Abstract
The transmission dynamics of Anaplasma phagocytophilum (Ap) and Borrelia burgdorferi (Bb) among Ixodes scapularis (Is) and mammalian hosts was investigated at Camp Ripley, an area representative of central Minnesota. Prevalence of white-footed mouse infection with Ap and Bb were 20% and 42%, respectively, with a coinfection level of 14%. Peak levels of infection with both agents occurred in May. The average levels of seropositivity to Ap and Bb were 29.3% and 48%, respectively. Of the mice infected with Ap, 47.5% were able to eliminate the pathogen as compared with 19.4% of mice infected with Bb. Ap was detected in 88.4% of 43 eastern chipmunks examined and isolated from 44.7% of the animals. Bb was present in 72.7% of 11 chipmunks examined, and 100% of the animals were also infected with Ap. The seasonality of tick activity differs from that reported for the New York area. Is infestation of mice began in May with peak nymphal infestation also occurring in May (7.4 per infested mouse) and overlapping with peak larval infestation in June (77.1 per infested mouse). Infestation ranged from 100% in May to 34.5% in October. Is comprised 98.4% of the ticks infesting the mice. The temporal pattern of the developmental stages of Is infesting chipmunks was the same as for mice, except that the tick burdens were greater. The nymphal stage peaked in May (81.3 per animal), and the larval stage peaked in June (164.7 per animal). Infestation was 100% in May–August, and >99% of the ticks were Is. Antibodies to Ap were present in >80% of the white-tailed deer examined, but they were infected with the Ap-1 variant rather than the Ap strain infecting mice and humans. Antibodies to Bb were detected in >80% of the deer, but Bb DNA was only detected in 1.5% of blood specimens.
Key Words: Anaplasma phagocytophilum, Borrelia burgdorferi, Ixodes scapularis, Transmission
Introduction
Human anaplasmosis (HA) and Lyme disease (LD) are emerging, vector-borne diseases present in the temperate regions of the northern hemisphere. In the United States, most cases of HA and LD are reported from the Northeastern and Northcentral states (United States Department of Health and Human Services 2000, Dumler et al. 2005, Bakken and Dumler 2008). Anaplasma phagocytophilum (Ap) and Borrelia burgdorferi (Bb) are the etiological agents of HA and LD, respectively. Both agents are transmitted through the bite of infected Ixodes scapularis (Is) in these regions. A habitat of woodlands with a leaf litter of high moisture content and the presence of mammalian hosts such as the white-footed mouse (Peromyscus leucopus) and the white-tailed deer (Odocoileus virginianus) provides the environment for the establishment of Is and the transmission cycle of Ap (Telford et al. 1996) and Bb (Piesman 2002).
Effective control programs for HA and LD are predicated on the transmission dynamics of the pathogens among the tick vector and mammalian hosts in their natural habitat (Stafford 2004). The seasonality of Is in the Northcentral states varies from that of the Northeastern states (Gatewood et al. 2009). The purpose of this study was to study the natural transmission cycle of Ap and Bb and the presence of dual infections by these agents in the different climate and habitats of central Minnesota.
Materials and Methods
Site selection
The region of Minnesota studied was Camp Ripley located in the central portion of Minnesota, approximately 160 km northwest of Minneapolis/Saint Paul (Figure 1). The study site was located in the southcentral area of the camp and consisted of mixed hardwood forest with an estimated white-tailed deer population of 24–31per sq km. For additional information on wildlife and habitat of Camp Ripley, please see website (http://www.minnesotanationalguard.org/assets/ConservationProgramReport2008FINAL.pdf).
FIG. 1.
Location of Camp Ripley study site.
Small mammal and tick collection
Small mammals were collected by Metropolitan Mosquito Control District and Camp Ripley personnel. Sherman Traps (H.B. Sherman Traps, Inc., Tallahassee, FL), baited with peanut butter and oats, were placed at 10–15 m intervals in mixed habitat and collected 24 h later. The animals were euthanized by carbon dioxide inhalation, identified, and ticks were removed. Tick burdens and developmental stage based on morphology data are for infested animals only.
