Abstract
Maintenance of extracellular K+ concentration within a narrow range is vital for numerous cell functions, particularly electrical excitability of heart and muscle. Potassium homeostasis during intermittent ingestion of K+ involves rapid redistribution of K+ into the intracellular space to minimize increases in extracellular K+ concentration, and ultimate elimination of the K+ load by renal excretion. Recent years have seen great progress in identifying the transporters and channels involved in renal and extrarenal K+ homeostasis. Here we apply these advances in molecular physiology to understand how acid-base disturbances affect serum potassium.
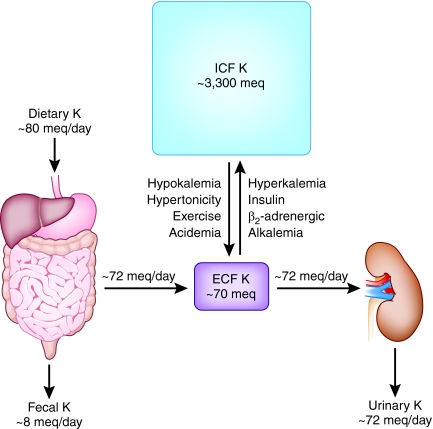
The effects of acid-base balance on serum potassium are well known.1 Maintenance of extracellular K+ concentration within a narrow range is vital for numerous cell functions, particularly electrical excitability of heart and muscle.2 However, maintenance of normal extracellular K+ (3.5 to 5 mEq/L) is under two potential threats. First, as illustrated in Figure 1, because some 98% of the total body content of K+ resides within cells, predominantly skeletal muscle, small acute shifts of intracellular K+ into or out of the extracellular space can cause severe, even lethal, derangements of extracellular K+ concentration. As described in Figure 1, many factors in addition to acid-base perturbations modulate internal K+ distribution including insulin, catecholamines, and hypertonicity.3,4 Rapid redistribution of K+ into the intracellular space is essential for minimizing increases in extracellular K+ concentration during acute K+ loads. Second, as also illustrated in Figure 1, in steady state the typical daily K+ ingestion of about 70 mEq/d would be sufficient to cause large changes in extracellular K+ were it not for continuous renal K+ excretion, because K+ loss from the gastrointestinal tract is quite modest under normal conditions. Thus, plasma K+ is at the mercy of the interplay between internal K+ distribution and external K+ balance mediated by renal K+ excretion.
Figure 1.
K+ concentration in the extracellular fluid (ECF) is affected by dietary intake, exchange with the intracellular fluid (ICF), and urinary excretion.
Recent years have seen remarkable advances in identifying the transport processes involved in renal and extrarenal K+ balance and their regulation. Here we apply these advances in molecular physiology to understand the basis for longstanding observations of the effects of acid-base disturbances on serum potassium. We do not address the large spectrum of clinical syndromes that mutually affect K+ and acid-base balance.
Effects of Acid-Base Status on Internal K+ Distribution
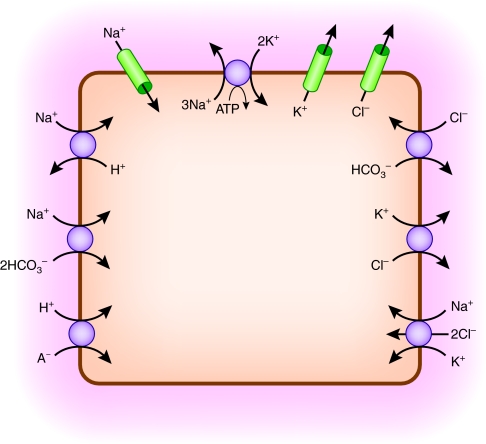
Most of the body K+ content resides in the intracellular space of skeletal muscle.2 An overview of ion transport pathways that directly or indirectly mediate shifts of K+ between muscle cells and the extracellular space in response to acid-base changes is shown in Figure 2.
Figure 2.
Multiple ion transport pathways directly or indirectly affect net K+ flux in skeletal muscle cells.
Muscle contraction is triggered by action potentials involving depolarizing Na+ entry through Na+ channels followed by membrane repolarization mediated by K+ efflux through K+ channels. Cl− channels play an important role in stabilizing the membrane potential and contributing to repolarization after action potentials. The electrochemical gradients of Na+ and K+ are restored by active Na+ extrusion and K+ uptake by the Na+,K+-ATPase.5 Accordingly, cell ion content is determined by the balance between pump and leak pathways for Na+ and K+.
However, muscle cells have additional pathways regulating intracellular pH homeostasis that can indirectly affect cellular Na+ and K+ balance.6 Quantitatively, the most important pathway regulating intracellular pH in skeletal muscle is Na+-H+ exchange,7 as shown in Figure 2. Na+-H+ exchange in skeletal muscle is highly dependent on intracellular pH, with marked activation by intracellular acidity and inhibition by alkalinity.8 Activity of this pathway in response to acid-base perturbations strongly affects intracellular Na+ loading.7 Na+-H+ exchanger isoform NHE1 is expressed in skeletal muscle and presumably accounts for most Na+-H+ exchange activity in this tissue.9
A lesser component of intracellular pH regulation in skeletal muscle is HCO3-dependent, because of Cl−-HCO3− exchange,7 also shown in Figure 2. In addition, isoforms of the Na+-bicarbonate cotransporter, NBCe1 and NBCe2, are expressed in muscle, raising the possibility that Na+-HCO3− cotransport contributes to intracellular pH regulation,10 as also indicated in Figure 2. Another pathway of potential importance for cellular acid-base homeostasis is monocarboxylate cotransport that mediates coupled flux of H+ with such organic anions as lactate (Figure 2). Monocarboxylate cotransporters, MCT1 and MCT4, are expressed in skeletal muscle.11 During conditions like lactic acidosis, this pathway will mediate influx of H+ and lactate, resulting in decreased intracellular pH. Cation-chloride cotransport pathways are also present. Expression of K+-Cl− cotransporters KCC1, KCC3, and KCC4, as well as Na+-K+-Cl− cotransporter NKCC1, has been detected in skeletal muscle.12–17 Interaction of K+-Cl− cotransport with acid-base transport will be discussed later.
