Abstract
Calcium (Ca2+) ions are important mediators of cellular homeostasis owing to their ability to elicit a dynamic, transient, and tightly regulated range of biochemical responses. More than a decade ago, a nonselective, Ca2+-permeable, cationic conductance was identified in podocytes downstream of angiotensin II (Ang II) signaling, but its molecular structure remained elusive. Six years ago, transient receptor potential canonical 6 (TRPC6) mutations were found in families with hereditary FSGS, and TRPC5 and TRPC6 channels are now known as the Ca2+ influx pathways for this previously described, nonselective, cationic current in podocytes. Ang II activation engages this Ca2+ influx to modulate the actin cytoskeleton in podocytes. These discoveries dovetail with previously described regulation of actin dynamics by the Ca2+-activated phosphatase, calcineurin, and the emergence of Rho GTPases as critical regulators of podocyte function in health and disease. Understanding the interconnected signaling regulated by Ca2+ currents offers potential new therapeutic targets and highlights the notion that synergistic therapies targeting multiple levels of biochemistry may be useful in treating proteinuric kidney disease.
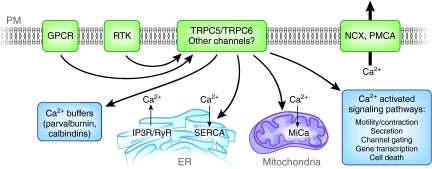
A single ion channel can negotiate the passage of over 10 million ions per second across the plasma membrane,1 and calcium (Ca2+) ions, in particular, are important mediators of cellular homeostasis.1,2 Ca2+ permeates the membrane of virtually every cell to regulate diverse vital processes such as muscle contraction, cytoskeletal structure, vesicle secretion, gene transcription, and programmed cell death, to name a few.1,2 Thousands of Ca2+ channels on the plasma membrane precisely control the timing and entry of Ca2+ ions and cellular homeostatic mechanisms modulate the tight control and compartmentalization of these intracellular Ca2+ transients (Figure 1).2
Figure 1.
Schematic representation of Ca2+ homeostasis in podocytes Ca2+ is a potent signaling molecule because of its ability to mediate a dynamic, dramatic, transient, and tightly regulated range of intracellular responses1,2,9 (PM: plasma membrane). Some proteins shown here have not yet been identified or studied in podocytes (calbindins, the mitochondrial uniporter or MiCa etc.), but they are likely to be present based on our understanding of calcium homeostasis in other cell types. The influx of Ca2+ is likely to be mediated by TRPC5 and TRPC6 channels, which were recorded at the single channel level in podocytes,37 but other influx pathways cannot be excluded. TRPC5 and TRPC6 are activated by upstream receptors such as G-protein coupled receptors (GPCR), including the AT1R, and receptor tyrosine kinases (RTK), similar to other cell types.62 Ca2+ is tightly regulated upon entry into the cytoplasm. Calcium homeostasis relies on the Na+-Ca2+ exchanger (NCX), which has been described in podocytes,110 the ATP-dependent plasma membrane Ca2+ pump (PMCA), plasma Ca2+ buffers (calbindins, parvalbumin, etc.) and internal Ca2+ stores (endoplasmic reticulum (ER), mitochondria) to maintain low cytoplasmic Ca2+ levels.8 When a Ca2+-permeable channel opens, whether in the plasma membrane or on a Ca2+-loaded organelle (e.g. the IP3R in the ER), Ca2+ ions flow transiently into the cytoplasm, until the homeostatic mechanisms take over once again to buffer or extrude the excess Ca2+ ions.
Here, we review the emerging role of Ca2+ signaling in the regulation of podocyte function in health and disease (Figure 2). In particular, we explore recently uncovered insights into the activation of transient receptor potential canonical (TRPC) channels by Ang II and the resulting effects on podocyte signaling under physiologic and pathologic conditions (Figure 3). Finally, we highlight the implications of balancing Ca2+-controlled signaling pathways in podocytes for the development of novel antiproteinuric therapies (Figure 4).
Figure 2.
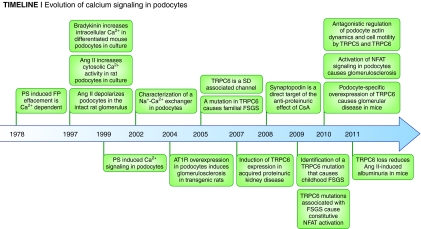
Evolution of calcium signaling in podocytes from 1978 to today.
Figure 3.
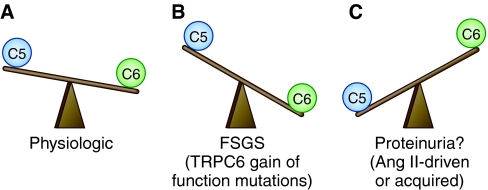
Antagonistic activities of TRPC5 versus TRPC6 signaling in podocytes in health and disease: Is it a balancing act? This working model attempts to synthesize published data and underscore the areas in which future experiments are likely to enhance our understanding of TRPC signaling in podocytes. (A) Under physiologic conditions, active TRPC6 channels are more abundant on the podocyte cell membrane, as demonstrated on the single channel level,37 which underscores their importance for maintaining podocyte integrity, through their selective activation of RhoA.37,46 (B) TRPC6 gain of function mutations20,36,111 result in overactive TRPC6 channels, the cell is overwhelmed by TRPC6-mediated Ca2+ influx, which ultimately leads to FSGS.83 The observed podocyte injury may result either broadly from Ca2+ cytotoxicity and cell death or specifically from excessive RhoA-mediated contraction, for example, increased “stiffness” leading to a “broken” actin cytoskeleton, and ultimately, cell death. (C) Given the experimental evidence that (a) constitutive Rac1 activity leads to proteinuria,76 (b) TRPC5 activates Rac1 in podocytes,37 and (c) Rac1 is required for TRPC5 insertion into the plasma membrane in podocytes,37 it is reasonable to hypothesize that, in states of excess AngII, TRPC5/Rac1–mediated overactivity drives proteinuria. This notion generates interest in TRPC5 channels as mediators of acquired, Ang II–driven proteinuria.
Figure 4.
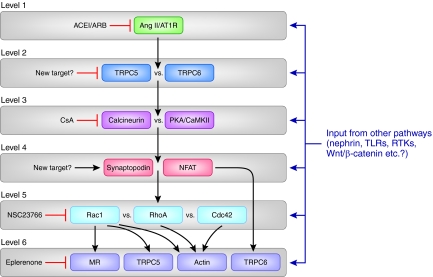
A model for multiple signaling pathways in podocyte injury: Is a multidrug, synergistic therapy the answer to proteinuria? A synthesis of work by many groups suggests that multiple signaling levels are involved in the Ang II–mediated regulation of podocyte function in health and disease. Level 1 consists of the binding of Ang II, whose availability is limited by ACE inhibitors, to AT1Rs, which are blocked by ARBs. Subsequent activation of TRPC5 and TRPC6 channels on level 2 results in Ca2+ influx into podocytes. TRPC5-driven signaling may predominate under pathologic conditions of excess Ang II and/or Rac1 activity. TRPC5-targeted agents may therefore be a novel therapeutic approach to proteinuria. On level 3, Ca2+-activated phosphatases (calcineurin) or kinases (PKA and CamKII) battle for downstream effects on their mutual targets, synaptopodin and NFAT (level 4). Synaptopodin-preserving agents such as CsA exert their antiproteinuric effect by blocking the calcineurin-initiated degradation of synaptopodin. Stabilization of synaptopodin protein abundance or inhibition of NFAT signaling may be another therapeutic approach to proteinuria. On level 5, Rac1, RhoA, and Cdc42 battle for downstream effects on the actin cytoskeleton. Inhibition of Rac1 has an antiproteinuric effect in vivo, perhaps because of the decreased activity of mineralocorticoid receptors (MR), similar to treatment with Eplerenone (level 6). Synergistic mechanisms on level 6 include (a) the Rac1-driven insertion of TRPC5 channels in the plasma membrane (level 6), which potentiates the activity of Rac1 in a positive feedback loop, and (b) the effects of Rac1 on the actin cytoskeleton, resulting in maladaptive podocyte foot process motility, which correlates with proteinuria (level 6). RhoA and Cdc42 also affect the actin cytoskeleton (level 6). NFAT promotes TRPC6 transcription (level 6). Although AngII and AT1Rs are centrally important in podocyte Ca2+ signaling, inputs from many other pathways are likely to modulate the molecular events in each of the signaling levels, for example, signaling through the Nephrin pathway. This model also offers an explanation why the combined inhibition of several levels is necessary for effective and sustained antiproteinuric treatment (TLRs, toll-like receptors; RTKs, receptor tyrosine kinases).
