Abstract
A 47-base-pair (bp) conserved sequence in the 5′-flanking regions of three transcriptional units coding for nodulation functions (nodABC, nodEFG, and nodH) has been identified in Rhizobium meliloti strain 41. The conserved region contains subsequences of 7 bp, 5 bp, and 25 bp. The conserved 25-bp sequence was synthesized and used as a hybridization probe; three additional copies of the sequence were identified in R. meliloti 41; all three were localized in the 135-kb nod/nif region of the symbiotic megaplasmid. Nucleotide sequence analysis of the six regions revealed that all contained the 47-bp conserved sequence but, with one exception, adjacent DNA regions did not have long conserved DNA stretches. The position of the 47-bp region was about 200-240 bp upstream of the translational start codons of the three nod genes. This conserved sequence is present in several other Rhizobium species and located adjacent to nod genes. We have demonstrated the involvement of this sequence in the expression of nodulation functions, which suggests that these extended promoter regions may have a role in the coordinated regulation of nodulation genes.
Keywords: symbiosis, gene expression, promoter sequence
Full text
PDF
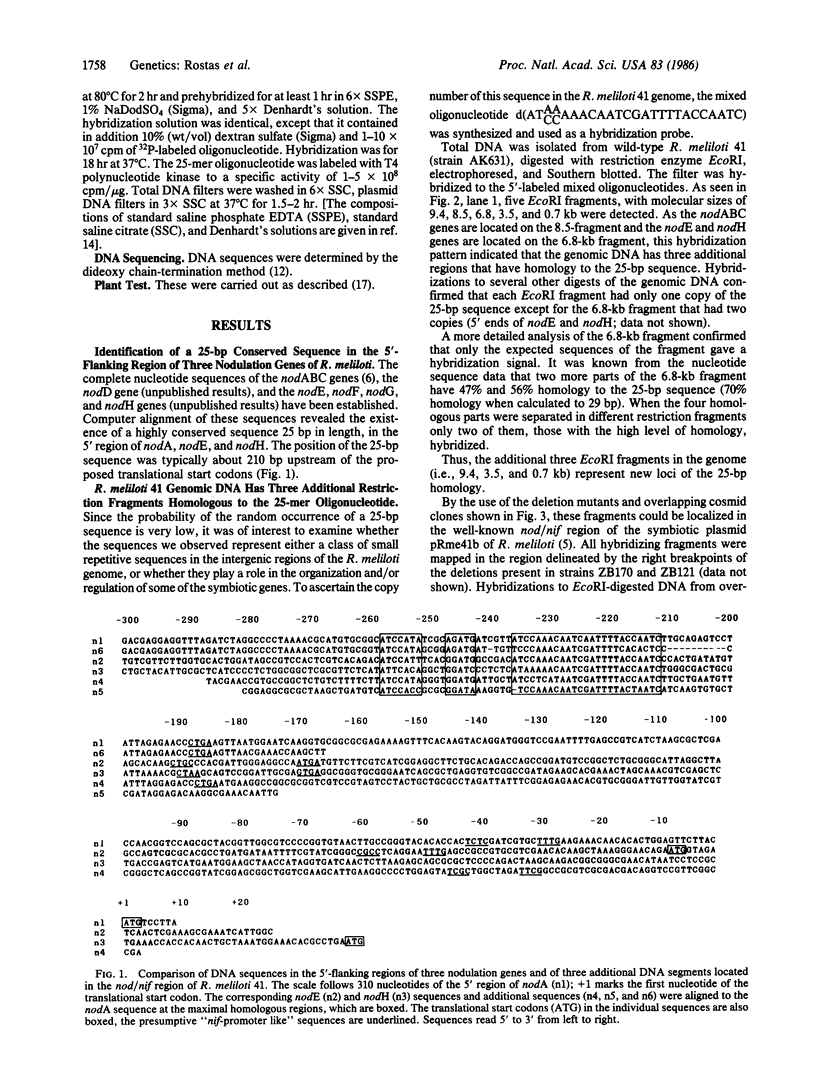
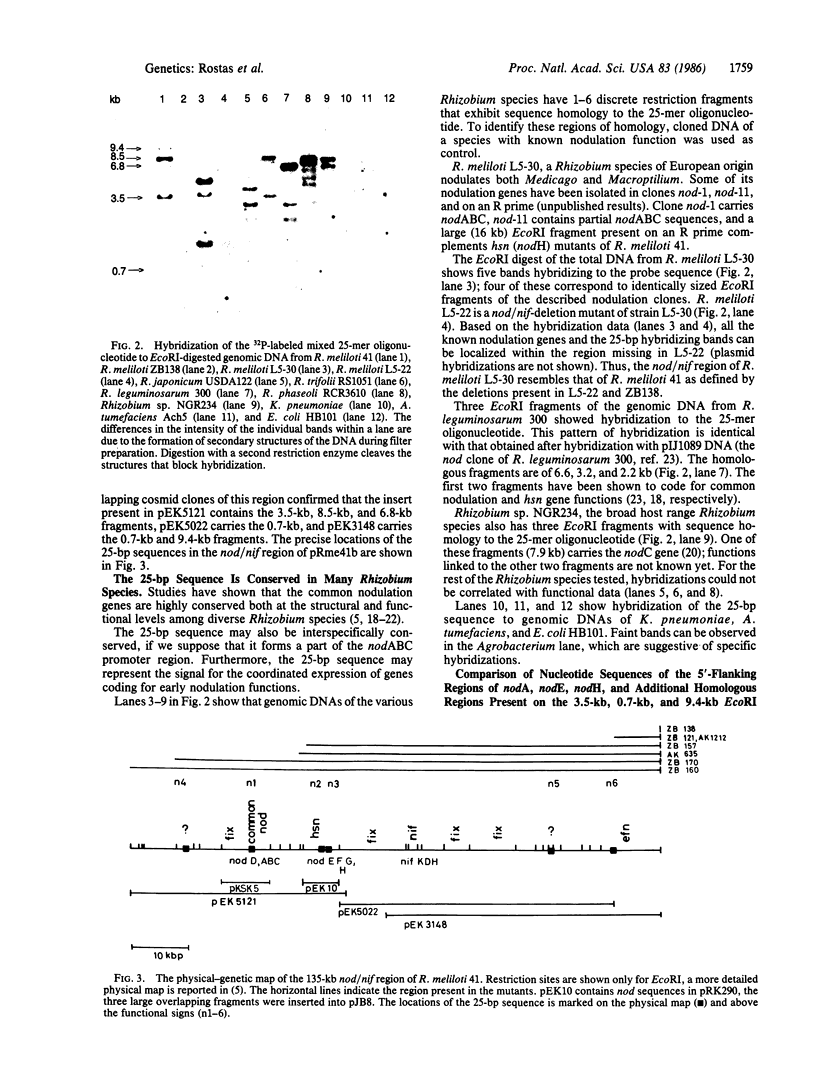


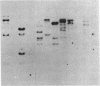
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banfalvi Z., Kondorosi E., Kondorosi A. Rhizobium meliloti carries two megaplasmids. Plasmid. 1985 Mar;13(2):129–138. doi: 10.1016/0147-619x(85)90065-4. [DOI] [PubMed] [Google Scholar]
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Lewis B., Corbin D., Ditta G., Helinski D. R. Structural relationships among Rhizobium meliloti symbiotic promoters. Cell. 1983 Dec;35(2 Pt 1):479–485. doi: 10.1016/0092-8674(83)90181-2. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bánfalvi Z., Sakanyan V., Koncz C., Kiss A., Dusha I., Kondorosi A. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of R. meliloti. Mol Gen Genet. 1981;184(2):318–325. doi: 10.1007/BF00272925. [DOI] [PubMed] [Google Scholar]