Acute effects of acid-base disturbances on K+ redistribution have long been known.1,4 In general, metabolic acidosis with acidemia causes a net shift of K+ from the intracellular to the extracellular space. Conversely, net cellular uptake of K+ is observed in metabolic alkalosis with alkalemia. The directional effects of acidemia and alkalemia on K+ redistribution are similar in respiratory acid-base disturbances as in metabolic derangements,4 but the effects of respiratory disorders on K+ redistribution tend to be smaller than metabolic acid-base disturbances.4
How can these effects of acid-base disturbances on K+ redistribution be explained in terms of the underlying cellular transport mechanisms? The general effect of acidemia to cause K+ loss from cells is often attributed to membrane K+-H+ exchange. However, directly coupled K+-H+ exchange is undetected in skeletal muscle.7 Nevertheless, reduction of extracellular pH results in net loss of K+ even from isolated muscle,18,19 indicating this phenomenon is at least in part intrinsic to muscle and independent of changes in hormonal milieu as might occur in vivo. What then explains the apparent K+-H+ exchange?
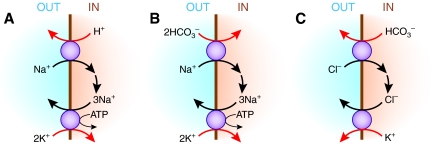
As illustrated in Figure 3, the multiple acid-base transport pathways mentioned above may give rise to apparent K+-H+ exchange. In the case of the predominant pH regulatory pathway, Na+-H+ exchange, Na+ that enters by this route must be extruded by the Na+,K+-ATPase (Figure 3A). Accordingly, K+ uptake by the Na+,K+-ATPase will be greater when Na+-H+ exchange activity is stimulated and will be diminished when the rate of Na+-H+ exchange is reduced. In the case of acidosis with acidemia, the fall in extracellular pH would result in inhibition of the rate of Na+-H+ exchange, leading to the accumulation of intracellular H+ and a decline in intracellular Na+. The latter would result in reduced Na+,K+-ATPase activity, leading to decreased active cellular K+ uptake to counteract passive K+ efflux through K+ channels.20 The final result would be as if H+ had entered the cell in exchange for K+.
Figure 3.
Apparent K+-H+ exchange (or K+-HCO3− cotransport) in skeletal muscle cells can arise from functional coupling between (A) Na+-H+ exchange and Na+,K+-ATPase, (B) Na+-HCO3− cotransport and Na+,K+-ATPase, or (C) Cl−-HCO3− exchange and K+-Cl− cotransport.
Similarly, as illustrated in Figure 3B, Na+-HCO3− cotransport operating in parallel with Na+,K+-ATPase may result in K+-HCO3− cotransport, which is equivalent to K+-H+ exchange. For example, in the case of metabolic acidosis with acidemia, the fall in extracellular HCO3− results in inhibition of the inward rate of Na+-bicarbonate cotransport, leading to a fall in intracellular Na+ and reduced Na+,K+-ATPase activity. Lower Na+,K+-ATPase activity would cause a net loss of cellular K+. Again, the result would be as if H+ had entered the cell in exchange for K+.
Finally, Cl−-HCO3− exchange also may contribute to apparent K+-H+ exchange if operating in parallel with K+-Cl− cotransport, as shown in Figure 3C. Metabolic acidosis with a fall in extracellular HCO3− would increase the inward movement of Cl− by Cl−-HCO3− exchange. The resulting rise in intracellular Cl− would then promote K+ efflux by K+-Cl− cotransport. The net result would be K+ efflux along with HCO3−, which is an equivalent process to exchanging intracellular K+ for extracellular H+.
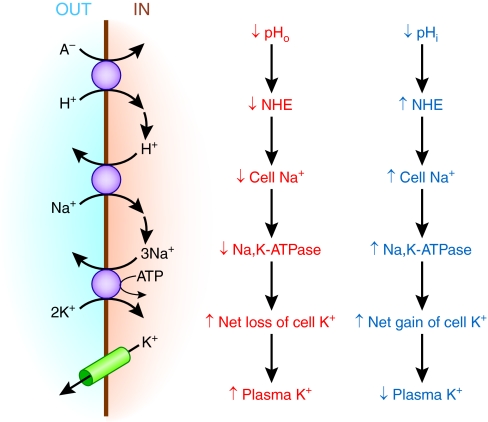
A striking observation has been that metabolic acidosis caused by mineral acid (hyperchloremic, nongap acidosis) causes a much larger shift of K+ into the extracellular fluid than does organic acidosis (lactic acidosis).21 The effect of hydrochloric acid but not organic acids to release K+ into the extracellular space had been observed using isolated muscle preparations, indicating this phenomenon can occur independently of systemic factors.22 In the case of acidemia caused by an organic acidosis like lactic acidosis, there would again be the effect of both low extracellular pH and HCO3− tending to inhibit Na+-H+ exchange and Na+-bicarbonate cotransport. This is illustrated for the case of Na+-H+ exchange in Figure 4, but in contrast to the situation with hyperchloremic acidosis, there would also be a strong inward flux of lactate and H+ through the monocarboxylate transporter, resulting in a larger fall in intracellular pH and HCO3−. The decrease in intracellular pH and HCO3− would tend to stimulate Na+ entry by Na+-H+ exchange and Na+-HCO3− cotransport, stimulating Na+,K+-ATPase activity. The net effect would be to drive net cellular uptake of K+.
Figure 4.
Opposing effects of extracellular and intracellular pH modify the influence of organic acidosis on plasma K+.