Similar to sodium (Na+) and potassium (K+) channels initially recorded in the classical Hodgkin-Huxley studies of the squid giant axon,1,3,4 Ca2+ channels were identified in studies of excitable cells from crustaceans. In the early 1950s, when Na+ was firmly established as the ion mediating action potentials, known as the Na+ theory of the action potential,1 Fatt and Katz accidentally stumbled on an important exception: the crayfish muscle.1,5,6 In pioneering two microelectrode intracellular recordings, the investigators were surprised when Na+ channel blockers did not abrogate action potentials, but instead engendered larger and more prolonged spikes.5,6 Further experiments revealed that the mysterious action potentials were generated by the entry of divalent Ca2+ ions through voltage-gated Ca2+ channels.1
Because of these initial experiments, important insights have been gained into the electrogenic role of Ca2+ in excitable cells: Electricity is used to open (or gate) the channels, and in turn the channels are used to make electricity (action potentials). Importantly, most excitable cells ultimately translate their electric excitation into another form of activity or cellular function. Although the list of processes controlled by Ca2+ is lengthy, classical studies in excitable cells focus on three: contraction, secretion, and gating.1 The role of Ca2+ in myocyte contraction is particularly important in the context of this review, as it provides insight into the Ca2+-dependent mechanisms underlying all contractile cells, including kidney podocytes.7
How are cells capable of sensing Ca2+? In a resting cell, the levels of free cytoplasmic Ca2+ are tightly controlled and held extremely low. The resting intracellular Ca2+ concentration [Ca2+] lies in the 20 to 300 nM range in virtually all cells.1 Calcium homeostasis mechanisms (Figure 1) such as the Na+-Ca2+ exchanger (NCX) and the ATP-dependent plasma membrane Ca2+ pump (PMCA), along with Ca2+ buffers (such as calbindins or parvalbumin) and internal Ca2+ stores (endoplasmic reticulum [ER], mitochondria uniporter [MiCa]), labor to maintain the cytoplasmic Ca2+ levels low.8 When a Ca2+-permeable channel opens, whether in the plasma membrane or on a Ca2+-loaded organelle (such as the inositol triphosphate receptor [IP3R] in the ER; Figure 1), Ca2+ ions flow transiently into the cytoplasm, until the homeostatic mechanisms prevail once again to tie up or extrude the excess Ca2+ ions. Ca2+ is a potent signaling molecule because of its ability to mediate a dynamic, dramatic, transient, and tightly regulated range of responses. Simply put, the entry or release of any amount of Ca2+ in the cytoplasm, large or small, is a call to action.1,2,9
PODOCYTES AND CALCIUM
The glomerular filtration unit of the kidney is a highly specialized corpuscle of capillaries capable of modulating hydrostatic ultrafiltration of blood plasma, allowing the passage of solutes but retaining vital proteins.10–12 Among the three layers of the glomerular filtration barrier, the podocyte layer comprises unique cells with a complex organizing phenotype, including characteristic interdigitating foot processes with an interposed slit diaphragm that cover the outer aspect of the glomerular basement membrane.10,12,13 Podocyte dysfunction and cytoskeletal disorganization leads to foot process effacement, disruption of the slit diaphragm, and proteinuria, and is often a starting point for progressive kidney disease.7,12 Thus, proteins regulating the plasticity of the podocyte actin cytoskeleton are critical for sustained function of the glomerulus.7,12 Mutations in genes encoding nephrin,14 podocin,15 or phospholipase C epsilon16 give rise to congenital proteinuria.14,15 Additionally, adult-onset focal segmental glomerulosclerosis (FSGS) is associated with podocyte mutations in genes encoding α-actinin 4,17 CD2AP,18 INF2,19 TRPC6,20 and synaptopodin.21 Most recently, common variations in glypican 5 associate with acquired nephrotic syndrome.22 Taken together, mutations in these moieties highlight the importance of the cytoskeleton in podocyte health and disease.
From the cell biologist's perspective, the podocyte's refined repertoire of cytoskeletal adaptations to environmental cues12,23–28 renders it an ideal model system to study Ca2+-dependent actin dynamics in a physiologically relevant context. A central working hypothesis has been that podocyte foot process effacement is mediated by rearrangement of the actin cytoskeleton.7,23,24 As early as 1978, elegant work by Kerjaschki proposed that an increase in [Ca2+]i may be an early event in podocyte injury29 (Figure 2). Complement C5b-9 complex-mediated podocyte damage also associates with an increase in [Ca2+]i and activation of phospholipase C (PLC).30 Protamine sulfate, which can cause foot process effacement in vivo,31 also increases [Ca2+]i in vitro32 (Figure 2). Differentiated mouse podocytes in culture increase [Ca2+]i in response to bradykinin,33 an effect also observed in cultured rat podocytes in response to Ang II34 (Figure 2). Importantly, in pioneering whole rat glomerular recordings, Pavenstadt and colleagues described how Ang II evokes a nonselective cationic current in podocytes35 (Figure 2). The molecular identity of this current remained elusive until 2005, when Winn and colleagues provided the first clues by describing a TRPC6 channel mutation in familial FSGS,20 followed by the identification of five additional mutations by Pollak and colleagues36 (Figure 2). Detailed electrophysiology recordings demonstrating TRPC channel activity in podocytes were not available until recently, when Tian and colleagues37 unveiled TRPC5 and TRPC6 as the channels downstream of Ang II–evoked nonselective cationic conductance initially identified more than a decade ago35 (Figure 2).
