Abstract
The Gulf Coast tick, Amblyomma maculatum, is a vector of Rickettsia parkeri, a recently identified human pathogen that causes a disease with clinical symptoms that resemble a mild form of Rocky Mountain spotted fever. Because the prevalence of R. parkeri infection in geographically distinct populations of A. maculatum is not fully understood, A. maculatum specimens collected as part of a tick and pathogen surveillance system in Fairfax County, Virginia, were screened to determine pathogen infection rates. Overall, R. parkeri was found in 41.4% of the A. maculatum that were screened. Additionally, the novel spotted fever group Rickettsia sp., tentatively named “Candidatus Rickettsia andeanae,” was observed for the first time in Virginia.
Key Words: Amblyomma maculatum, Rickettsia andeanae, Rickettsia parkeri, Virginia
Introduction
The Gulf Coast tick, Amblyomma maculatum Koch, is an ixodid tick that has been recognized for its increasing veterinary and medical importance. In the United States the historic range of A. maculatum was reported to be within 160 km (100 miles) of the Gulf Coast and coastal southern Atlantic States from South Carolina to Texas (Bishopp and Trembley 1945). However, inland populations of the tick have been recognized in Oklahoma and Kansas since the 1970s (Semtner and Hair 1973, Teel et al. 2010), and more recently in Arkansas (Trout et al. 2010). Additionally, incidental collections of A. maculatum throughout the later half of the 20th century, thought to have been due to migratory bird drop-offs (Scott et al. 2001), have been reported from Iowa to Maine (Teel et al. 2010), including Virginia (Sonenshine et al. 1965), where historically four to five specimens are collected annually in Fairfax County (unpublished data), but it has only been within the last decade that the tick has been recognized as a vector of public health importance.
Rickettsia parkeri, a spotted fever group (SFG) Rickettsia, has been associated with A. maculatum since 1937 when it was isolated from Gulf Coast ticks in Texas (Parker et al. 1939). Although the bacterium was pathogenic for guinea pigs (Parker et al. 1939), it was thought to be nonpathogenic for humans until the first confirmed case of human infection was described in 2002 (Paddock et al. 2004). R. parkeri causes a disease similar to, but milder than, Rocky Mountain spotted fever (RMSF) (Paddock et al. 2008), which is caused by R. rickettsii infection. Since the initial human case, more than 20 additional instances of R. parkeri rickettsiosis have been reported (Paddock et al. 2010), including two published case reports from southeast Virginia (Paddock et al. 2004, Whitman et al. 2007).
The occurrence and frequency of R. parkeri infection in Gulf Coast ticks is still not fully understood. R. parkeri has been found infecting A. maculatum throughout much of its range. Positive specimens have been detected from at least nine states: Alabama, Arkansas, Florida, Georgia, Kentucky, Mississippi, Oklahoma, South Carolina, and Texas (Parker et al. 1939, Philip and White 1955, Philip et al. 1978, Sumner et al. 2007, Edwards et al. 2011, Paddock et al. 2010, Trout et al. 2010). Here we report high rates of R. parkeri infection in Gulf Coast ticks collected from Fairfax County, Virginia.
Materials and Methods
Tick collection
Gulf Coast ticks were collected alongside the American dog tick, Dermacentor variabilis (Say), the black-legged deer tick, Ixodes scapularis Say, and the lone star tick, Amblyomma americanum (Linnaeus), from various locations in Fairfax County, Virginia, from June 2008 to September 2010. Questing ticks were collected using either a drag cloth or flagging (June–August), or carbon dioxide traps (January–December). Additional specimens were obtained from an injured deer rescued from the I-66 landfill site that was brought into the Fairfax County animal shelter, 27 deer culled from the I-66 landfill site, and from a deer from Mason Neck obtained during a controlled deer hunt. Deer hunts were performed from August to January. Ticks were morphologically identified to species, sex, and life stage and then kept frozen at −20°C until processing for DNA extraction.