Thus, as illustrated in Figure 4, extracellular and intracellular acidosis are predicted to have opposing effects on the distribution of K+ because of their differing effects on cellular Na+ loading. During organic acidosis, there will be greater cellular acidification and Na+ entry than during hyperchloremic acidosis, resulting in higher Na+,K+-ATPase activity compared with hyperchloremic acidosis. However, in several tissues, Na+,K+-ATPase activity is affected by intracellular pH, with reduced activity when intracellular pH is lower than normal.23–25 For intracellular acidification to stimulate net K+ uptake, it would require that the effect of low intracellular pH to inhibit Na+,K+-ATPase activity is less significant than the effect of intracellular Na+ loading to stimulate pump activity.
The acid-base mechanisms illustrated in Figures 2 and 3 also provide a possible explanation for the observation that bicarbonate can affect K+ redistribution independent of the effect of extracellular pH.26,27 Na+ entry by Na+-HCO3− cotransport would be enhanced whenever extracellular HCO3− is increased, resulting in increased cell Na+ uptake, stimulation of Na+,K+-ATPase activity, and net cellular K+ uptake (Figure 3B). Conversely, inhibition of Na+-HCO3− cotransport when extracellular HCO3− is reduced leads to a net loss of cell K+. Analogously, the rate of Cl− entry by Cl−-HCO3− exchange would be higher when extracellular HCO3− is reduced, increasing cell Cl− and enhancing the exit of K+ by K+-Cl− cotransport (Figure 3C). Conversely, Cl− entry by Cl−-HCO3− exchange would be lower when extracellular HCO3− is increased, leading to reduced K+ efflux by K+-Cl− cotransport.
Similar considerations may also account for the smaller shifts in K+ observed with respiratory acidosis compared with metabolic acidosis.4 In respiratory acidosis, there is a fall in extracellular pH, but extracellular bicarbonate is elevated. One would therefore expect that Na+-H+ exchange is inhibited as in metabolic acidosis with equivalent acidemia, but Na+-bicarbonate cotransport would not be reduced. Accordingly, as compared with metabolic acidosis, respiratory acidosis would be associated with a smaller decrement in intracellular Na+, less inhibition of Na+,K+-ATPase activity, and reduced net K+ loss from the cell. In addition, during respiratory acidosis with elevated pCO2, rapid cell entry of CO2 will acidify intracellular pH. As discussed above for the case of organic acidosis, acidification of intracellular pH, by stimulating Na+ entry by Na+-H+ exchange tends to enhance Na+,K+-ATPase activity and oppose a net loss of intracellular K+.
In view of the longstanding observations discussed above on K+ redistribution in acid-base disorders, one would expect alkalinization by HCO3− administration to be an effective modality for acute treatment of hyperkalemia. However, some investigators have failed to find an effect of HCO3− administration to lower plasma K+ in hyperkalemic patients.28–30 An effect of HCO3− administration to lower plasma K+ has been more striking in patients with more severe degrees of pre-existing acidosis than in those with only minimal reductions of plasma HCO3−.31 One possible factor modifying the effect of extracellular HCO3− and pH on K+ distribution is the level of intracellular pH and HCO3−. At any given extracellular pH and HCO3−, Na+ entry by Na+-H+ exchange and Na+-bicarbonate cotransport is greater when intracellular pH and HCO3− are reduced, as discussed earlier. Patients with appreciable pre-existing metabolic acidosis would be expected to have lower intracellular pH and HCO3−. This may account for the fact that the effect of HCO3− administration to reduce plasma K+ has been more striking in patients with pre-existing acidosis.31
Effects of pH and HCO3− on internal K+ distribution may be modified by hormonal systems that affect cellular K+ uptake and release. For example, net cellular uptake of K+ is strongly stimulated by insulin because of increased Na+,K+-ATPase activity.3,2 There is evidence that stimulation of insulin secretion by acidosis diminishes the hyperkalemia otherwise resulting from acidosis.32 Moreover, differential effects of organic versus hyperchloremic acidosis on insulin and glucagon secretion may contribute to the differing effects of these forms of acidosis on plasma K+ as discussed earlier.33 Although skeletal muscle is the predominant source of intracellular K+ content, there is evidence that the effect of organic acid-induced insulin secretion on plasma K+ is mediated at least in part by hepatic K+ uptake.33 The interactions of acid-base disturbances with other hormonal systems are at present incompletely defined.
Effects of Acid-Base on Renal K+ Excretion
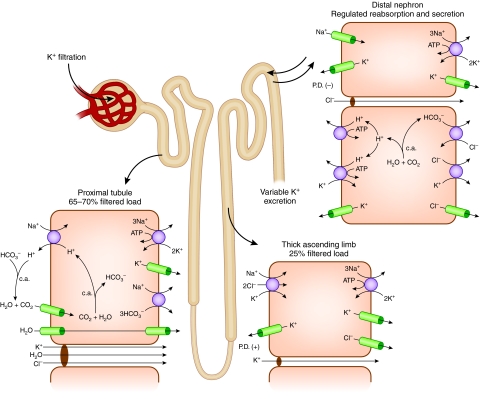
The geography of net K+ transport along the nephron is shown in Figure 5. The bulk of filtered potassium is reabsorbed in the proximal tubule, where its reabsorption is predominantly passive and paracellular.34 Such passive reabsorption ultimately depends on and is driven by fluid reabsorption, which in turn is a function of Na+ reabsorption in this highly water-permeable nephron segment. Accordingly, the proximal tubule reabsorbs K+ in approximate proportion to reabsorption of filtered Na+ and water, accounting for retrieval of some 70% of the filtered load of K+.
Figure 5.
Urinary K+ excretion is the resultant of filtration, reabsorption and secretion along the nephron. Mechanisms of K+ transport in proximal tubule, thick ascending limb, and distal nephron (connecting and collecting tubule) are shown in the inset.