ANG II–MEDIATED Ca2+ SIGNALING IN PODOCYTES
In the context of proteinuric kidney disease, angiotensin 1 receptor–mediated (AT1R-mediated) signaling is of particular importance. In classical studies of diabetic nephropathy, angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) delay renal progression.38,39 In 2004, Hoffmann and colleagues provided direct evidence that podocyte-specific overexpression of AT1R in transgenic rats is sufficient to cause proteinuria and FSGS-type lesions (Figure 2).40 At a cellular level, AT1R signaling is upstream of a number of pathways that may be important to TRPC signaling. One is the AT1R-dependent activation of calcineurin.41 Furthermore, AT1R signaling causes transactivation of the EGF receptor (EGFR) in tubular epithelia42 and podocytes.43 AT1R-EGFR interactions also activate downstream serine/threonine kinases such as the MAPK pathway in a process that is known to require an increase in cytosolic [Ca2+].44 Ang II induces membrane ruffling and loss of stress fibers,45 thereby phenocopying the depletion of synaptopodin46,47 or TRPC6.37
TRPC CHANNELS AND Ca2+ SIGNALING
Transient receptor potential (TRP) channels are receptor-operated, nonselective, cationic channels first identified in Drosophila48 (see below for some technical considerations regarding canonical TRP [TRPC] channels). Although nonselective for a particular cation, from a cellular signaling perspective, the central focus in the study of TRPC channels has been their ability to negotiate the influx of small but potent amounts of Ca2+ through the cell membrane.9,49 The TRP superfamily consists of 28 members segregated into 7 families with diverse mechanisms of activation, tissue-specific distribution, and functional properties.9,49 The canonical TRPC channels include seven members, TRPC1 to TRPC7.9,49 On the basis of functional similarities and sequence alignment, two major subfamilies emerge, TRPC1/4/5 and TRPC3/6/7 (TRPC2 is a pseudogene in humans).49 Four TRP subunits are required to form a functional channel, rendering a cation-conducting pore that is either a homomeric or heteromeric tetramer.49 TRPC channel activation occurs primarily through activation of phospholipase C (PLC), and the cleavage of phosphatidylinositol bisphosphate (PIP2) which gates the channels directly, as in the case of TRPC4 and TRPC5, or indirectly through its hydrolysis into diacylglycerol, as in the case of TRPC3,6,7.9,50
TRPC6 is closely related to TRPC7, with overlapping expression patterns in the kidney.50 TRPC6 channels form homomers with a characteristic doubly rectifying current-voltage relationship and are six times more permeable for Ca2+ than Na+ with a single-channel conductance of 35 pS.49,51 TRPC6 channels, which inhibit endothelial cell migration,52 are implicated in pulmonary arterial hypertension53 and cardiac hypertrophy and fibrosis.41,54–56 TRPC6 also increases smooth muscle cell contraction.57–59 TRPC6 null mice were therefore expected to have decreased vascular smooth muscle tone resulting in hypotension. Surprisingly, however, Birnbaumer and colleagues observed elevated BP and increased vascular smooth muscle contractility in aortic rings from TRPC6 null mice.60 These unexpected effects were the result of compensatory upregulation of constitutively active TRPC3 channels.60 In the kidney, TRPC channels came to the forefront when gain-of-function TRPC6 mutations were linked to familial FSGS20,36 and TRPC6 was shown to be associated with the slit diaphragm.36
Recent work has also implicated TRPC5, in addition to TRPC6, as an important mediator of cytoskeletal changes in podocytes.37 Although TRPC5 is ubiquitously expressed, its highest levels of expression are in brain and kidney.9,50 TRPC5 channels generate Ca2+ transients implicated in neuronal growth cone motility61,62 and vascular smooth muscle cell migration.63 Vesicular insertion of TRPC5 from a reserve pool is downstream of EGF-RTK signaling in a pathway that involves Rac1 and phosphatidylinositol 4-phosphate 5-kinase.62 TRPC5 subunits form homomeric channels with a unique current-voltage (I–V) signature curve in response to stimulation through G protein coupled receptors (GPCR).64,65 TRPC5 channels conduct ten Ca2+ ions for one Na+ ion into cells with a single-channel conductance of 38 pS.66 The current-voltage relation is doubly rectifying, with substantial inward current, little outward current up to +40 mV, and steeply outwardly rectifying current above +40 mV.
TRPC5-mediated currents are increased in the presence of 100 μM La3+, in contrast to virtually all other TRPC channels (except TRPC4), which are inhibited by La3+ at even lower concentrations.67 TRPC5 is also strongly potentiated by intracellular Ca2+.66 Interestingly, coexpression of TRPC1 and TRPC5 in HEK293 cells results in heteromeric TRPC1/5 channels with voltage dependence similar to N-methylD-aspartate receptor channels: At polarized potentials the inward current is gently inwardly rectifying, but steeply outwardly rectifying above 0 mV. Loss of TRPC5 in the amygdala results in impaired fear responses in young mice,68 suggesting a role for these channels in fear conditioning and anxiety during postnatal brain development. In podocytes, TRPC5 channels have been studied in vitro,37 but no in vivo studies have been performed to date.
CALCINEURIN SIGNALING IN PODOCYTES
An important link between Ca2+ and podocyte injury is the finding that activation of the Ca2+-dependent phosphatase, calcineurin, leads to cathepsin L-mediated cleavage of synaptopodin and proteinuria47 (Figure 2). The calcineurin inhibitor, cyclosporine A (CsA), prevents synaptopodin degradation in vitro, and mice resistant to cathepsin-mediated synaptopodin degradation are protected from proteinuria in vivo.47 Conversely, the activation of calcineurin in podocytes is sufficient to cause degradation of synaptopodin and proteinuria.47 Taken together, these findings reveal a T cell and NFAT-independent mechanism for the long known antiproteinuric effect of CsA: Preservation of synaptopodin and the podocyte actin cytoskeleton.47
In light of the proposed central role of synaptopodin in podocytes, we must take a moment to revisit the question of synaptopodin in null mice. Synaptopodin mutant mice lacking Synpo-short and Synpo-long upregulate Synpo-T protein expression in podocytes, thereby rescuing kidney filter function during development.69 Moreover, bigenic heretozygosity for synaptopodin and CD2AP results in proteinuria and FSGS-like glomerular damage, underscoring the importance of synaptopodin and CD2AP for sustained kidney filter function.70 In humans, heterozygous mutations in the promoter of the synpo gene in patients with idiopathic FSGS reduce gene transcription in vitro and protein abundance in vivo.21
FSGS-causing TRPC6 mutations but not wild-type TRPC6 induce constitutive activation of calcineurin-NFAT–dependent gene transcription.71 Although not clearly related to cytoskeletal regulation, this study supports an important role for TRPC channel-mediated Ca2+ influx in activating calcineurin and its downstream effectors in podocytes. One hypothesis involves a positive feedback loop, whereby calcineurin-NFAT–mediated increases in transcription lead to more TRPC channels on the membrane, thus more Ca2+ influx and perpetuation of the cycle, leading to podocyte injury. Such a feed forward cycle has been demonstrated in a mouse model of cardiac hypertrophy, where calcineurin-NFAT–mediated increases in TRPC6 transcription promote pathologic cardiac remodeling.41 More recently, however, other TRPC channels, including TRPC4 (which is highly homologous and functionally similar to TRPC564,65), were also shown to mediate calcineurin-NFAT activation in cardiac myocytes downstream of Ang II and AT1R signaling,41,55,56 strongly suggesting this pathway is not specific to TRPC6. Recent work by Chen and colleagues also shows that activation of NFAT signaling in podocytes is sufficient to cause glomerulosclerosis in mice.72 This finding begs the question of whether, by analogy to cardiac myocytes, podocytes have also evolved a feed forward loop linking TRPC channels to NFAT activation and subsequent TRPC transcriptional upregulation. As it stands now, however, there is no direct evidence showing that TRPC6 or another member of the TRPC family causes FSGS through the activation of NFAT in podocytes.
