Abstract
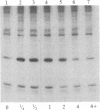
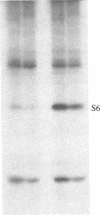
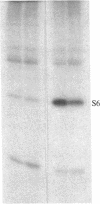
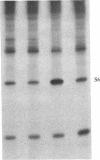
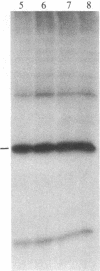
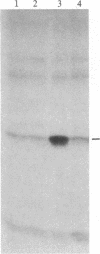
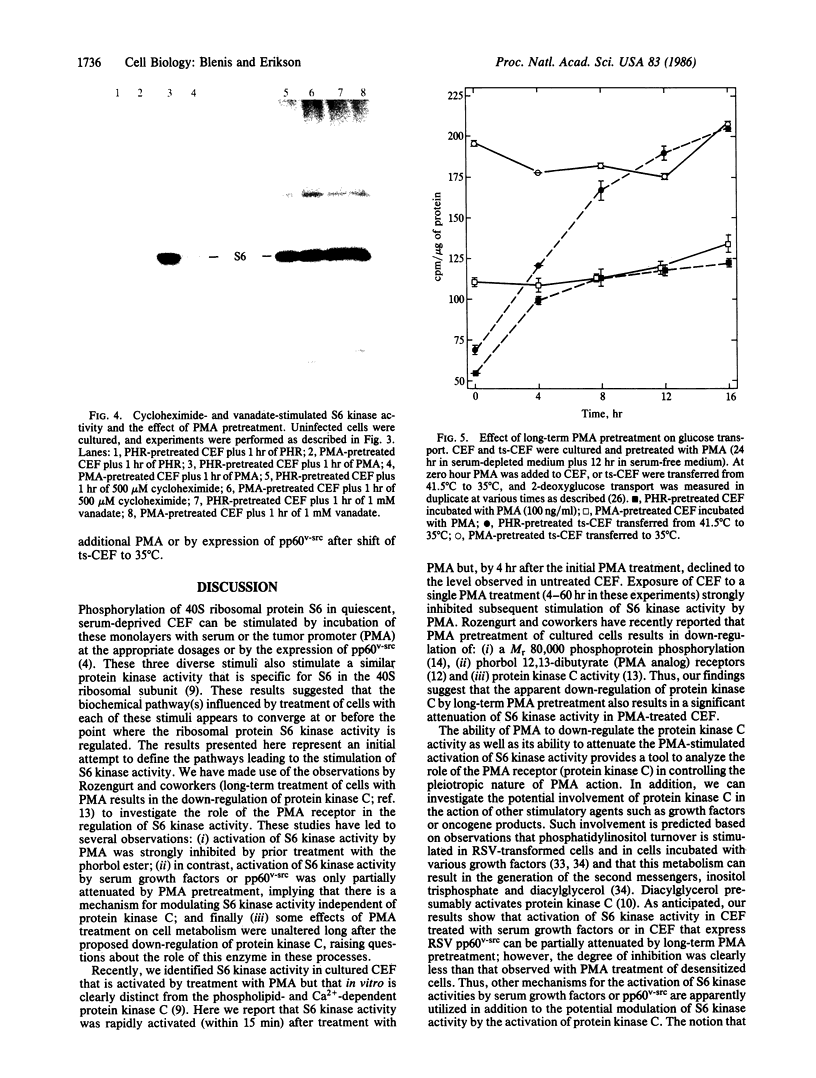
Ribosomal protein S6 kinase activity was measured in lysates prepared from serum-deprived chicken embryo fibroblasts (CEF) treated for various times with phorbol 12-myristate 13-acetate (PMA). Maximal activity was observed within 15 min, and it declined to the initial level by 4 hr. Incubation of these cells with PMA 4-60 hr after the initial treatment did not result in an additional increase in S6 protein kinase activity. These results are consistent with down-regulation of the PMA receptor, protein kinase C, and the dependence of PMA-stimulated S6 kinase activity on this enzyme. Long-term pretreatment of CEF with PMA only partially attenuated the stimulation of the S6 protein kinase activity by serum or by expression of the Rous sarcoma virus transforming gene product, pp60v-src. A similar protein kinase activity also was stimulated in cells treated with cycloheximide or sodium vanadate. Pretreatment with PMA had little effect on this response. These data indicate that it is likely that there are at least two mechanisms through which S6 kinase activity can be regulated, one of which apparently utilizes protein kinase C whereas the other(s) does not. Additional experiments show PMA-stimulated glucose transport was not attenuated by long-term incubation with phorbol ester, suggesting that another mechanism, which is not dependent on the presence of protein kinase C, maintains this response after the proposed down-regulation of the PMA receptor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., Hatié C., Calvin M. Is the product of the src gene a promoter? Proc Natl Acad Sci U S A. 1979 Jan;76(1):348–352. doi: 10.1073/pnas.76.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenis J., Erikson R. L. Regulation of a ribosomal protein S6 kinase activity by the Rous sarcoma virus transforming protein, serum, or phorbol ester. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7621–7625. doi: 10.1073/pnas.82.22.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenis J., Spivack J. G., Erikson R. L. Phorbol ester, serum, and rous sarcoma virus transforming gene product induce similar phosphorylations of ribosomal protein S6. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6408–6412. doi: 10.1073/pnas.81.20.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burkhard S. J., Traugh J. A. Changes in ribosome function by cAMP-dependent and cAMP-independent phosphorylation of ribosomal protein S6. J Biol Chem. 1983 Nov 25;258(22):14003–14008. [PubMed] [Google Scholar]
- Chen B. D., Wilkins K. L. Role of phorbol ester receptors in the 12-0-tetradecanoyl-phorbol-13-acetate (TPA)-induced down-regulation of colony-stimulating factor (CSF-1) binding to murine peritoneal exudate macrophages. J Cell Physiol. 1985 Aug;124(2):305–312. doi: 10.1002/jcp.1041240221. [DOI] [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Homologous and heterologous mitogenic desensitization of Swiss 3T3 cells to phorbol esters and vasopressin: role of receptor and postreceptor steps. J Cell Physiol. 1984 Feb;118(2):133–142. doi: 10.1002/jcp.1041180205. [DOI] [PubMed] [Google Scholar]
- Diringer H., Friis R. R. Changes in phosphatidylinositol metabolism correlated to growth state of normal and Rous sarcoma virus-transformed Japanese quail cells. Cancer Res. 1977 Sep;37(9):2979–2984. [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. The effect of phorbol diesters on chicken embryo fibroblasts. Cancer Res. 1977 Sep;37(9):3257–3265. [PubMed] [Google Scholar]