Most of the remaining fraction of filtered K+ (approximately 25%) is reabsorbed in the thick ascending limb by a transcellular route involving Na+-K+-Cl− cotransport across the apical membrane and extrusion by the Na+,K+-ATPase across the basolateral membrane.34 As a consequence, only about 5% of the filtered K+ reaches the distal tubule.
The critical sites in the nephron responsible for determining K+ excretion are the connecting tubule and collecting tubule.35 Under conditions of normal or elevated dietary K+ intake, these segments mediate net secretion of K+. However, under conditions of K+ depletion, net K+ reabsorption may occur in this portion of the nephron.
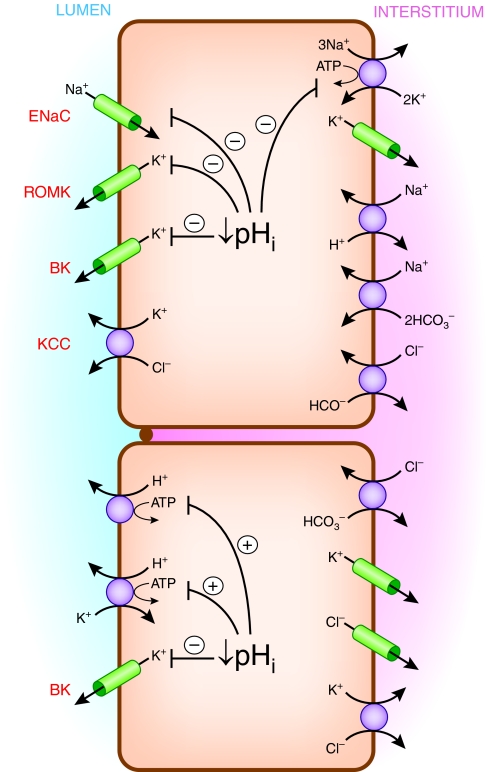
Cell models for K+ secretion and reabsorption are illustrated in Figure 6. K+ secretion, which takes place through connecting tubule cells and principal cells of the collecting tubule, involves active uptake across the basolateral membrane mediated by Na+,K+-ATPase, followed by passive exit across the apical membrane through K+ channels. The ENaC Na+ channel in the apical membrane indirectly plays a critical role in K+ secretion by providing intracellular Na+ as substrate for the Na+,K+-ATPase and by depolarizing the apical membrane, thereby increasing the driving force for K+ efflux into the tubule fluid. At least two types of apical K+ channels have been implicated in K+ secretion, ROMK and BK.36–39 In addition, effects of luminal Cl− on K+ secretion suggest that a component of apical membrane K+ efflux takes place by electroneutral K+-Cl− cotransport,40,41 although its molecular mechanism is unclear.
Figure 6.
Multiple ion transport pathways directly or indirectly affect K+ transport in the connecting tubule and cortical collecting tubule and are affected by pH.
Multiple factors modulate K+ secretion in these distal nephron segments. First, plasma K+ concentration is an important determinant of the rate of K+ secretion. External K+ is substrate for the Na+,K+-ATPase, and there is saturable dependence of K+ secretion on plasma K+ concentration.42 In addition, the higher the plasma K+, the less backflux of K+ takes place through basolateral K+ channels. Second, aldosterone, whose secretion is stimulated by elevated plasma K+, is a major regulator of K+ secretion in distal nephron segments. Aldosterone acts through several signaling pathways and effector mechanisms to bring about increased activities of apical Na+ and K+ channels and increased activity of basolateral Na+,K+-ATPase.35,43,44 Third, K+ secretion is highly affected by luminal Na+ and fluid delivery to distal K+ secretory sites.45,46 K+ secretion is dependent directly and indirectly on luminal Na+ entry through ENaC, as discussed above, which in turn is a function of luminal Na+ concentration.47 K+ secretion is enhanced at higher luminal flow rates because of activation of BK channels.46 Recent studies suggest that flow activation of BK channels involves intracellular Ca++ signaling most likely in response to mechanosensing by apical cilia.46 Finally, net K+ secretion is modified by modulation of active K+ reabsorption mediated by apical H+,K+-ATPase in intercalated cells.48,49 Chronic K+ depletion causes upregulation of K+ reabsorption mediated by apical H+,K+-ATPase in the collecting tubule.50,51
Acid-base disturbances have complex effects on renal K+ excretion. For example, acute acidemia caused by metabolic acidosis reduces renal K+ excretion, whereas metabolic acidosis at a later phase promotes urinary K+ excretion and development of K+ deficiency.52 How can these seemingly contradictory effects of acidemia be explained?
The principal cells of the cortical collecting tubule have transporters regulating intracellular pH that can indirectly affect K+ secretion. Specifically, activities of Na+-H+ exchange, Na+-HCO3− cotransport and Cl−-HCO3− exchange at the basolateral membrane of principal cells have been observed.53–56 Na+-H+ exchanger isoform NHE1 is strongly expressed on the basolateral membrane of connecting tubule and principal cells.57 As discussed earlier for the case of muscle, activities of Na+-H+ exchange and Na+-HCO3− cotransport can affect intracellular Na+ loading and Na+, K+-ATPase activity. Changes in Na+, K+-ATPase activity would affect basolateral K+ uptake and transtubular K+ secretion. In fact, basolateral Na+ entry by Na+-H+ exchange can support K+ secretion in the absence of luminal Na+.58 Thus, inhibition of Na+-H+ exchange and Na+-HCO3− cotransport during metabolic acidosis with acidemia reduces cell Na+ and thereby inhibits K+ secretion. Conversely, metabolic alkalosis with alkalemia would tend to stimulate K+ secretion.