Ca2+-DEPENDENT RHO GTPase SIGNALING IN PODOCYTES
The Rho family of GTPases (RhoA, Rac1, and Cdc42) controls pathways that modulate cytoskeletal dynamics.73,74 Rac1 and Cdc42 promote cell motility through the formation of lamellipodia and filopodia at the leading edge, respectively.74 In contrast, RhoA promotes the formation of stress fibers and focal contacts, generating a contractile phenotype.74 In podocytes, predominance of RhoA activity produces a stationary phenotype, suggesting stable foot processes, whereas predominance of Cdc42/Rac1 activity mediates a disease-associated motile phenotype, suggesting unstable or retracted foot processes.7
Synaptopodin promotes RhoA signaling through competitive inhibition of Smurf1-mediated ubiquitination of RhoA.46 Synaptopodin thus protects RhoA from proteosomal degradation, and preserves stress fibers in vitro, while safeguarding against proteinuria in vivo.46,47 This notion is supported by the observation that synaptopodin-depleted podocytes display loss of stress fiber formation and aberrant filopodia.69 Synaptopodin also suppresses Cdc42 signaling through the inhibition of Cdc42:IRSp53:Mena complexes.75 Overall, synaptopodin stabilizes the kidney filter by preventing the reorganization of the podocyte foot process cytoskeleton into a migratory phenotype.47
Elegant work by Fujita and colleagues76 recently showed that Rho GDP dissociation inhibitor α null mice77 also develop heavy albuminuria, which is attributed to increased, constitutively active Rac1 signaling in podocytes.76 The proposed mechanism involves the Rac1-dependent accumulation of mineralocorticoid receptor into the podocyte nucleus through p21 activated kinase phosphorylation.76 Pharmacologic intervention with a Rac1-specific small molecule inhibitor (NSC23766) diminishes mineralocorticoid receptor hyperactivity and ameliorates proteinuria and renal damage in this mouse model of proteinuria.76
Although Ca2+ and synaptopodin-mediated Rho GTPase signaling are important in the modulation of the podocyte cytoskeleton, the question remains as to how these pathways intersect. Previous studies in other cell types reveal that spatially and temporally restricted changes in the concentration of free calcium (Ca2+ flickers) are enriched near the leading edge of migrating cells.78,79 Rho GTPases can be regulated by Ca2+ and by GTPase-activating proteins (GAPs), catalyzing the hydrolysis of GTP to GDP, and guanine nucleotide exchange factors (GEFs), catalyzing the exchange of bound GDP with free GTP.74 In vascular smooth muscle cells, the Rho GEF, Arhgef1, mediates the effects of Ang II on vascular tone and BP in a Ca2+-dependent manner.80 Although these studies establish an intimate association between Ca2+ influx and Rho GTPase-mediated cytoskeletal reorganization in other cell types, the mechanisms by which the podocyte senses and transduces extracellular cues that modulate synaptopodin and Rho GTPase activity, cell shape, and motility remained unclear. In particular, the Ca2+ entry mechanism responsible for mediating these signaling pathways had been elusive.
ANTAGONISTIC REGULATION OF PODOCYTE ACTIN DYNAMICS BY TRPC CHANNELS: A BALANCING ACT?
Recently, TRPC5 and TRPC6 were unveiled as conserved antagonistic regulators of actin dynamics and cell motility in podocytes and fibroblasts through the regulation of RhoA and Rac1, respectively.37 The latter study identified conserved, antagonistic, mutually inhibitory signaling pathways triggered by AT1R-mediated TRPC5 and TRPC6 channel activity to control the balance between motile and contractile phenotypes. Gene silencing of TRPC6 results in loss of stress fibers, activation of Rac1, and increased motility, which is rescued by constitutively active RhoA.37 Conversely, gene silencing of TRPC5 results in enhanced stress fiber formation, activation of RhoA, and decreased motility, which is reversed by constitutively active Rac1.37 Remarkably, TRPC5 specifically interacts with and activates Rac1, whereas TRPC6 specifically interacts with and activates RhoA in another, distinct molecular complex.37 Consistent with previous studies,47 CsA rescues the loss of synaptopodin in TRPC6-depleted cells.37 In contrast, synaptopodin expression is preserved in TRPC5-depleted podocytes.37 The antagonistic relationship between TRPC5 and TRPC6 suggests that unopposed TRPC5 activity is responsible for the Ca2+-calcineurin–dependent degradation of synaptopodin and loss of stress fibers in TRPC6-depleted podocytes. These results significantly extended our mechanistic understanding of TRPC channelopathies in podocytes by revealing that rather than focusing on a single channel, we have to consider the balance between TRPC5 and TRPC6 in understanding the effects of Ca2+ signaling in podocytes (Figure 3).
How can this new insight be placed in the context of what we have collectively learned thus far? Biochemical and detailed electrophysiology experiments identify TRPC6 as the predominant TRPC channel in the plasma membrane of podocytes, which supports the notion that at baseline podocytes maintain an adaptive TRPC6 and RhoA-predominant, contractile actin cytoskeleton37 (Figure 3). This baseline condition, characterized by relative TRPC6 predominance, is likely to become severely biased toward TRPC6 in the presence of gain of function TRPC6 mutations, where the podocyte's homeostatic mechanisms may be overwhelmed, leading to Ca2+ overload–mediated cellular injury or death, and ultimately, to FSGS (Figure 3). Alternatively, an unopposed or overactive TRPC6-RhoA pathway may tip the balance too far in favor of a contractile phenotype: Podocytes that are too stiff may have trouble adapting to fluctuations in glomerular filtration pressure, and may suffer from a disrupted cytoskeleton, disassembly of the actin filament network, and cell death. Interestingly, TRPC6 activity above or below physiologic levels may lead to disease, a notion supported by the observation that TRPC6 overexpression in podocytes results in loss of stress fibers,81 similarly to the silenced TRPC6 morphology.37 This concept is in keeping with neuronal plasticity models, where either increased or decreased Ca2+ channel activity ultimately has the same effect on the cytoskeleton, resulting in the weakening or pruning of a synaptic contact.82 Whatever the precise mechanism for podocyte loss may be, an in vivo study showed that mice overexpressing wild-type TRPC6 or TRPC6 gain-of-function mutants develop albuminuria and FSGS-type lesions.83 Although proteinuria in these mice was modest with low and variable penetrance, and structural abnormalities were not observed before 6 to 8 months of age,83 these results are consistent with a dominant TRPC6 hypothesis (Figure 3). Clearly, more mechanistic work is required to delineate the precise mechanisms leading to FSGS in TRPC6 transgenic mice.
Constitutively active Rac1 signaling in podocytes results in enhanced translocation of the mineralocorticoid receptor to the nucleus, an effect efficiently blocked by a Rac1 inhibitor.76 Intriguingly, TRPC5 channels depend on Rac1 for their insertion into the plasma membrane.62 If TRPC5 is indeed an important mediator of Ca2+ signaling in podocytes, could the enhanced insertion and overactivity of TRPC5 channels in the podocyte plasma membrane contribute to the proteinuria observed in RhoGDIα null mice (Figure 3)? Clearly, future in vivo studies are needed to delineate the role of TRPC5 in podocytes in health and disease.
