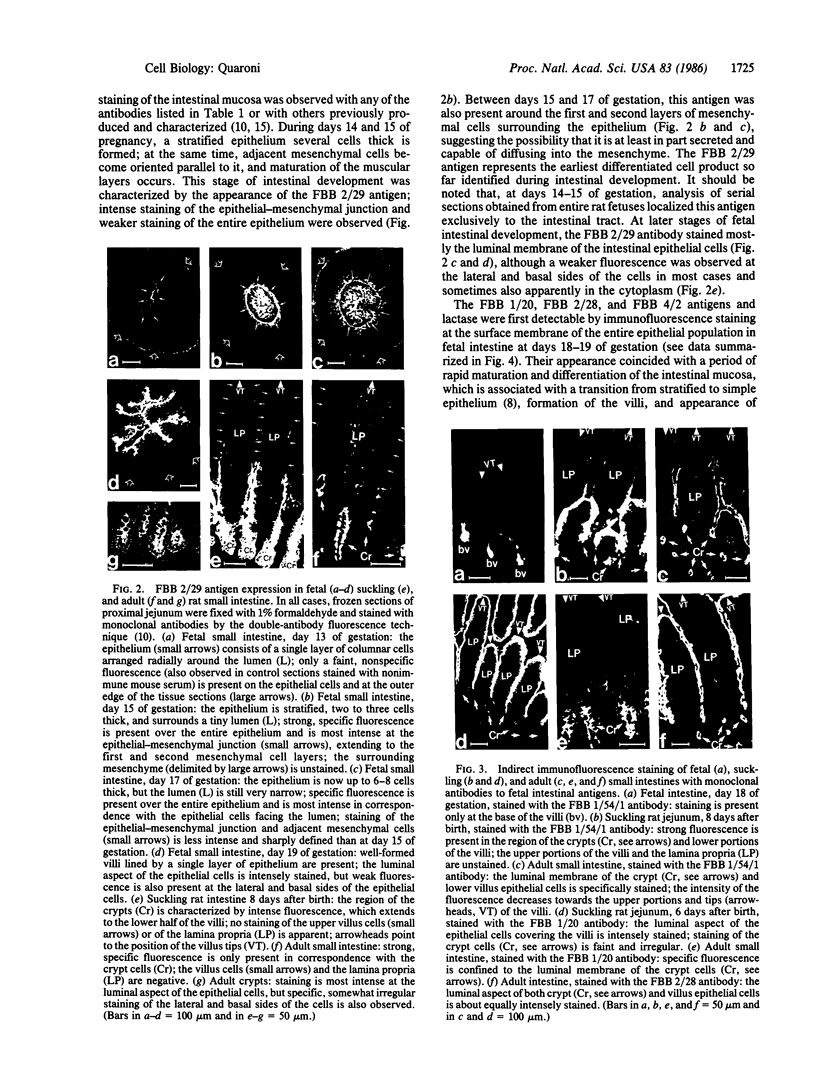
Nine monoclonal antibodies were prepared against luminal membranes purified from rat intestinal cells at day 19 of gestation, and seven of them were found to define antigens common to adult crypt cells and fetal or embryonic intestinal epithelial cells. The FBB 2/29 antigen was first detected over the entire intestinal epithelial population at days 14-15 of gestation, a period of development characterized by formation of a stratified intestinal epithelium and differentiation of the surrounding mesenchyme. This antigen, identified as a set of high molecular mass proteins, became restricted to the crypt and lower villus cells after birth and was exclusively expressed by the crypt cells in adult intestine. It also was found to be expressed by the epithelial cells of the distal tubuli in the kidney of adult rats and by cultured human tumor colonic cells. The FBB 1/54/1, FBB 3/46, and FBB 3/78/9 antibodies stained only the epithelial cells present at the base of the villi in fetal intestine, starting at days 20-21 of gestation (about 1-2 days before birth), and stained the crypt and lower villus cells in newborn and adult intestine; these antigens may be regarded as specific markers for the developing crypt cells in fetal intestine shortly before birth. The FBB 1/20 and FBB 4/2 antigens were first detected on the fetal intestinal cells at day 18 of gestation; they were located over the entire epithelium in newborn rats and became restricted to the crypts after weaning. The FBB 2/28 antigen was expressed by the entire intestinal epithelium at all stages of development, starting from days 18-19 of gestation in the fetus. Two antibodies, FBB 3/4 and FBB 3/24, were found to be specific for lactase. These results have demonstrated the expression of cell- and tissue-specific components in rat intestine during early embryonic development and revealed a marked similarity in surface membrane antigens between fetal intestinal epithelial cells and adult crypt cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cairnie A. B., Lamerton L. F., Steel G. G. Cell proliferation studies in the intestinal epithelium of the rat. I. Determination of the kinetic parameters. Exp Cell Res. 1965 Sep;39(2):528–538. doi: 10.1016/0014-4827(65)90055-8. [DOI] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974 Dec;141(4):537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Haffen K., Kedinger M., Simon P. M., Raul F. Organogenetic potentialities of rat intestinal epithelioid cell culture. Differentiation. 