Sample processing
DNA was extracted from individual ticks using a MasterPure DNA Purification Kit (EPICENTRE Biotechnologies, Madison, WI), with a modified extraction procedure. Briefly, a tick was placed into a round-bottom tube with a 5-mm stainless steel bead and 50 μL of Tissue and Cell Lysis Solution. Samples were disrupted using a TissueLyser II (Qiagen, Valencia, CA) at 30 Hz for 3 min and then centrifuged for 1 min at 16,100 g at room temperature in a microcentrifuge. To each sample 250 μL of Tissue and Cell Lysis Solution, containing 1 μL of Proteinase K (50g/μL), were added, and beads were removed with a magnetic tool. Samples were thoroughly mixed and incubated at 65°C for 60 min. After placing the samples on ice for 5 min, 150 μL of MPC Protein Precipitation Reagent was added to each lysed sample, which was then vigorously vortexed. Debris was pelleted by centrifugation at 4°C for 10 min at 17,000 g in a microcentrifuge, and supernatants were transferred to clean microcentrifuge tubes. Subsequently, 500 μL of ice-cold isopropanol were added to each sample, and DNA was pelleted by centrifugation at 4°C for 10 min at 17,000 g. DNA pellets were washed twice with 500 μL of 75% ice-cold ethanol and after air-drying were resuspended in 30 μL of molecular grade water.
Mitochondrial 12S and 16S primers were used to confirm extraction quality and tick species by PCR and DNA sequencing (Norris et al. 1999). Further, amplicons obtained using the 16S+2 and 16S-1 primers were evaluated by restriction fragment length polymorphism (RFLP) analysis as an additional means of ixodid species confirmation. For each 25 μL reaction, 15 μL of PCR product was incubated with 1 μL of FastDigest® AseI and 2.5 μL of 10×FastDigest® Green Buffer (Fermentas, Glen Burnie, MD) for 20 min at 37°C. Digested products were separated on a 2.5% agarose gel, stained with ethidium bromide.
Molecular detection and analysis of SFG rickettsiae
DNA extractions were screened for SFG Rickettsia using a nested PCR (Blair et al. 2004) that amplifies a segment of the rickettsial outer membrane protein A (ompA) gene. Amplicons from ompA-positive specimens were evaluated by RFLP analysis using the endonuclease PstI (Roux et al. 1996). For each 25 μL reaction, 20 μL of PCR product was incubated with 1 μL PstI, 2.5 μL of 10×NE buffer 3, and 0.3 μL of 100×bovine serum albumin (New England BioLabs, Ipswich, MA). Characterization of the genetic variability of R. parkeri was performed by sequencing the ompA amplicon obtained using the Rr190.70p and Rr190.602n primers (Regnery et al. 1991). The unknown SFG Rickettsia was identified by sequence comparison of its ompA, citrate synthase (gltA) (Blair et al. 2004), and the rickettsial outer membrane protein B (ompB) (Roux and Raoult 2000) genes with sequences in GenBank (National Center for Biotechnology Information, Bethesda, MD).
Cell culture isolation
A subset of 18 Gulf Coast ticks, collected from the I-66 landfill, was evaluated to obtain isolates of R. parkeri. In brief, live ticks were washed in sequential disinfectant solutions as described (Paddock et al. 2010) and bisected longitudinally. One half of each tick was placed in an individual sterile tube and frozen at −80°C. DNA was extracted from each corresponding half of the frozen tick specimens and tested by PCR for molecular evidence of infection with R. parkeri, as described previously. The remaining halves of each specimen that tested positive by PCR were thawed and triturated with a sterile scalpel blade in 0.5 mL of Minimal Essential Media and inoculated onto a semiconfluent monolayer of Vero E6 cells (Paddock et al. 2010). Cell cultures were monitored for evidence of infection by using 0.01% acridine orange stain on cytospin preparations of cell culture supernatant. The identity of each isolate was confirmed by ompA PCR and RFLP analysis of the amplicon.
Results
A total of 507 A. maculatum were screened by nested PCR for the presence of SFG Rickettsia. Of the 217 Gulf Coast ticks that produced ompA amplicons, 210 specimens, 41.4% of A. maculatum collected, were determined to be infected with R. parkeri by digestion with PstI. R. parkeri-positive ticks were found from all six locations in Fairfax County where A. maculatum were collected and during each of the three collection years (Table 1). There was no significant difference between the proportion of males and females that were infected (p=0.86). Two stable isolates of R. parkeri (strains Fairfax and I-66) were obtained in Vero E6 cells from a male and a female A. maculatum tick collected at the I-66 landfill. Rickettsiae were visible by acridine orange stain in cell culture supernatants within 5 days after inoculation of tick triturates.