A recent study reported that TRPC6 null mice were significantly protected from the proteinuric effects of Ang II.84 A well-designed protocol included the analysis of 18 null animals, showing significantly mitigated proteinuria to Ang II infusion for the first 4 weeks of infusion. The observed protective role of TRPC6 deletion is surprising given that TRPC6 null mice were initially reported to be hypertensive at baseline,60 and thus one would expect increased albuminuria, if not at baseline, certainly after Ang II infusion. In the original TRPC6 deletion study, TRPC3 (over)compensated for the loss of TRPC6.60 Significant upregulation of mRNA encoding TRPC3 was also found in the latter study.84 Indeed, the well-known compensation by the various TRPC channels has plagued the TRPC deletion field for more than a decade, making it impossible to assign specific functions to specific channels.85
Although this study has extended our understanding of TRPC6 channels in podocytes in vivo, it also raises some interesting questions: Is the significance of the protective effect lost because of the inherent variability in proteinuria that some animal models display or could this be the sign of a biologic phenomenon? Is perhaps TRPC3 (over)compensating for the loss of TRPC6, thus conferring a protective effect from exposure to AngII? In light of the antagonistic effects of TRPC5 and TRPC6 on podocyte actin dynamics,37 it will be interesting to study the role of TRPC5 in podocytes derived from TRPC6 knockout mice. Future studies will be necessary to elucidate the role of TRPC6 channels in podocytes in vivo, perhaps by developing podocyte-specific, inducible TRPC6 knockout mice.
TRPC CHANNEL TRAFFICKING IN PODOCYTES
Another important aspect of TRPC channel biology is the regulation by upstream receptor pathways. GPCR signaling promotes TRPC6 plasma membrane insertion in endothelial cells.86 TRPC6 can traffic to the cell membrane as part of a large molecular complex that includes the large conductance Ca2+-activated K+ (BK) channel.87,88 In neurons, BK conductance results in hyperpolarization, which terminates voltage gated Ca2+ channel activity.89 The same inhibitory role for BK has been shown for TRPC1-BK interactions in vascular smooth muscle cells,90 and TRPM4/5-BK interactions in mast cells.91,92 In contrast, the speculation in podocytes is that BK promotes TRPC6 channel activity through membrane hyperpolarization, which allows for increased Ca2+ permeability through TRPC6 pores.87,93 This would effectively prolong TRPC6 channel activity,87,93 thereby tipping the balance in favor of a RhoA-dominant, contractile phenotype. This hypothesis has not yet been tested experimentally in podocytes, and thus we cannot exclude the alternate possibility that Ca2+-mediated inactivation of TRPC6 channels precedes or prevails over the effects of BK activity. Additionally, BK-TRPC5 interactions have not yet been tested in podocytes. Future studies will be required to address these unresolved issues.
Receptor-mediated insertion of TRPC5 into the plasma membrane depends on Rac1.62 The reciprocal dependence between TRPC5 and Rac1 activities suggests there may be a feed forward loop: Rac1 promotes channel localization to the membrane,37,62 which in turn triggers enhanced TRPC5-mediated Ca2+ influx, thereby increasing Rac1 activity.37 The cycle may be terminated when the Ca2+ signal wanes, perhaps because of depletion of the pool of TRPC5-containing vesicles, or because of channel inactivation. What could be the physiologic significance of such a feed forward model? When the hydrostatic pressure across the glomerular filter changes, dictated by the cardiac cycle or under conditions of increased systemic BP, foot process remodeling is required to maintain an intact filter. According to this model, BP-driven increases in Ang II may signal to TRPC5 channels, triggering their insertion into the plasma membrane, thereby increasing Rac1 activity. This event would counter the effects of the more abundant TRPC6/RhoA pathway and therefore promote transient, adaptive foot process actin remodeling. Under maladaptive pathophysiology of excessive AT1R40 or Rac176 signaling, excess TRPC5 channel insertion may promote excessive foot process remodeling, which causes podocyte injury (Figure 3). Further work is needed to elucidate the precise mechanisms for TRPC5 channel trafficking in podocyte health and disease.
TRPC CHANNELS IN PODOCYTES: A FEW TECHNICAL CONSIDERATIONS
Although TRPC channels are firmly established as receptor-operated channels (ROCs)50 in a wide array of cell types including podocytes,37 some investigators have implicated TRPC-mediated store-operated Ca2+ entry in podocytes.93,94 In the past decade, there were indeed reports suggesting that various TRPC channels could serve as store-operated channels (SOCs). However, the identification of Orai-STIM as the best-characterized SOC channel complex and the channel responsible for Ca2+ release activated current (ICRAC)95–97 has cast serious doubt on the idea that TRPC channels are components of SOCs.9,50 It is worth noting that most data postulating TRPCs as SOCs are solely based on Ca2+ imaging, whereas most of the electrophysiology studies refute the idea that TRPCs are SOCs.9 Ca2+ imaging alone is prone to false positives, and electrophysiology alone is prone to false negatives.9 Ca2+ imaging is an indirect readout of channel function because it reflects the total cytosolic [Ca2+] regardless of the cause. Ca2+ entry into the unbuffered cytoplasm can affect the activity of numerous Ca2+-activated channels, transporters, or other molecules, which in turn influence the imaging results.9 In Ca2+ imaging experiments, voltage levels, which fuel the entry of Ca2+ ions into the cell, are uncontrolled. In contrast, patch clamp electrophysiology offers a direct measurement of channel activity if and when these channels are present in the plasma membrane.9 Perfusion of the membrane with defined solutions, tight control (clamping) of the voltage, and the opportunity to apply compounds on either side of the membrane, coupled with excellent time and current resolution, are clear advantages of this technique.9 However, in standard patch clamp, the intracellular contents are dialyzed out, removing important diffusible signaling molecules.9 Perforated patch recordings can be performed to overcome this difficulty, but even in their absence, electrophysiology is the technique of choice to record TRP channel activity.9 Thus, a combination of Ca2+ imaging and electrophysiology may ultimately be the most illuminating approach for the characterization of TRP channels in podocytes, but Ca2+ imaging alone should be avoided, as experience in other cell types has shown that it is prone to erroneous conclusions. As it stands, there is compelling evidence that TRPC channels are receptor-operated and there is no need to evoke them as components of SOCs.
Another important consideration involves the idea that TRPC6 channels may act as mechanosensors in podocytes.98 The term mechanosensor describes a channel directly gated by mechanical force, thus acting as its own force sensor, to be distinguished from a mechanically sensitive channel that is activated by second messengers downstream of true mechanical force sensors.99 By this definition, a review of the literature reveals little evidence to support the notion that TRPC6 channels are mechanosensors. One study reported that the interaction of podocin with TRPC6 results in enhanced TRPC6 channel activity in oocytes in a cholesterol-dependent manner.100 Another report suggested that receptor and mechanically mediated TRPC6 activity share a common underlying mechanism, which involves lipid sensing by the channel.101 Similarly, in an elegant study, Mederos y Schnitzler and colleagues102 showed that TRPC6 is not a mechanosensitive channel, but rather, that the AT1R imparts mechanosensitivity to vascular smooth muscle cells through its effects on TRPC6 channels, to mediate the Bayliss effect,103 the intrinsic property of arterial blood vessels to constrict in response to rises in intraluminal pressure. Thus, with regard to TRPC6, the weight of the evidence suggests that, rather than direct mechanical gating, there is likely indirect sensitivity to mechanical forces through signaling mechanisms involving plasma membrane lipids. In light of the discovery of TRPC5 channels as important mediators of Ca2+ influx in podocytes,37 it is also worth considering this question more broadly. There is one report of osmomechanical stimulation of TRPC5, which was however dependent on alterations in PIP2 levels,104 and thereby not directly gated by mechanical forces.
In general, TRP ion channels are emerging as candidate transduction channels in a wide variety of sensory systems, particularly those involved in sensing mechanical stimuli. As reviewed in great detail by Christensen and Corey,99 for these TRP channels, and generally for all mechanically gated channel candidates, it is essential to differentiate between channels that are directly gated by mechanical forces and those that are downstream of a force sensor. It is also essential to show these candidate proteins fulfill a number of specific criteria (reviewed in detail99). To date, therefore, the question of whether TRPC5, TRPC6, or any other ion channel in podocytes serves as a mechanosensor lacks experimental evidence and is thus in need of further investigation.