Several of the pathways involved in distal nephron K+ secretion are directly affected by pH, as illustrated in Figure 6. A fall in intracellular pH reduces activity of Na+,K+-ATPase on the basolateral membrane23–25 and diminishes activities of ENaC, ROMK, and BK on the apical membrane.59–62 These effects could contribute to inhibition of active K+ secretion in response to acidemia to the extent that there is parallel intracellular acidosis. ENaC abundance is decreased when luminal or basolateral HCO3− (and pH) is reduced.63 In addition, stimulation of electrogenic H+ secretion by the vacuolar ATPase in intercalated cells during acidemia would tend to reduce the lumen-negative transepithelial potential difference,64 inhibiting K+ secretion. Furthermore, acidemia upregulates apical H+,K+-ATPase in intercalated cells,65 thereby enhancing K+ reabsorption and reducing net K+ secretion.
However, metabolic acidosis causes increased distal Na+ delivery and flow rate, as well as increased urinary Na+ excretion.42,52 There are at least four possible factors contributing to this phenomenon. First, Na+,K+-ATPase in all nephron segments may be inhibited by acidemia with reduced intracellular pH, leading to diminished renal tubular Na+ reabsorption upstream of the K+ secretory sites. Second, although acidosis causes upregulation of NHE3 activity in the proximal tubule,66,67 the absolute amount of Na+ reabsorbed with HCO3− in the proximal tubule is actually reduced in metabolic acidosis because of the diminished filtered load of HCO3−.68 Third, the reduction in absolute fluid reabsorption coupled to NaHCO3 reabsorption in metabolic acidosis results in a less than normal increment in luminal Cl− concentration along the length of the proximal tubule, lowering the driving force for passive paracellular NaCl reabsorption.68 Fourth, apical membrane Cl−-base exchange activity is downregulated in metabolic acidosis, reducing transcellular NaCl reabsorption in the proximal tubule.69
These effects of metabolic acidosis to enhance distal Na+ delivery and flow rate and to augment urinary Na+ excretion tend to cause volume depletion, resulting in increased renin and aldosterone secretion.70 In addition, acidosis may directly stimulate aldosterone secretion independent of renin secretion.70,71 As discussed above, K+ secretion in the distal nephron is strongly stimulated by aldosterone and by increased luminal Na+ delivery and flow rate. These factors eventually predominate over the local inhibitory effect of acidemia on the K+ secreting cells, resulting in increased K+ excretion and negative K+ balance. Ultimately, a new steady state is established as K+ balance is restored at the expense of hypokalemia.
This pathophysiology is well illustrated by the disorder of classic distal RTA. Although there are exceptions,72 these patients generally have hypokalemia caused by renal K+ wasting during chronic acidosis, which is corrected by base administration.73,74 Another clinical example in which the effect of distal Na+ delivery and flow to stimulate urinary K+ excretion predominates over the inhibitory effect of acidemia is diabetic ketoacidosis with osmotic diuresis.75 Moreover, organic acidosis with high rates of excretion of nonchloride anions in the urine will enhance distal K+ secretion caused by the reduced luminal Cl− concentration through presumed apical membrane K+-Cl− cotransport.40,41
Acidemia caused by respiratory acidosis results in similar directional changes in urinary K+ excretion as does metabolic acidosis. Acute acidemia caused by respiratory acidosis inhibits renal K+ secretion in the distal nephron76 and reduces urinary K+ excretion.52 Over the following days, respiratory acidosis results in urinary K+ wasting associated with increased Na+ excretion.52 It is likely these effects are mediated by many of the same mechanisms discussed above for metabolic acidosis. However, the effects of chronic acidemia to augment K+ excretion caused by increased distal Na+ and fluid delivery will tend to be milder for respiratory acidosis than for metabolic acidosis.52 First, renal compensation of chronic respiratory acid-base disorders restores blood pH much closer to normal than does respiratory compensation of metabolic acid-base disorders.77,78 Thus, acidemia will be milder in chronic respiratory acidosis than in chronic metabolic acidosis, resulting in less inhibition of Na+,K+-ATPase and less augmentation of Na+ and fluid delivery to the distal nephron. Second, the role of low filtered load of HCO3− in limiting absolute NaHCO3 reabsorption along the proximal tubule in metabolic acidosis as discussed above would not be a contributing factor in respiratory acidosis.
In general, alkalemia stimulates distal nephron K+ secretion and urinary K+ excretion.52 These effects are larger for metabolic than for respiratory alkalosis and are in essence the converse of the effects of acidemia on the K+ secretory pathways, because elevated intracellular pH tends to increase activities of ENaC, ROMK, and BK.59–62 ENaC abundance is increased when luminal or basolateral HCO3− (and pH) is elevated.63 Moreover, in the case of acute metabolic alkalosis, there is inhibition of fractional NaHCO3 and fluid reabsorption in the proximal tubule, leading to increased distal delivery of Na+ and HCO3− and enhanced fluid flow.79 As already discussed, increased Na+ delivery and fluid flow stimulate K+ secretion. In addition, increased luminal delivery of HCO3− as a nonchloride anion stimulates a component of distal K+ secretion because of the reduced luminal Cl− concentration as mentioned earlier.41 Of course, in the maintenance phase of metabolic alkalosis with volume contraction, distal Na+ and HCO3− delivery will decline, thereby mitigating the rate of K+ wasting.80
Summary and Conclusions
We have used new information about the molecular physiology of extrarenal and renal potassium transport to explain longstanding observations of the complex effects of acid-base disturbances on serum potassium. It should be emphasized that many aspects of these explanations are somewhat speculative because the quantitative contributions of specific pathways to mediating effects of pH on extrarenal and renal K+ transport are not known with certainty. Moreover, we have not considered new developments concerning molecular sensors and signaling mechanisms that respond to changes in extracellular and intracellular pH, CO2, and HCO3−.81 The roles of these sensor and signaling mechanisms in mediating the effects of acid-base disturbances on extrarenal and renal K+ homeostasis are not yet known.