Table 1.
Distribution of Rickettsia parkeri–Positive Amblyomma maculatum from Fairfax County, Virginia
| |
|
Total number of ticks tested (number positive for Rickettsia parkeri) |
|
||
|---|---|---|---|---|---|
| Site | Year | Male | Female | Nymph | Overall infection rate (%) |
| Huntley meadows | 2008 | 1 (1) | 0 | 0 | 100 |
| 2010 | 1 (1) | 0 | 0 | 100 | |
| I-66 Landfill | 2010 | 337 (139) | 156 (63) | 3 (0) | 40.7 |
| Lorton | 2008 | 2 (1) | 2 (1) | 0 | 50 |
| 2010 | 1 (1) | 2 (1) | 0 | 66.6 | |
| Mason neck | 2009 | 0 | 1 (1) | 0 | 100 |
| Northumberland Road | 2009 | 1 (1) | 0 | 0 | 100 |
| Total | 343 (144) | 161 (66) | 3 (0) | 41.4 | |
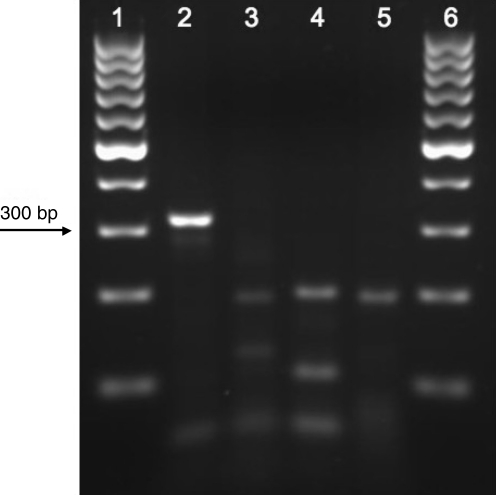
The largest number of R. parkeri–positive A. maculatum came from the I-66 landfill collection site (Table 1). When D. variabilis collected from the landfill were screened for SFG Rickettsia, none were infected with R. rickettsii, but 2 of 783 specimens (0.26%) were positive for R. parkeri by PstI digest and sequencing. The morphological identifications of the R. parkeri–positive D. variabilis were confirmed by AseI digestion of PCR amplicons corresponding to a segment of their 16S mitochondrial DNA (Table 2 and Fig. 1), as well as by direct sequencing.
Table 2.
Predicted Fragment Lengths of Ixodid 16S Mitochondrial DNA When Digested with AseI
| Species | Undigested length (bp) | Fragment sizes (bp) |
|---|---|---|
| Amblyomma maculatum | 321 | 192 and 129 |
| Amblyomma americanum | 323 | 323 [no restriction sites] |
| Dermacentor variabilis | 318 | 197, 67, 36, 18 |
| Ixodes scapularis | 320 | 195, 103, 22 |
FIG. 1.
Ethidium bromide-stained 2.5% agarose gel of AseI restriction enzyme digests of 16S mitochondrial rDNA amplicons. Lane 1, 100 bp DNA ladder; lane 2, Amblyomma americanum; lane 3, Amblyomma maculatum; lane 4, Ixodes scapularis; lane 5, Dermacentor variabilis; lane 6, 100 bp DNA ladder.
The genetic variability of selected R. parkeri samples was assessed by sequencing 507 bp of the ompA gene. Complete identity was observed between R. parkeri sequences from A. maculatum collected at each site listed in Table 1 and with partial sequences of R. parkeri from North and South America (GenBank accession nos. U43802, FJ986616, and EF102238). Further, the R. parkeri sequences obtained from D. variabilis were 100% identical to the sequences obtained from A. maculatum.
Of the 282 A. maculatum collected off deer from the I-66 landfill site, 4 ticks (1.42%) were found to be positive for Rickettsia amblyommii and 3 ticks (1.06%), 2 male and 1 female, produced ompA amplicons that when digested with PstI were of unknown origin. Partial ompA sequences (573 bp) were identical for the three samples and completely matched other partial ompA sequences from published (GenBank accession nos. EF372578, EF451004, and EU826513) (Pacheco et al. 2007, Sumner et al. 2007, Tomassone et al. 2010) and unpublished (GenBank accession nos. EF524203 and EF689729) sources, corresponding to what has been identified as a novel SFG Rickettsia species (Paddock et al. 2010), also designated “Candidatus Rickettsia andeanae” (Blair et al. 2004, Jiang et al. 2005) and Rickettsia sp. Argentina (Pacheco et al. 2007). Evaluation of partial ompB (785 bp) and gltA sequences (356 bp) from the unknown Rickettsia specimens revealed complete identity with sequences of “Candidatus Rickettsia andeanae” (GenBank accession nos. AY652981 and GU169050) (Blair et al. 2004, Jiang et al. 2005) and Rickettsia sp. Argentina (GenBank accession no. EF451001) (Pacheco et al. 2007).