CONCLUSIONS AND CLINICAL IMPLICATIONS
A fundamental question in cell biology is how a signal maintains specificity in time and space. This is particularly important in contractile cells such as podocytes, where the continuous remodeling of the actin cytoskeleton is required to adapt to ever-changing environmental cues. This review highlights the critical role played by the tiny but potent Ca2+ ion in this dynamic process. Although more work is needed to develop a mechanistic understanding of TRPC channelopathies in podocytes, a novel emerging paradigm suggests the balance between TRPC5 and TRPC6 may be particularly important in understanding the effects of Ca2+ signaling in podocytes.
Unresolved questions of current interest include the role of synaptopodin in the TRPC-dependent regulation of Rho GTPase activity in podocytes. Long-term studies should reveal if and how, similar to myocytes, podocytes employ Ca2+ as a selective activator of kinases or phosphatases to mediate contraction versus relaxation or increased foot process motility. Importantly, a systematic investigation of all Ca2+ influx, release, and efflux mechanisms is needed to ultimately obtain a comprehensive view of Ca2+ signaling in podocytes (Figures 1 and 3).
Another open question is the relation of TRPC/Rho GTPase-mediated actin remodeling to nephrin and Nck-mediated remodeling of podocyte actin dynamics.7,23,105,106 A recent report suggests that nephrin may suppress TRPC6 channel activity under physiologic conditions, whereas TRPC6 mutations causing FSGS escape the inhibitory effect of nephrin.107 Future studies should test whether slit diaphragm-mediated actin dynamics can modulate TRPC signaling, and vice versa, whether TRPC signaling can modulate slit diaphragm protein function.
Proteinuria is a cardinal sign and a prognostic marker of kidney disease, and also an independent risk factor for cardiovascular morbidity and mortality.108 For decades, proteinuric kidney disease, such as hypertensive and diabetic nephropathy, have been treated with essentially the same agents, targeting the renin-angiotensin system.109 How can our enhanced knowledge of Ca2+ signaling in podocytes translate into new therapeutic interventions that may help our patients in the clinic? The discovery that Ang II acts through TRPC5 and TRPC6 to target Rho GTPase activity37 offers a molecular, mechanistic understanding of how ACEi and ARB treat proteinuria in a podocyte-specific manner, above and beyond their systemic BP lowering effects (Figure 4).39
A new disease paradigm thus emerges whereby proteinuria arises as a result of dysfunctions in a multilevel signaling cascade, which bring together upstream receptor pathways, TRPCs, synaptopodin, NFAT, Rho GTPases, and downstream targets such as the actin cytoskeleton or the mineralocorticoid receptor (Figure 4). The detailed mechanistic understanding of podocyte-specific signaling validates the podocyte as the target of choice for the treatment of proteinuria. Although still speculative, this schematic understanding of key molecules provides a framework and should pave the road for the identification of novel drug targets in podocytes that may act synergistically with established agents (Figure 4). For example, we can imagine a new agent acting as an inhibitor of TRPC5 channels: This would abrogate Rac1 signaling, shut down Rac1-mediated TRPC5 membrane insertion, and thus act synergistically with Rac1 inhibitors or mineralocorticoid receptor blockers (eplerenone)76 to stem proteinuria (Figure 4). Similarly, one can envision synaptopodin-stabilizing agents, which, much like CsA,47 may act by promoting the RhoA-dependent preservation of the stationary actin cytoskeleton (Figure 4). The Ang II–mediated regulation of podocyte actin dynamics involves at least six signaling levels, each of which can receive additional input from multiple other signaling pathways (Figure 4). This model may help explain why ACEi and ARBs have only limited efficacy as antiproteinurics. It also highlights why the combined or synergistic effect of specific agents targeted to specific levels within the signaling cascade, similar to oncologic treatments, may ultimately be our best answer for the effective treatment of proteinuria.
Acknowledgments
We apologize to our colleagues whose work we were not able to cite in this review because of space limitations. Reviews were often quoted at the expense of original work. We thank D. Corey, W. Kriz, M. Winn, and M.A. Arnaout for helpful discussions. A.G. is funded by a Nephcure Young Investigator Grant and NIH Grant DK083511 and P.M. by NIH Grants DK57683 and DK062472.
NOTE ADDED IN PROOF
The following studies were published after acceptance of this manuscript: Vassiliadis J, Bracken C, Matthews D, O'Brien S, Schiavi S, Wawersik S: Calcium mediates glomerular filtration through calcineurin and mTORC2/Akt signaling. J Am Soc Nephrol 22: 1453–1461, 2011; Zhu L, Jiang R, Aoudjit L, Jones N, Takano T: Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1621–1630, 2011.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Hille B: Ionic channels of excitable membranes, Sunderland, MA, Sinauer Associates, Inc., 1992 [Google Scholar]
- 2. Clapham DE: Calcium signaling. Cell 131: 1047–1058, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Hodgkin AL, Huxley AF: Potassium leakage from an active nerve fibre. Nature 158: 376, 1946 [DOI] [PubMed] [Google Scholar]
- 4. Hodgkin AL, Huxley AF: Resting and action potentials in single nerve fibres. J Physiol 104: 176–195, 1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fatt P, Katz B: The effect of inhibitory nerve impulses on a crustacean muscle fibre. J Physiol 121: 374–389, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fatt P, Katz B: The electrical properties of crustacean muscle fibres. J Physiol 120: 171–204, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Schwaller B: Cytosolic Ca2+ buffers. Cold Spring Harb Perspect Biol 2, 2010, a004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clapham DE: TRP channels as cellular sensors. Nature 426: 517–524, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Kriz W, LeHir M: Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Mundel P, Shankland SJ: Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Somlo S, Mundel P: Getting a foothold in nephrotic syndrome. Nat Genet 24: 333–335, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Mundel P, Kriz W: Structure and function of podocytes: An update. Anat Embryol (Berl) 192: 385–397, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Hinkes B, Wiggins RC, Gbadegesin R, Vlangos CN, Seelow D, Nürnberg G, Garg P, Verma R, Chaib H, Hoskins BE, Ashraf S, Becker C, Hennies HC, Goyal M, Wharram BL, Schachter AD, Mudumana S, Drummond I, Kerjaschki D, Waldherr R, Dietrich A, Ozaltin F, Bakkaloglu A, Cleper R, Basel-Vanagaite L, Pohl M, Griebel M, Tsygin AN, Soylu A, Muller D, Sorli CS, Bunney TD, Katan M, Liu J, Attanasio M, O'toole JF, Hasselbacher K, Mucha B, Otto EA, Airik R, Kispert A, Kelley GG, Smrcka AV, Gudermann T, Holzman LB, Nürnberg P, Hildebrandt F: Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat Genet 38: 1397–1405, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodríguez-Pérez JC, Allen PG, Beggs AH, Pollak MR: Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS: CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300: 1298–1300, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Brown EJ, Schlondorff JS, Becker DJ, Tsukaguchi H, Tonna SJ, Uscinski AL, Higgs HN, Henderson JM, Pollak MR: Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet 42: 72–76, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB: A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Dai S, Wang Z, Pan X, Wang W, Chen X, Ren H, Hao C, Han B, Chen N: Functional analysis of promoter mutations in the ACTN4 and SYNPO genes in focal segmental glomerulosclerosis. Nephrol Dial Transplant 25: 824–835, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Okamoto K, Tokunaga K, Doi K, Fujita T, Suzuki H, Katoh T, Watanabe T, Nishida N, Mabuchi A, Takahashi A, Kubo M, Maeda S, Nakamura Y, Noiri E: Common variation in GPC5 is associated with acquired nephrotic syndrome. Nat Genet 43: 459–463, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB: Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol 27: 8698–8712, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB: Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB: Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem 278: 20716–20723, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Barletta GM, Kovari IA, Verma RK, Kerjaschki D, Holzman LB: Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem 278: 19266–19271, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Kurihara H, Anderson JM, Farquhar MG: Diversity among tight junctions in rat kidney: glomerular slit diaphragms and endothelial junctions express only one isoform of the tight junction protein ZO-1. Proc Natl Acad Sci U S A 89: 7075–7079, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kerjaschki D: Polycation-induced dislocation of slit diaphragms and formation of cell junctions in rat kidney glomeruli: The effects of low temperature, divalent cations, colchicine, and cytochalasin B. Lab Invest 39: 430–440, 1978 [PubMed] [Google Scholar]