Nevertheless, it should be apparent that there is no simple relationship between pH and serum potassium because of the multiplicity of factors affecting internal and external K+ balance. For example, consider the patient with diabetic ketoacidosis. Acidemia will tend to shift K+ out of cells and cause hyperkalemia, but this effect is less pronounced in organic acidosis than in mineral acidosis. On the other hand, hypertonicity in the absence of insulin will promote K+ release into the extracellular space. Renal K+ excretion will be acutely inhibited by acidemia but ultimately enhanced by the increased distal Na+ delivery and flow rate caused by metabolic acidosis and osmotic diuresis in the setting of high aldosterone. Indeed, the patient may present with marked K+ depletion if osmotic diuresis has been going on for some time. Renal K+ excretion may later become reduced when GFR falls as volume depletion ensues. Accordingly, there will not be a straightforward relationship between serum potassium and acid-base status in such a patient. The clinician will need to be knowledgeable about the many factors affecting internal and external K+ balance to provide optimal patient care.
DISCLOSURES
Work in the authors' laboratories on this topic was supported by National Institutes of Health Grants DK17433 and DK33793. We are grateful for the careful review of the manuscript by Drs. Lisa Satlin and Alicia McDonough and for their helpful comments.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Burnell JM, Scribner BH, Uyeno BT, Villamil MF: The effect in humans of extracellular pH change on the relationship between serum potassium concentration and intracellular potassium. J Clin Invest 35: 935–939, 1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Youn JH, McDonough AA: Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol 71: 381–401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bia MJ, DeFronzo RA: Extrarenal potassium homeostasis. Am J Physiol 240: F257–F268, 1981 [DOI] [PubMed] [Google Scholar]
- 4. Adrogue HJ, Madias NE: Changes in plasma potassium concentration during acute acid-base disturbances. Am J Med 71: 456–467, 1981 [DOI] [PubMed] [Google Scholar]
- 5. Clausen T: Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev 83: 1269–1324, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Juel C: Regulation of pH in human skeletal muscle: Adaptations to physical activity. Acta Physiol 193: 17–24, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Aickin CC, Thomas RC: An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol 273: 295–316, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Juel C: Skeletal muscle Na+/H+ exchange in rats: pH dependency and the effect of training. Acta Physiol Scand 164: 135–140, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Juel C: Expression of the Na(+)/H(+) exchanger isoform NHE1 in rat skeletal muscle and effect of training. Acta Physiol Scand 170: 59–63, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Kristensen JM, Kristensen M, Juel C: Expression of Na+/HCO3- co-transporter proteins (NBCs) in rat and human skeletal muscle. Acta Physiol Scand 182: 69–76, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Juel C: Lactate-proton cotransport in skeletal muscle. Physiol Rev 77: 321–358, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Gillen CM, Brill S, Payne JA, Forbush B, 3rd: Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human: A new member of the cation-chloride cotransporter family. J Biol Chem 271: 16237–16244, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Race JE, Makhlouf FN, Logue PJ, Wilson FH, Dunham PB, Holtzman EJ: Molecular cloning and functional characterization of KCC3, a new K-Cl cotransporter. Am J Physiol 277: C1210–C1219, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Mount DB, Mercado A, Song L, Xu J, George AL, Jr, Delpire E, Gamba G: Cloning and characterization of KCC3 and KCC4, new members of the cation-chloride cotransporter gene family. J Biol Chem 274: 16355–16362, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Hiki K, D'Andrea RJ, Furze J, Crawford J, Woollatt E, Sutherland GR, Vadas MA, Gamble JR: Cloning, characterization, and chromosomal location of a novel human K+-Cl- cotransporter. J Biol Chem 274: 10661–10667, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Wong JA, Fu L, Schneider EG, Thomason DB: Molecular and functional evidence for Na(+)-K(+)-2Cl(-) cotransporter expression in rat skeletal muscle. Am J Physiol 277: R154–R161, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Kristensen M, Hansen T, Juel C: Membrane proteins involved in potassium shifts during muscle activity and fatigue. Am J Physiol Regul Integr Comp Physiol 290: R766–R772, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Rogers TA: Tissue buffering in rat diaphragm. Am J Physiol 191: 363–366, 1957 [DOI] [PubMed] [Google Scholar]
- 19. Fenn WO, Rogers TA, Ohr EA: Muscle electrolytes in acid and alkaline solutions. Am J Physiol 194: 373–378, 1958 [DOI] [PubMed] [Google Scholar]
- 20. Kamel KS, Wei C: Controversial issues in the treatment of hyperkalaemia. Nephrol Dial Transplant 18: 2215–2218, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Oster JR, Perez GO, Vaamonde CA: Relationship between blood pH and potassium and phosphorus during acute metabolic acidosis. Am J Physiol 235: F345–F351, 1978 [DOI] [PubMed] [Google Scholar]
- 22. Rogers TA, Wachenfeld AE: Effect of physiologic acids on electrolytes in rat diaphragm. Am J Physiol 193: 623–626, 1958 [DOI] [PubMed] [Google Scholar]