- Duncan R., McConkey E. H. Preferential utilization of phosphorylated 40-S ribosomal subunits during initiation complex formation. Eur J Biochem. 1982 Apr;123(3):535–538. doi: 10.1111/j.1432-1033.1982.tb06564.x. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Purchio A. F., Erikson E., Collett M. S., Brugge J. S. Molecular events in cells transformed by Rous Sarcoma virus. J Cell Biol. 1980 Nov;87(2 Pt 1):319–325. doi: 10.1083/jcb.87.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B., Frackelton A. R., Jr, Ross A. H., Connors J. M., Fujiki H., Sugimura T., Rosner M. R. Tumor promoters block tyrosine-specific phosphorylation of the epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1984 May;81(10):3034–3038. doi: 10.1073/pnas.81.10.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard P. R., Mazzei G. J., Kuo J. F. Immunological quantitation of phospholipid/Ca2+-dependent protein kinase and its fragments. Tissue levels, subcellular distribution, and ontogenetic changes in brain and heart. J Biol Chem. 1986 Jan 5;261(1):370–375. [PubMed] [Google Scholar]
- Girard P. R., Mazzei G. J., Wood J. G., Kuo J. F. Polyclonal antibodies to phospholipid/Ca2+-dependent protein kinase and immunocytochemical localization of the enzyme in rat brain. Proc Natl Acad Sci U S A. 1985 May;82(9):3030–3034. doi: 10.1073/pnas.82.9.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Tyrosine protein kinases and their substrates: an overview. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:443–455. [PubMed] [Google Scholar]
- Iwashita S., Fox C. F. Epidermal growth factor and potent phorbol tumor promoters induce epidermal growth factor receptor phosphorylation in a similar but distinctively different manner in human epidermoid carcinoma A431 cells. J Biol Chem. 1984 Feb 25;259(4):2559–2567. [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kelleher D. J., Pessin J. E., Ruoho A. E., Johnson G. L. Phorbol ester induces desensitization of adenylate cyclase and phosphorylation of the beta-adrenergic receptor in turkey erythrocytes. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4316–4320. doi: 10.1073/pnas.81.14.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Venuta S., Weber M., Rubin H. Temperature-dependent alterations in sugar transport in cells infected by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2739–2741. doi: 10.1073/pnas.68.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil P. L., McKenna M. P., Taylor D. L. A transient rise in cytosolic calcium follows stimulation of quiescent cells with growth factors and is inhibitable with phorbol myristate acetate. J Cell Biol. 1985 Aug;101(2):372–379. doi: 10.1083/jcb.101.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. J., Thomas G., Maller J. L. Increased phosphorylation of ribosomal protein S6 during meiotic maturation of Xenopus oocytes. Proc Natl Acad Sci U S A. 1982 May;79(9):2937–2941. doi: 10.1073/pnas.79.9.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Owen N. E. Effect of TPA on ion fluxes and DNA synthesis in vascular smooth muscle cells. J Cell Biol. 1985 Aug;101(2):454–459. doi: 10.1083/jcb.101.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G., Hawkes S. P. Detection of an early surface change during oncogenic transformation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3703–3707. doi: 10.1073/pnas.75.8.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebois R. V., Patel J. Phorbol ester causes desensitization of gonadotropin-responsive adenylate cyclase in a murine Leydig tumor cell line. J Biol Chem. 1985 Jul 5;260(13):8026–8031. [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena A., Coombs M., Sinnett-Smith J. Diacylglycerol stimulates DNA synthesis and cell division in mouse 3T3 cells: role of Ca2+-sensitive phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5748–5752. doi: 10.1073/pnas.81.18.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Siegmann M., Kubler A. M., Gordon J., Jimenez de Asua L. Regulation of 40S ribosomal protein S6 phosphorylation in Swiss mouse 3T3 cells. Cell. 1980 Apr;19(4):1015–1023. doi: 10.1016/0092-8674(80)90092-6. [DOI] [PubMed] [Google Scholar]