White-tailed deer and black bear specimens
Deer-blood-impregnated filter paper specimens were provided by deer hunters at Camp Ripley (October 1999–2004). The Minnesota Department of Natural Resources Wildlife Management personnel provided whole blood from live-captured deer and hibernating black bear from Camp Ripley and northeastern Minnesota (December–February, 2000–2006).
Culture and polymerase chain reaction (PCR) assay
DNA was obtained from specimens using the IsoQuick Nucleic Acid Extraction Kit (# MXT-020-100, ORCA Research Inc., Bothell, WA). Blood specimens were examined for Ap by culture (Ravyn et al. 2001) and/or PCR for the presence of the P44 gene of Ap using the primers 5′ AGCGTAATGATGTCT ATGGC-3′ and 5′ ACCCTAA CACCACCAAATTCCC-3′ (Carter et al. 2001). Deer whole-blood specimens that were PCR positive for Ap were subjected to nested PCR to amplify a 260 bp from the 16S rRNA region to identify the AP-variant 1 described by Massung et al. (2005). DNA sequencing of the PCR product indicated that the Ap present in deer was the AP-I variant.
Mammal specimens were also examined for the presence of Bb by culture and/or PCR. Blood was collected by cardiac puncture, and urinary bladder tissue was dissected and triturated in Barbour-Stoenner-Kelly (BSK) medium (Barbour 1984). One to two drops of blood or a 1:10 dilution of homogenized tissue were inoculated into 7 ml of BSK medium in a 10 mL tube and examined by darkfield microscopy after 3 and 6 weeks incubation at 32°C. Specimens were examined for the flagellin gene of Bb by nested PCR as described by Picken et al. (1996) using the following primers: FL3b, CGA GCT TCT GAT GAT GCT GGC ATG GGA G; FL5b, GGG GAA CTT GAT TAG CCT GCG CAA TCA TTG CC; FL6b TTC AGG GTC TCA AGC GTC TTG GAC T; and FL7b GCA TTT TCA ATT TTA GCA AGT GAT G.
Enzyme immunoassay (EIA) and immunoblot analysis
Serum samples were analyzed for Anti-Ap and anti-Bb antibodies by enzyme immunoassay and immunoblot as previously described (Engstrom et al. 1995, Ravyn et al. 1998). Goat anti-P. leucopus IgG heavy and light chains antibody and rabbit anti-deer IgG conjugated to horseradish peroxidase (Kirkegaard & Perry Laboratories, Gaithersburg, MD) were used for the detection of mouse and deer antibodies, respectively. Goat anti-P. leucopus IgG heavy and light chains antibody and rabbit anti-deer IgG conjugated to alkaline phosphatase (Kirkegaard & Perry Laboratories, Gaithersburg, MD) were used in the immunoblot assay for the detection of mouse and deer antibodies, respectively. All seropositive mouse sera are both EIA and immunoblot positive. Deer antibody assays were conducted on eluates from blood-impregnated filter paper as described by Kamal et al. (1994) or on whole-blood specimens.
Statistics
Excel (Microsoft Corporation) was used for statistical analysis.