- 23. Russell JM, Boron WF, Brodwick MS: Intracellular pH and Na fluxes in barnacle muscle with evidence for reversal of the ionic mechanism of intracellular pH regulation. J Gen Physiol 82: 47–78, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eaton DC, Hamilton KL, Johnson KE: Intracellular acidosis blocks the basolateral Na-K pump in rabbit urinary bladder. Am J Physiol 247: F946–F954, 1984 [DOI] [PubMed] [Google Scholar]
- 25. Breitwieser GE, Altamirano AA, Russell JM: Effects of pH changes on sodium pump fluxes in squid giant axon. Am J Physiol 253: C547–C554, 1987 [DOI] [PubMed] [Google Scholar]
- 26. Fraley DS, Adler S: Isohydric regulation of plasma potassium by bicarbonate in the rat. Kidney Int 9: 333–343, 1976 [DOI] [PubMed] [Google Scholar]
- 27. Fraley DS, Adler S: Correction of hyperkalemia by bicarbonate despite constant blood pH. Kidney Int 12: 354–360, 1977 [DOI] [PubMed] [Google Scholar]
- 28. Blumberg A, Weidmann P, Shaw S, Gnadinger M: Effect of various therapeutic approaches on plasma potassium and major regulating factors in terminal renal failure. Am J Med 85: 507–512, 1988 [DOI] [PubMed] [Google Scholar]
- 29. Blumberg A, Weidmann P, Ferrari P: Effect of prolonged bicarbonate administration on plasma potassium in terminal renal failure. Kidney Int 41: 369–374, 1992 [DOI] [PubMed] [Google Scholar]
- 30. Gutierrez R, Schlessinger F, Oster JR, Rietberg B, Perez GO: Effect of hypertonic versus isotonic sodium bicarbonate on plasma potassium concentration in patients with end-stage renal disease. Miner Electrolyte Metab 17: 297–302, 1991 [PubMed] [Google Scholar]
- 31. Schwarz KC, Cohen BD, Lubash GD, Rubin AL: Severe acidosis and hyperpotassemia treated with sodium bicarbonate infusion. Circulation 19: 215–220, 1959 [DOI] [PubMed] [Google Scholar]
- 32. Wiederseiner JM, Muser J, Lutz T, Hulter HN, Krapf R: Acute metabolic acidosis: Characterization and diagnosis of the disorder and the plasma potassium response. J Am Soc Nephrol 15: 1589–1596, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Adrogue HJ, Chap Z, Ishida T, Field JB: Role of the endocrine pancreas in the kalemic response to acute metabolic acidosis in conscious dogs. J Clin Invest 75: 798–808, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giebisch G, Krapf R, Wagner C: Renal and extrarenal regulation of potassium. Kidney Int 72: 397–410, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Wang WH, Giebisch G: Regulation of potassium (K) handling in the renal collecting duct. Pflugers Arch 458: 157–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palmer LG: Potassium secretion and the regulation of distal nephron K channels. Am J Physiol 277: F821–F825, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Hebert SC, Desir G, Giebisch G, Wang W: Molecular diversity and regulation of renal potassium channels. Physiol Rev 85: 319–371, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Welling PA, Ho K: A comprehensive guide to the ROMK potassium channel: Form and function in health and disease. Am J Physiol Renal Physiol 297: F849–F863, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pluznick JL, Sansom SC: BK channels in the kidney: Role in K(+) secretion and localization of molecular components. Am J Physiol Renal Physiol 291: F517–F529, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Ellison DH, Velazquez H, Wright FS: Stimulation of distal potassium secretion by low lumen chloride in the presence of barium. Am J Physiol 248: F638–F649, 1985 [DOI] [PubMed] [Google Scholar]
- 41. Amorim JB, Bailey MA, Musa-Aziz R, Giebisch G, Malnic G: Role of luminal anion and pH in distal tubule potassium secretion. Am J Physiol Renal Physiol 284: F381–F388, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Stanton BA, Giebisch G: Effects of pH on potassium transport by renal distal tubule. Am J Physiol 242: F544–F551, 1982 [DOI] [PubMed] [Google Scholar]
- 43. Vinciguerra M, Mordasini D, Vandewalle A, Feraille E: Hormonal and nonhormonal mechanisms of regulation of the NA,K-pump in collecting duct principal cells. Semin Nephrol 25: 312–321, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Verrey F, Fakitsas P, Adam G, Staub O: Early transcriptional control of ENaC (de)ubiquitylation by aldosterone. Kidney Int 73: 691–696, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Khuri RN, Strieder WN, Giebisch G: Effects of flow rate and potassium intake on distal tubular potassium transfer. Am J Physiol 228: 1249–1261, 1975 [DOI] [PubMed] [Google Scholar]
- 46. Satlin LM, Carattino MD, Liu W, Kleyman TR: Regulation of cation transport in the distal nephron by mechanical forces. Am J Physiol Renal Physiol 291: F923–F931, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Palmer LG, Sackin H, Frindt G: Regulation of Na+ channels by luminal Na+ in rat cortical collecting tubule. J Physiol 509: 151–162, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Codina J, DuBose TD, Jr: Molecular regulation and physiology of the H+,K+-ATPases in kidney. Semin Nephrol 26: 345–351, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Gumz ML, Lynch IJ, Greenlee MM, Cain BD, Wingo CS: The renal H+-K+-ATPases: Physiology, regulation, and structure. Am J Physiol Renal Physiol 298: F12–F21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wingo CS: Active proton secretion and potassium absorption in the rabbit outer medullary collecting duct: Functional evidence for proton-potassium-activated adenosine triphosphatase. J Clin Invest 84: 361–365, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheval L, Barlet-Bas C, Khadouri C, Feraille E, Marsy S, Doucet A: K(+)-ATPase-mediated Rb+ transport in rat collecting tubule: Modulation during K+ deprivation. Am J Physiol 260: F800–F805, 1991 [DOI] [PubMed] [Google Scholar]
- 52. Gennari FJ, Cohen JJ: Role of the kidney in potassium homeostasis: Lessons from acid-base disturbances. Kidney Int 8: 1–5, 1975 [DOI] [PubMed] [Google Scholar]