Discussion
As seen in other geographically distinct collections, high rates of R. parkeri infection were observed in A. maculatum ticks collected from Fairfax County, Virginia. In particular, a 40.7% infection rate was seen in the largest A. maculatum collection from the I-66 landfill site. This prevalence is relatively high compared to previously published reports. R. parkeri has been found in approximately 10%–40% of questing adult A. maculatum from four locations in Florida and Mississippi (Paddock et al. 2010) and in 11%–12% of A. maculatum from three collections in Mississippi and Florida (Sumner et al. 2007). In contrast, R. rickettsii is usually found in less than 1% of ticks tested (Paddock 2009).
Accompanying the high infection rates of A. maculatum, two coexisting D. variabilis specimens were positive for R. parkeri. To our knowledge, infection of D. variabilis ticks with R. parkeri has been described only once, in 4 (2.3%) of 176 adult American dog ticks collected in Texas (Williamson et al. 2010). R. parkeri has also been detected in lone star ticks from Mississippi and Kentucky (Goddard and Norment 1986), as well as Tennessee and Georgia (Cohen et al. 2009). While A. americanum has experimentally transmitted R. parkeri transstadially and transovarially (Goddard 2003), the presence of R. parkeri in D. variabilis observed in this study might have been due to incidental infection during cofeeding since D. variabilis and A. maculatum were coexistent at the landfill collection site and are likely to have shared hosts. Pathogen transmission via tick cofeeding has been observed for a wide range of viruses (Randolph et al. 1996), but has also been demonstrated for bacteria, including Borrelia burgdorferi (Gern and Rais 1996, Patrican 1997) and, more recently, R. conorii (Zemtsova 2010).
In addition to R. parkeri, the SFG screen of A. maculatum detected the novel SFG Rickettsia species tentatively designated “Candidatus Rickettsia andeanae” (Blair et al. 2004, Jiang et al. 2005, Paddock et al. 2010). Of the 496 A. maculatum tested from the landfill site, 0.60% were positive for “Candidatus Rickettsia andeanae.” This novel SFG Rickettsia has been identified previously in the United States in A. maculatum collections from Florida, Georgia, and Mississippi (Sumner et al. 2007), with prevalence rates ranging from 2% to 5% (Paddock et al. 2010), but this is the first report of the bacterium in Gulf Coast ticks from Virginia. As has been noted previously, the pathogenic potential of this novel SFG rickettsial species as well as its potential interactions with other Rickettsia species, including R. parkeri, are unknown and will need to be studied further (Paddock et al. 2010).
Although there have been two published case reports of R. parkeri infection in humans from Virginia (Paddock et al. 2004, Whitman et al. 2007), this is the first description of a population of A. maculatum infected with high rates of R. parkeri from the state. Further, while the reported human cases of R. parkeri rickettsiosis were from southeastern Virginia, the ticks collected for this study were from Fairfax County in northern Virginia, bordering Maryland and the District of Columbia. Serological cross-reactivity exists among SFG Rickettsia species (Paddock et al. 2008), especially between R. rickettsii and R. parkeri (Raoult and Paddock 2005), and care should be taken to perform proper diagnostic tests on patients from northern Virginia presenting with symptoms of RMSF. The situation is compounded by the observation that DNA of R. rickettsii was not found in any of 783 D. variabilis ticks collected in the same geographic area. Our findings are consistent with many other past and recent reports describing the relative infrequency of ticks infected with R. rickettsii in nature (Paddock et al. 2009, Stromdahl et al. 2011). Recognition of the potential for R. parkeri infection in Fairfax County will allow proper RMSF and R. parkeri infection incidence rates to be reported and will help further the understanding of emerging rickettsioses in the United States
Disclaimer
The findings and conclusions presented herein are those of the authors and do not necessarily reflect the views and opinions of the U.S. Department of Health and Human Services.