1981;18(2):97–103. doi: 10.1111/j.1432-0436.1981.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Hermos J. A., Mathan M., Trier J. S. DNA synthesis and proliferation by villous epithelial cells in fetal rats. J Cell Biol. 1971 Jul;50(1):255–258. doi: 10.1083/jcb.50.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- LEBLOND C. P., MESSIER B. Renewal of chief cells and goblet cells in the small intestine as shown by radioautography after injection of thymidine-H3 into mice. Anat Rec. 1958 Nov;132(3):247–259. doi: 10.1002/ar.1091320303. [DOI] [PubMed] [Google Scholar]
- Mathan M., Moxey P. C., Trier J. S. Morphogenesis of fetal rat duodenal villi. Am J Anat. 1976 May;146(1):73–92. doi: 10.1002/aja.1001460104. [DOI] [PubMed] [Google Scholar]
- Montgomery R. K., Zinman H. M., Smith B. T. Organotypic differentiation of trypsin-dissociated fetal rat intestine. Dev Biol. 1983 Nov;100(1):181–189. doi: 10.1016/0012-1606(83)90209-9. [DOI] [PubMed] [Google Scholar]
- Ponder B. A., Schmidt G. H., Wilkinson M. M., Wood M. J., Monk M., Reid A. Derivation of mouse intestinal crypts from single progenitor cells. Nature. 1985 Feb 21;313(6004):689–691. doi: 10.1038/313689a0. [DOI] [PubMed] [Google Scholar]
- Quaroni A. Crypt cell development in newborn rat small intestine. J Cell Biol. 1985 May;100(5):1601–1610. doi: 10.1083/jcb.100.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A. Development of fetal rat intestine in organ and monolayer culture. J Cell Biol. 1985 May;100(5):1611–1622. doi: 10.1083/jcb.100.5.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A. Expression of YBB 3/10 antigen in human tumor colon cell lines and its induction by N,N-dimethylformamide. J Natl Cancer Inst. 1985 Mar;74(3):591–602. [PubMed] [Google Scholar]
- Quaroni A., Isselbacher K. J. Study of intestinal cell differentiation with monoclonal antibodies to intestinal cell surface components. Dev Biol. 1985 Oct;111(2):267–279. doi: 10.1016/0012-1606(85)90482-8. [DOI] [PubMed] [Google Scholar]
- Quaroni A. Pre- and postnatal development of differentiated functions in rat intestinal epithelial cells. Dev Biol. 1985 Oct;111(2):280–292. doi: 10.1016/0012-1606(85)90483-x. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. II. Glycosyltransferases and endogenous acceptors of the undifferentiated cell surface membrane. J Biol Chem. 1973 Apr 10;248(7):2542–2548. [PubMed] [Google Scholar]