Results
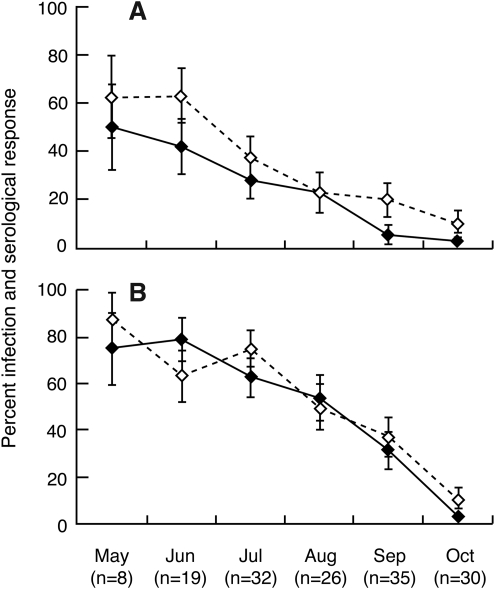
May through October of 2000, 98 white-footed mice (infested and noninfested) were examined for infection with Ap. The level of infection ranged from a high of 55.6% in May to a low of 28% in October with an average infection level of 46.8%. Forty-nine percent of the mice were seropositive, and 73% of seropositive mice were infected. In 2001, we expanded the anaplasmosis study of 150 white-footed mice to include infection with Bb and coinfection. Infections with Ap or Bb and coinfections were highest in May and gradually decreased to their lowest levels in October (Figure 2). The average levels of infection for six months were as follows: Ap, 20%; Bb, 42.7%; and coinfection, 14.7%. The monthly pattern of the antibody response and infection were similar (Figure 2). The percentage of animals with antibodies to Ap and Bb were 29.3% and 48%, respectively.
FIG. 2.
The percent infection (–♦–) and serological response (–◊–) of white-footed mice (infested and noninfested) to Anaplasma phagocytophilum (A) and Borrelia burgdoferi (B). Bars indicate±two standard deviations. The numbers of animals tested are below the graphs.
Since Bb has a greater propensity to cause chronic infections in mice than Ap, we compared the ability of mice to eliminate the agents. We considered animals that were PCR negative and antibody positive for the pathogens to have eliminated them. Of the mice infected with Ap, 47.7% were able to eliminate the pathogen from the blood. In contrast, only19.4% of the mice infected with Bb eliminated the pathogen.
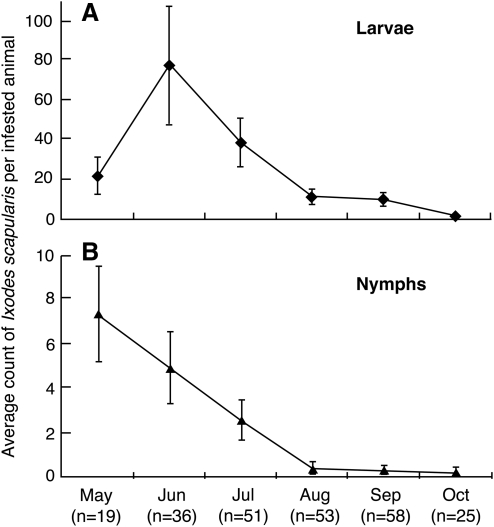
We investigated the seasonality of larval and nymphal Is activity. Is comprised 98.4% of the 6,598 ticks removed from white-footed mice captured in 2000 and 2001. The remaining ticks were the American dog tick, Dermacentor variabilis. Is was not present on mice captured in April. The percent of mice infested with Is ranged from 100% in May to 34.5% in October with an average infestation level of 77%. The greatest larval parasitism of mice occurred in June at 77.08 per animal and declined to 1.56 in October (Figure 3). The maximal nymphal activity of 7.37 per infested mouse occurred in May (Figure 3) and overlapped with peak larval activity (Figure 3).
FIG. 3.
Number of Ixodes scapularis larvae (A) and nymphs (B) per infested white-footed mouse. Bars indicate±two standard deviations. The numbers of animals tested are below the graphs.
The blood of 43 chipmunks (2000–2001) was examined for the presence of Ap. Ap DNA was detected in 38 chipmunks (88.4%) and was isolated from 17 (44.7%). The bladders of 11 chipmunks captured in 2001were analyzed for Bb DNA. Eight animals (72.7%) were infected, and these animals were also infected with Ap (100% coinfection).
The ticks removed from 52 chipmunks consisted of 4,917 Is and 7 D. variabilis. Infestation with Is was 100% in May through August with a decrease to 71.4% in September. Is was not present on the two chipmunks captured in April or on the single animal captured in October. The temporal pattern of the developmental stages of Is infesting chipmunks was the same as for mice (Figure 3). The larval infestation peaked at 164.7 per animal in June and declined to 1.2 per animal in September. The nymphal stage peaked in May at a level of 81.3 per animal and declined to 1.7 per animal in September. Although the temporal pattern of the developmental stages of the tick on chipmunks was the same as mice, the tick burden was greater. The larval burden of chipmunks in June was twice that of mice, and the nymphal infestation in May was more than ten times greater than mice.