Acknowledgments
We would like to thank Sonya Graves, Chris Eliff, Sara Bennett, Bryce De Sostoa, Ada García, Aubrey Gamboa, Dan Good, John Orr, Matt Prosser, and Rachel Severson for their help in the field collecting ticks and in the lab sorting ticks. Research funding and support, in part, was provided to DEN from the County of Fairfax, Virginia (Contract RQ10-151007-31A) and NIH (1R21AI67386-1A2 and 1R03AI079297-01).
Disclosure Statement
No competing financial interests exist.
References
- Bishopp FC. Trembley HL. Distribution and hosts of certain North American ticks. J Parasitol. 1945;31:1–54. [Google Scholar]
- Blair PJ. Jiang J. Schoeler GB. Moron C, et al. Characterization of spotted fever group rickettsiae in flea and tick specimens from northern Peru. J Clin Microbiol. 2004;42:4961–4967. doi: 10.1128/JCM.42.11.4961-4967.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SB. Yabsley MJ. Garrison LE. Freye JD, et al. Rickettsia parkeri in Amblyomma americanum ticks, Tennessee and Georgia, USA. Emerg Infect Dis. 2009;15:1471–1473. doi: 10.3201/eid1509.090330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KT. Goddard J. Jones TL. Paddock CD, et al. Cattle and the natural history of Rickettsia parkeri in Mississippi. Vector Borne Zoonotic Dis. 2011;11:485–491. doi: 10.1089/vbz.2010.0056. [DOI] [PubMed] [Google Scholar]
- Gern L. Rais O. Efficient transmission of Borrelia burgdorferi between cofeeding Ixodes ricinus ticks (Acari: Ixodidae) J Med Entomol. 1996;33:189–192. doi: 10.1093/jmedent/33.1.189. [DOI] [PubMed] [Google Scholar]
- Goddard J. Experimental infection of lone star ticks, Amblyomma americanum (L.), with Rickettsia parkeri and exposure of guinea pigs to the agent. J Med Entomol. 2003;40:686–689. doi: 10.1603/0022-2585-40.5.686. [DOI] [PubMed] [Google Scholar]
- Goddard J. Norment BR. Spotted fever group rickettsiae in the lone star tick, Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 1986;23:465–472. doi: 10.1093/jmedent/23.5.465. [DOI] [PubMed] [Google Scholar]
- Jiang J. Blair PJ. Felices V. Moron C, et al. Phylogenetic analysis of a novel molecular isolate of spotted fever group Rickettsiae from northern Peru: Candidatus Rickettsia andeanae. Ann N Y Acad Sci. 2005;1063:337–342. doi: 10.1196/annals.1355.054. [DOI] [PubMed] [Google Scholar]
- Norris DE. Klompen JSH. Black WC. Comparison of the mitochondrial 12S and 16S ribosomal DNA genes in resolving phylogenetic relationships among hard ticks (Acari: Ixodidae) Ann Entomol Soc Am. 1999;92:117–129. [Google Scholar]
- Pacheco RC. Moraes-Filho J. Nava S. Brandao PE, et al. Detection of a novel spotted fever group rickettsia in Amblyomma parvum ticks (Acari: Ixodidae) from Argentina. Exp Appl Acarol. 2007;43:63–71. doi: 10.1007/s10493-007-9099-5. [DOI] [PubMed] [Google Scholar]
- Paddock CD. The science and fiction of emerging rickettsioses. Ann N Y Acad Sci. 2009;1166:133–143. doi: 10.1111/j.1749-6632.2009.04529.x. [DOI] [PubMed] [Google Scholar]
- Paddock CD. Finley RW. Wright CS. Robinson HN, et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis. 2008;47:1188–1196. doi: 10.1086/592254. [DOI] [PubMed] [Google Scholar]
- Paddock CD. Fournier PE. Sumner JW. Goddard J, et al. Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. Appl Environ Microbiol. 2010;76:2689–2696. doi: 10.1128/AEM.02737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD. Sumner JW. Comer JA. Zaki SR, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- Parker RR. Kohls GM. Cox GW. Davis GE. Observations on an infectious agent from Amblyomma maculatum. Public Health Rep. 1939;54:1482–1484. [Google Scholar]
- Patrican LA. Acquisition of Lyme disease spirochetes by cofeeding Ixodes scapularis ticks. Am J Trop Med Hyg. 1997;57:589–593. doi: 10.4269/ajtmh.1997.57.589. [DOI] [PubMed] [Google Scholar]