- 30. Cybulsky AV, Bonventre JV, Quigg RJ, Lieberthal W, Salant DJ: Cytosolic calcium and protein kinase C reduce complement-mediated glomerular epithelial injury. Kidney Int 38: 803–811, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Seiler MW, Venkatachalam MA, Cotran RS: Glomerular epithelium: Structural alterations induced by polycations. Science 189: 390–393, 1975 [DOI] [PubMed] [Google Scholar]
- 32. Rudiger F, Greger R, Nitschke R, Henger A, Mundel P, Pavenstadt H: Polycations induce calcium signaling in glomerular podocytes. Kidney Int 56: 1700–1709, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Henger A, Huber T, Fischer KG, Nitschke R, Mundel P, Schollmeyer P, Greger R, Pavenstadt H: Angiotensin II increases the cytosolic calcium activity in rat podocytes in culture. Kidney Int 52: 687–693, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Gloy J, Henger A, Fischer KG, Nitschke R, Mundel P, Bleich M, Schollmeyer P, Greger R, Pavenstadt H: Angiotensin II depolarizes podocytes in the intact glomerulus of the rat. J Clin Invest 99: 2772–2781, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reiser J, Polu KR, Moller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR: TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, Anagnostou T, Pavenstadt H, Hsu HH, Schlondorff J, Ramos A, Greka A: Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal 3: ra77, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N: Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol 15: 1475–1487, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN: TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 116: 3114–3126, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen J, Chen JK, Neilson EG, Harris RC: Role of EGF receptor activation in angiotensin II-induced renal epithelial cell hypertrophy. J Am Soc Nephrol 17: 1615–1623, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Flannery PJ, Spurney RF: Transactivation of the epidermal growth factor receptor by angiotensin II in glomerular podocytes. Nephron Exp Nephrol 103: e109–e118, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Inagami T, Eguchi S, Numaguchi K, Motley ED, Tang H, Matsumoto T, Yamakawa T: Cross-talk between angiotensin II receptors and the tyrosine kinases and phosphatases. J Am Soc Nephrol 10 [Suppl 11]: S57–S61, 1999 [PubMed] [Google Scholar]
- 45. Hsu HH, Hoffmann S, Endlich N, Velic A, Schwab A, Weide T, Schlatter E, Pavenstadt H: Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. J Mol Med 86: 1379–1394, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P: Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol 8: 485–491, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Montell C: TRP channels in Drosophila photoreceptor cells. J Physiol 567: 45–51, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramsey IS, Delling M, Clapham DE: An introduction to TRP channels. Annu Rev Physiol 68: 619–647, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Gees M, Colsoul B, Nilius B: The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol 2: a003962, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G: Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397: 259–263, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Chaudhuri P, Colles SM, Bhat M, Van Wagoner DR, Birnbaumer L, Graham LM: Elucidation of a TRPC6-TRPC5 channel cascade that restricts endothelial cell movement. Mol Biol Cell 19: 3203–3211, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX: Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci U S A 101: 13861–13866, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nishida M, Onohara N, Sato Y, Suda R, Ogushi M, Tanabe S, Inoue R, Mori Y, Kurose H: Galpha12/13-mediated up-regulation of TRPC6 negatively regulates endothelin-1-induced cardiac myofibroblast formation and collagen synthesis through nuclear factor of activated T cells activation. J Biol Chem 282: 23117–23128, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, Mori Y, Nagao T, Kurose H: TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J 25: 5305–5316, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wu X, Eder P, Chang B, Molkentin JD: TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci U S A 107: 7000–7005, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y: The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ Res 88: 325–332, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Jung S, Strotmann R, Schultz G, Plant TD: TRPC6 is a candidate channel involved in receptor-stimulated cation currents in A7r5 smooth muscle cells. Am J Physiol Cell Physiol 282: C347–C359, 2002 [DOI] [PubMed] [Google Scholar]
- 59. Welsh DG, Morielli AD, Nelson MT, Brayden JE: Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Dietrich A, Mederos Y, Schnitzler M, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L: Increased vascular smooth muscle contractility in TRPC6-/- mice. Mol Cell Biol 25: 6980–6989, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Greka A, Navarro B, Oancea E, Duggan A, Clapham DE: TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci 6: 837–845, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE: Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol 6: 709–720, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, Dedman AM, Flemming PK, McHugh D, Naylor J, Cheong A, Bateson AN, Munsch CM, Porter KE, Beech DJ: A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res 98: 1381–1389, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE: TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29: 645–655, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE: Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem 278: 39014–39019, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Blair NT, Kaczmarek JS, Clapham DE: Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol 133: 525–546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jung S, Mühle A, Schaefer M, Strotmann R, Schultz G, Plant TD: Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem 278: 3562–3571, 2003 [DOI] [PubMed] [Google Scholar]
- 68. Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, Gapon S, Yao GL, Tsvetkov E, Rodig SJ, Van't Veer A, Meloni EG, Carlezon WA, Jr, Bolshakov VY, Clapham DE: Essential role for TRPC5 in amygdala function and fear-related behavior. Cell 137: 761–772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, Reiser J, Mundel P: Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest 115: 1188–1198, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Huber TB, Kwoh C, Wu H, Asanuma K, Godel M, Hartleben B, Blumer KJ, Miner JH, Mundel P, Shaw AS: Bigenic mouse models of focal segmental glomerulosclerosis involving pairwise interaction of CD2AP, Fyn, and synaptopodin. J Clin Invest 116: 1337–1345, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schlondorff J, Del Camino D, Carrasquillo R, Lacey V, Pollak MR: TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am J Physiol Cell Physiol 296: C558–C569, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang Y, Jarad G, Tripathi P, Pan M, Cunningham J, Martin DR, Liapis H, Miner JH, Chen F: Activation of NFAT signaling in podocytes causes glomerulosclerosis. J Am Soc Nephrol 21: 1657–1666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Aspenstrom P, Fransson A, Saras J: Rho GTPases have diverse effects on the organization of the actin filament system. Biochem J 377: 327–337, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Etienne-Manneville S, Hall A: Rho GTPases in cell biology. Nature 420: 629–635, 2002 [DOI] [PubMed] [Google Scholar]