In addition to white-footed mice and chipmunks, a number of other rodents were captured and examined for infection with Ap. The rodents captured and level of infection were as follows: southern red backed vole, 11 of 73 (15.1%); short tailed shrew, 5 of 29 (17.2%); meadow jumping mouse, 9 of 18 (50%); and meadow vole, 2 of 14 (14.3%).
Blood was obtained from 1117 white-tailed deer (October 1999–2004) and examined for antibodies and infection with Ap and Bb. Eighty-five per cent (947) of the specimens were EIA positive for antibodies to Ap. Of the 397 randomly selected EIA positive specimens assayed by immunoblot, 98.7% were positive. During the six-year study, the level of EIA positive deer sera ranged from 71% to 98.5%. One hundred and twenty-four (47%) of the deer were infected (PCR positive) with Ap. The Ap DNA was analyzed and was found to be the AP-1 variant that is not infectious for rodents and humans.
Blood specimens from deer were also examined for antibodies and infection with Bb. Eight hundred and ninety-five (80.1%) samples were EIA positive. Of the 216 randomly selected EIA positive sera, 211 (97.7%) were positive by immunoblot assay. During the six-year study, the level of deer sera EIA positive for Bb ranged from 59.3% to 92.6%. The white-tailed deer blood specimens were examined by PCR for infection with Bb. Of the 266 randomly selected blood samples examined, Bb DNA was only detected in 2.5% of the specimens.
We also examined blood specimens from 161 black bear by PCR for infection with Ap and Bb. All blood specimens were negative for Ap and Bb DNA.
Discussion
We found Camp Ripley to be strongly endemic for the tick-borne diseases HA and LD. The levels of Ap and Bb infections of white-footed mice were ∼twice greater than in the Minneapolis/Saint Paul area (Walls et al. 1997, unpublished data).
The full seasonal curve of Ap and Bb infections was similar, reflecting vector tick activity. None of the mice were infested with Is in April. Tick activity and transmission of these agents rapidly increased in May, manifesting maximal levels of infection and coinfection with both agents. This peak level of activity was followed by gradual decreasing levels of infection. We found that active infections of mice by Bb was 2.1 times more frequent than infections by Ap. Our average level of coinfection was 14.7% (0%–35%), which is only slightly less than infection by Ap (av. 20.0%, range 3.3%–50%). Previous reports using serology as a measure of infection found that infections of mice by Bb were ∼1.3–5× more frequent than infections by Ap and coinfections ranged from 7.1% to 64.4% (Nieto and Foley 2009a).
Once Bb is established in reservoir host tissues, antibodies do not effectively eliminate them (Johnson et al. 1986, Hodzic et al. 2003) and Bb can persist for a year or longer (Barthold et al. 1993). Ap can persist for 35 to 60 days in experimentally infected mice (Massung et al. 2004). We compared the immune response and the ability of the two pathogens to persist in mice infected in nature. The results suggest that in naturally infected mice, both Ap and Bb can persist for extended periods of time.
We found that the level of spirochetemia in mice was quite high with Bb present in blood of 27.7% of the mice. This level spirochetemia is considerably higher than the 11.3% and 9.5% reported for a hyperendemic site in Connecticut (Bunikis et al. 2004). A possible reason for this difference may be that analyses at Connecticut were conducted on stored serum specimens, whereas we analyzed whole-blood samples. The Connecticut site appears to be more endemic than Camp Ripley, as their Bb seropositivity of 57%–93% is higher than our seropositivity of 47.3%–50.0%.