- Philip CB. White JS. Disease agents recovered incidental to a tick survey of the Mississippi Gulf Coast. J Econ Entomol. 1955;48:396–400. [Google Scholar]
- Philip RN. Casper EA. Burgdorfer W. Gerloff RK, et al. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978;121:1961–1968. [PubMed] [Google Scholar]
- Randolph SE. Gern L. Nuttall PA. Co-feeding ticks: epidemiological significance for tick-borne pathogen transmission. Parasitol Today. 1996;12:472–479. doi: 10.1016/s0169-4758(96)10072-7. [DOI] [PubMed] [Google Scholar]
- Raoult D. Paddock CD. Rickettsia parkeri infection and other spotted fevers in the United States. N Engl J Med. 2005;353:626–627. doi: 10.1056/NEJM200508113530617. [DOI] [PubMed] [Google Scholar]
- Regnery RL. Spruill CL. Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux V. Fournier PE. Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol. 1996;34:2058–2065. doi: 10.1128/jcm.34.9.2058-2065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux V. Raoult D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB) Int J Syst Evol Microbiol. 2000;50:1449–1455. doi: 10.1099/00207713-50-4-1449. [DOI] [PubMed] [Google Scholar]
- Scott JD. Fernando K. Banerjee SN. Durden LA, et al. Birds disperse ixodid (Acari: Ixodidae) and Borrelia burgdorferi-infected ticks in Canada. J Med Entomol. 2001;38:493–500. doi: 10.1603/0022-2585-38.4.493. [DOI] [PubMed] [Google Scholar]
- Semtner PJ. Hair JA. Distribution, seasonal abundance, and hosts of the Gulf Coast tick in Oklahoma. Ann Entomol Soc Am. 1973;66:1264–1268. [Google Scholar]
- Sonenshine DE. Lamb JT., Jr Anastos G. The distribution, hosts and seasonal activity of Virginia ticks. Va J Sci. 1965;16:26–91. [Google Scholar]
- Stromdahl EY. Jiang J. Vince M. Richards AL. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group rickettsiae in the United States. Vector Borne Zoonotic Dis. 2011;11:969–977. doi: 10.1089/vbz.2010.0099. [DOI] [PubMed] [Google Scholar]
- Sumner JW. Durden LA. Goddard J. Stromdahl EY, et al. Gulf coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis. 2007;13:751–753. doi: 10.3201/eid1305.061468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teel PD. Ketchum HR. Mock DE. Wright RE, et al. The Gulf coast tick: a review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J Med Entomol. 2010;47:707–722. doi: 10.1603/me10029. [DOI] [PubMed] [Google Scholar]
- Tomassone L. Nuñez P. Ceballos LA. Gürtler RE, et al. Detection of “Candidatus Rickettsia sp. strain Argentina” and Rickettsia bellii in Amblyomma ticks (Acari: Ixodidae) from Northern Argentina. Exp Appl Acarol. 2010;52:93–100. doi: 10.1007/s10493-010-9339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout R. Steelman CD. Szalanski AL. Williamson PC. Rickettsiae in Gulf Coast Ticks, Arkansas, USA. Emerg Infect Dis. 2010;16:830–832. doi: 10.3201/eid1605.091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman TJ. Richards AL. Paddock CD. Tamminga CL, et al. Rickettsia parkeri infection after tick bite, Virginia. Emerg Infect Dis. 2007;13:334–336. doi: 10.3201/eid1302.061295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson PC. Billingsley PM. Teltow GJ. Seals JP, et al. Borrelia, Ehrlichia, and Rickettsia spp. in Ticks Removed from Persons, Texas, USA. Emerg Infect Dis. 2010;16:441–446. doi: 10.3201/eid1603.091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemtsova G. Killmaster LF. Mumcuoglu KY. Levin ML. Co-feeding as a route for transmission of Rickettsia conorii israelensis between Rhipicephalus sanguineus ticks. Exp Appl Acarol. 2010;52:383–392. doi: 10.1007/s10493-010-9375-7. [DOI] [PubMed] [Google Scholar]