- 75. Yanagida-Asanuma E, Asanuma K, Kim K, Donnelly M, Young Choi H, Hyung Chang J, Suetsugu S, Tomino Y, Takenawa T, Faul C, Mundel P: Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am J Pathol 171: 415–427, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T: Modification of mineralocorticoid receptor function by Rac1 GTPase: Implication in proteinuric kidney disease. Nat Med 14: 1370–1376, 2008 [DOI] [PubMed] [Google Scholar]
- 77. Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, Nishioka H, Yoshida H, Doi T, Mizoguchi A, Matsuura N, Niho Y, Nishimune Y, Nishikawa S, Takai Y: Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene 18: 5373–5380, 1999 [DOI] [PubMed] [Google Scholar]
- 78. Collins SR, Meyer T. Calcium flickers lighting the way in chemotaxis? Dev Cell 16: 160–161, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H: Calcium flickers steer cell migration. Nature 457: 901–905, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Guilluy C, Bregeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, Henrion D, Scalbert E, Bril A, Torres RM, Offermanns S, Pacaud P, Loirand G: The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat Med 16: 183–190, 2010 [DOI] [PubMed] [Google Scholar]
- 81. Moller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, Reiser J: Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol 18: 29–36, 2007 [DOI] [PubMed] [Google Scholar]
- 82. Holtmaat A, Svoboda K: Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10: 647–658, 2009 [DOI] [PubMed] [Google Scholar]
- 83. Krall P, Canales CP, Kairath P, Carmona-Mora P, Molina J, Carpio JD, Ruiz P, Mezzano SA, Li J, Wei C, Reiser J, Young JI, Walz K: Podocyte-specific overexpression of wild type or mutant trpc6 in mice is sufficient to cause glomerular disease. PLoS One 5: e12859, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Eckel J, Lavin PJ, Finch EA, Mukerji N, Burch J, Gbadegesin R, Wu G, Bowling B, Byrd A, Hall G, Sparks M, Zhang ZS, Homstad A, Barisoni L, Birbaumer L, Rosenberg P, Winn MP: TRPC6 enhances angiotensin II-induced albuminuria. J Am Soc Nephrol 22: 526–535, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Freichel M, Vennekens R, Olausson J, Stolz S, Philipp SE, Weissgerber P, Flockerzi V: Functional role of TRPC proteins in native systems: Implications from knockout and knock-down studies. J Physiol 567: 59–66, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cayouette S, Lussier MP, Mathieu EL, Bousquet SM, Boulay G: Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. J Biol Chem 279: 7241–7246, 2004 [DOI] [PubMed] [Google Scholar]
- 87. Kim EY, Alvarez-Baron CP, Dryer SE: Canonical transient receptor potential channel (TRPC)3 and TRPC6 associate with large-conductance Ca2+-activated K+ (BKCa) channels: role in BKCa trafficking to the surface of cultured podocytes. Mol Pharmacol 75: 466–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kim EY, Choi KJ, Dryer SE: Nephrin binds to the COOH terminus of a large-conductance Ca2+-activated K+ channel isoform and regulates its expression on the cell surface. Am J Physiol Renal Physiol 295: F235–F246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fakler B, Adelman JP: Control of K(Ca) channels by calcium nano/microdomains. Neuron 59: 873–881, 2008 [DOI] [PubMed] [Google Scholar]
- 90. Kwan HY, Shen B, Ma X, Kwok YC, Huang Y, Man YB, Yu S, Yao X: TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ Res 104: 670–678, 2009 [DOI] [PubMed] [Google Scholar]
- 91. Shimizu T, Owsianik G, Freichel M, Flockerzi V, Nilius B, Vennekens R: TRPM4 regulates migration of mast cells in mice. Cell Calcium 45: 226–232, 2009 [DOI] [PubMed] [Google Scholar]
- 92. Vennekens R, Olausson J, Meissner M, Bloch W, Mathar I, Philipp SE, Schmitz F, Weissgerber P, Nilius B, Flockerzi V, Freichel M: Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol 8: 312–320, 2007 [DOI] [PubMed] [Google Scholar]
- 93. Dryer SE, Reiser J: TRPC6 channels and their binding partners in podocytes: Role in glomerular filtration and pathophysiology. Am J Physiol Renal Physiol 299: F689–F701, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Foster RR, Zadeh MA, Welsh GI, Satchell SC, Ye Y, Mathieson PW, Bates DO, Saleem MA: Flufenamic acid is a tool for investigating TRPC6-mediated calcium signalling in human conditionally immortalised podocytes and HEK293 cells. Cell Calcium 45: 384–390, 2009 [DOI] [PubMed] [Google Scholar]
- 95. Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP: CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312: 1220–1223, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS: Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 454: 538–542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG: Orai1 is an essential pore subunit of the CRAC channel. Nature 443: 230–233, 2006 [DOI] [PubMed] [Google Scholar]
- 98. Moller CC, Flesche J, Reiser J: Sensitizing the Slit Diaphragm with TRPC6 ion channels. J Am Soc Nephrol 20: 950–953, 2009 [DOI] [PubMed] [Google Scholar]
- 99. Christensen AP, Corey DP: TRP channels in mechanosensation: Direct or indirect activation? Nat Rev Neurosci 8: 510–521, 2007 [DOI] [PubMed] [Google Scholar]
- 100. Huber TB, Schermer B, Müller RU, Höhne M, Bartram M, Calixto A, Hagmann H, Reinhardt C, Koos F, Kunzelmann K, Shirokova E, Krautwurst D, Harteneck C, Simons M, Pavenstädt H, Kerjaschki D, Thiele C, Walz G, Chalfie M, Benzing T: Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci U S A 103: 17079–17086, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL: A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A 103: 16586–16591, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mederos y, Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T: Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 27: 3092–3103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Voets T, Nilius B: TRPCs, GPCRs and the Bayliss effect. EMBO J 28: 4–5, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gomis A, Soriano S, Belmonte C, Viana F: Hypoosmotic- and pressure-induced membrane stretch activate TRPC5 channels. J Physiol 586: 5633–5649, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tryggvason K, Pikkarainen T, Patrakka J: Nck links nephrin to actin in kidney podocytes. Cell 125: 221–224, 2006 [DOI] [PubMed] [Google Scholar]
- 106. Schlondorff J: Nephrin AKTs on actin: The slit diaphragm-actin cytoskeleton signaling network expands. Kidney Int 73: 524–526, 2008 [DOI] [PubMed] [Google Scholar]
- 107. Kanda S, Harita Y, Shibagaki Y, Sekine T, Igarashi T, Inoue T, Hattori S: Tyrosine phosphorylation-dependent activation of TRPC6 regulated by PLC-{gamma}1 and Nephrin: Effect of mutations associated with focal segmental glomerulosclerosis. Mol Biol Cell 22: 1824–1835, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Agrawal V, Marinescu V, Agarwal M, McCullough PA: Cardiovascular implications of proteinuria: An indicator of chronic kidney disease. Nat Rev Cardiol 6: 301–311, 2009 [DOI] [PubMed] [Google Scholar]
- 109. Casas JP, Chua W, Loukogeorgakis S, Vallance P, Smeeth L, Hingorani AD, MacAllister RJ: Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: Systematic review and meta-analysis. Lancet 366: 2026–2033, 2005 [DOI] [PubMed] [Google Scholar]
- 110. Fischer KG, Jonas N, Poschenrieder F, Cohen C, Kretzler M, Greiber S, Pavenstädt H: Characterization of a Na(+)-Ca(2+) exchanger in podocytes. Nephrol Dial Transplant 17: 1742–1750, 2002 [DOI] [PubMed] [Google Scholar]
- 111. Heeringa F, Moller CC, Du J, Yue L, Hinkes B, Chernin G, Vlangos CN, Hoyer PF, Reiser J, Hildebrandt F: A novel TRPC6 mutation that causes childhood FSGS. PLoS One 4: e7771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]