- Corbin D., Barran L., Ditta G. Organization and expression of Rhizobium meliloti nitrogen fixation genes. Proc Natl Acad Sci U S A. 1983 May;80(10):3005–3009. doi: 10.1073/pnas.80.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Ma Q. S., Knight C. D., Hombrecher G., Johnston A. W. Cloning of the symbiotic region of Rhizobium leguminosarum: the nodulation genes are between the nitrogenase genes and a nifA-like gene. EMBO J. 1983;2(6):947–952. doi: 10.1002/j.1460-2075.1983.tb01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M., Clements J., Merrick M., Dixon R. Positive control and autogenous regulation of the nifLA promoter in Klebsiella pneumoniae. Nature. 1983 Jan 27;301(5898):302–307. doi: 10.1038/301302a0. [DOI] [PubMed] [Google Scholar]
- Egelhoff T. T., Fisher R. F., Jacobs T. W., Mulligan J. T., Long S. R. Nucleotide sequence of Rhizobium meliloti 1021 nodulation genes: nodD is read divergently from nodABC. DNA. 1985 Jun;4(3):241–248. doi: 10.1089/dna.1985.4.241. [DOI] [PubMed] [Google Scholar]
- Jacobs T. W., Egelhoff T. T., Long S. R. Physical and genetic map of a Rhizobium meliloti nodulation gene region and nucleotide sequence of nodC. J Bacteriol. 1985 May;162(2):469–476. doi: 10.1128/jb.162.2.469-476.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miyada C. G., Soberón X., Itakura K., Wilcox G. The use of synthetic oligodeoxyribonucleotides to produce specific deletions in the araBAD promoter of Escherichia coli B/r. Gene. 1982 Feb;17(2):167–177. doi: 10.1016/0378-1119(82)90070-1. [DOI] [PubMed] [Google Scholar]
- Moore J. V., Dixon B. Metastasis of a transplantable mammary tumour in rats treated with cyclophosphamide and/or irradiation. Br J Cancer. 1977 Aug;36(2):221–226. doi: 10.1038/bjc.1977.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Rossen L., Johnston A. W., Downie J. A. DNA sequence of the Rhizobium leguminosarum nodulation genes nodAB and C required for root hair curling. Nucleic Acids Res. 1984 Dec 21;12(24):9497–9508. doi: 10.1093/nar/12.24.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Török I., Kondorosi E., Stepkowski T., Pósfai J., Kondorosi A. Nucleotide sequence of Rhizobium meliloti nodulation genes. Nucleic Acids Res. 1984 Dec 21;12(24):9509–9524. doi: 10.1093/nar/12.24.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]