- 53. Chaillet JR, Lopes AG, Boron WF: Basolateral Na-H exchange in the rabbit cortical collecting tubule. J Gen Physiol 86: 795–812, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang X, Kurtz I: H+/base transport in principal cells characterized by confocal fluorescence imaging. Am J Physiol 259: C365–C373, 1990 [DOI] [PubMed] [Google Scholar]
- 55. Weiner ID, Hamm LL: Regulation of intracellular pH in the rabbit cortical collecting tubule. J Clin Invest 85: 274–281, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Silver RB, Frindt G, Palmer LG: Regulation of principal cell pH by Na/H exchange in rabbit cortical collecting tubule. J Membr Biol 125: 13–24, 1992 [DOI] [PubMed] [Google Scholar]
- 57. Biemesderfer D, Reilly RF, Exner M, Igarashi P, Aronson PS: Immunocytochemical characterization of Na(+)-H+ exchanger isoform NHE-1 in rabbit kidney. Am J Physiol 263: F833–F840, 1992 [DOI] [PubMed] [Google Scholar]
- 58. Muto S, Tsuruoka S, Miyata Y, Fujimura A, Kusano E, Wang W, Seldin D, Giebisch G: Basolateral Na+/H+ exchange maintains potassium secretion during diminished sodium transport in the rabbit cortical collecting duct. Kidney Int 75: 25–30, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Palmer LG, Frindt G: Effects of cell Ca and pH on Na channels from rat cortical collecting tubule. Am J Physiol 253: F333–F339, 1987 [DOI] [PubMed] [Google Scholar]
- 60. Wang WH, Schwab A, Giebisch G: Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol 259: F494–F502, 1990 [DOI] [PubMed] [Google Scholar]
- 61. Hirsch J, Leipziger J, Frobe U, Schlatter E: Regulation and possible physiological role of the Ca(2+)-dependent K+ channel of cortical collecting ducts of the rat. Pflugers Arch 422: 492–498, 1993 [DOI] [PubMed] [Google Scholar]
- 62. Schlatter E, Haxelmans S, Hirsch J, Leipziger J: pH dependence of K+ conductances of rat cortical collecting duct principal cells. Pflugers Arch 428: 631–640, 1994 [DOI] [PubMed] [Google Scholar]
- 63. Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, Eaton DC, Wall SM: Pendrin modulates ENaC function by changing luminal HCO3. J Am Soc Nephrol 21: 1928–1941, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Koeppen BM, Helman SI: Acidification of luminal fluid by the rabbit cortical collecting tubule perfused in vitro. Am J Physiol 242: F521–F531, 1982 [DOI] [PubMed] [Google Scholar]
- 65. Silver RB, Mennitt PA, Satlin LM: Stimulation of apical H-K-ATPase in intercalated cells of cortical collecting duct with chronic metabolic acidosis. Am J Physiol 270: F539–F547, 1996 [DOI] [PubMed] [Google Scholar]
- 66. Wu MS, Biemesderfer D, Giebisch G, Aronson PS: Role of NHE3 in mediating renal brush border Na+-H+ exchange: Adaptation to metabolic acidosis. J Biol Chem 271: 32749–32752, 1996 [DOI] [PubMed] [Google Scholar]
- 67. Ambuhl PM, Amemiya M, Danczkay M, Lotscher M, Kaissling B, Moe OW, Preisig PA, Alpern RJ: Chronic metabolic acidosis increases NHE3 protein abundance in rat kidney. Am J Physiol 271: F917–F925, 1996 [DOI] [PubMed] [Google Scholar]
- 68. Cogan MG, Rector FC, Jr: Proximal reabsorption during metabolic acidosis in the rat. Am J Physiol 242: F499–F507, 1982 [DOI] [PubMed] [Google Scholar]
- 69. Wang T, Egbert AL, Jr, Aronson PS, Giebisch G: Effect of metabolic acidosis on NaCl transport in the proximal tubule. Am J Physiol 274: F1015–F1019, 1998 [DOI] [PubMed] [Google Scholar]
- 70. Schambelan M, Sebastian A, Katuna BA, Arteaga E: Adrenocortical hormone secretory response to chronic NH4Cl-induced metabolic acidosis. Am J Physiol 252: E454–E460, 1987 [DOI] [PubMed] [Google Scholar]
- 71. Loon NR, Wilcox CS: Mild metabolic alkalosis impairs the natriuretic response to bumetanide in normal human subjects. Clin Sci 94: 287–292, 1998 [DOI] [PubMed] [Google Scholar]
- 72. Sebastian A, McSherry E, Morris RC, Jr: Renal potassium wasting in renal tubular acidosis (RTA): Its occurrence in types 1 and 2 RTA despite sustained correction of systemic acidosis. J Clin Invest 50: 667–678, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pines KL, Mudge GH: Renal tubular acidosis with osteomalacia: Report of 3 cases. Am J Med 11: 302–311, 1951 [DOI] [PubMed] [Google Scholar]
- 74. Gill JR, Jr, Bell NH, Bartter FC: Impaired conservation of sodium and potassium in renal tubular acidosis and its correction by buffer anions. Clin Sci 33: 577–592, 1967 [PubMed] [Google Scholar]
- 75. Bergenstal RM: Diabetic ketoacidosis: How to treat and, when possible, prevent. Postgrad Med 77: 151–157, 161, 1985 [DOI] [PubMed] [Google Scholar]
- 76. Malnic G, De Mello Aires M, Giebisch G: Potassium transport across renal distal tubules during acid-base disturbances. Am J Physiol 221: 1192–1208, 1971 [DOI] [PubMed] [Google Scholar]
- 77. Brackett NC, Jr, Wingo CF, Muren O, Solano JT: Acid-base response to chronic hypercapnia in man. N Engl J Med 280: 124–130, 1969 [DOI] [PubMed] [Google Scholar]
- 78. Gennari FJ, Goldstein MB, Schwartz WB: The nature of the renal adaptation to chronic hypocapnia. J Clin Invest 51: 1722–1730, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Malnic G, De Mello Aires M, Giebisch G: Micropuncture study of renal tubular hydrogen ion transport in the rat. Am. J. Physiol. 222: 147–158, 1972 [DOI] [PubMed] [Google Scholar]
- 80. Kassirer JP, Schwartz WB: The response of normal man to selective depletion of hydrochloric acid: Factors in the genesis of persistent gastric alkalosis. Am J Med 40: 10–18, 1966 [DOI] [PubMed] [Google Scholar]
- 81. Tresguerres M, Buck J, Levin LR: Physiological carbon dioxide, bicarbonate, and pH sensing. Pflugers Arch 460: 953–964, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]