Apparently, infection with B. burgdorferi does not affect the survival time of mice (Hofmeister et al. 1999). However, our studies at Camp Ripley suggest that the life span of mice is very short, as seropositivity of mice decreased from 70.2% in July to 17.5% in October. A similar pattern is seen in bladder infections. The increase in the mouse population in fall and/or a high level of predation could account for these results.
The vector for the transmission of the agents of HA and LD is Is and the white-footed mouse appears to be the primary reservoir host for both pathogens (Levine et al. 1985, Telford et al. 1988, Piesman 2002). Is larvae become infected by feeding on reservoir hosts previously infected by the Is nymphal stage. In the coastal areas of New York, the seasonal activity of larvae is preceded by nymphal activity (Gatewood et al. 2009). This inversion of larval and nymphal seasonal activity is believed to result in a very effective transmission of Ap and Bb among the reservoir rodent population and a corresponding increase in the number of infected ticks (Piesman 2002). The seasonality of Is activity at Camp Ripley differs from that reported for the New York area (Gatewood et al. 2009). We found that peak larval activity overlapped with peak nymphal activity. In spite of the overlapping of larval and nymphal activities, we found a very effective transmission of both agents to mice and chipmunks. Infection of larval ticks co-feeding with infected nymphal ticks may enhance the transmission of these two agents. This difference in seasonality of immature tick activity is strongly related to the climate of Minnesota. Gatewood et al. (2009) reported that the seasonal synchrony of the immature stages of the tick is related to the average maximum and minimum temperatures. This overlapping of larval and nymphal activity allows for efficient and costeffective means of reducing the risk of LD in this region. One application of acaricides (Stafford 2004) in early summer would control both nymphal and larval Is.
The second potentially major small mammal host for immature Ixodes ticks is the chipmunk (Main et al. 1882, Mather et al. 1989, McLean et al. 1993, Nieto and Foley 2009a). Chipmunks are readily infected with Bb and Ap and effectively transmit the agents to ticks (Mather et al. 1989, McLean et al. 1993, LoGiudice et al. 2003, Nieto and Foley 2009b). We found infections of the chipmunk with these two agents to be much greater than those in mice. The percent of chipmunks infected with Ap and Bb were 4.4× and 1.7× greater than in mice, respectively. The level of chipmunk coinfection was 4.9× greater than mouse coinfection. Also, in contrast to mice, the Ap level of infection of chipmunks was greater than that with Bb. The tick burden of the chipmunk and mice differed. Larval tick burdens are generally higher on mice than they are on chipmunks, and nymphal tick burdens are higher on chipmunks than they are on mice (Davidar et al. 1989, Schulze et al. 2005). Since the larval burden of infested chipmunks was 1–3× greater than that of mice, the overall tick burden of infested chipmunks was greater than that of infested mice. Since chipmunks have a high level of infection, they provide an abundant source of the pathogens. Although we found the mouse population to be 15× greater than that of the chipmunk, this may be an under estimate of the chipmunk population, because our traps are more effective for mice. The role of chipmunks in the transmission dynamics of these agents at this site has the potential of being substantial. At a Wisconsin site (unpublished data) where the chipmunk population is approximately one half of the mouse population, we found (based on tick burdens and number of Bb infected animals) that the chipmunk was the major source (42.4%) of the potentially infected ticks, followed by the mouse (31.5%). The chipmunk may serve a similar role in northwestern Illinois (Mannelli et al. 1993, Slajchert et al. 1997, Jones and Kitron 2000) and New Jersey (Schulze et al. 2005), where the chipmunk capture outnumbers mice. The importance of nonmouse hosts in the LD transmission cycle is unknown. LoGuidice et al. (2003) reported that the most common nonmouse hosts are relatively poor reservoirs for Bb. However, Tsao et al. (2004) found that nonmouse hosts for Bb contributed more to infecting Is than was expected.
Adult Is parasitizes larger mammals, and its preferred host is the white-tailed deer (Wilson et al. 1985). Accordingly, deer are exposed to ticks infected with AP and Bb. We found that although deer were seropositive and PCR positive for Ap, genetic analysis of the Ap DNA revealed that they were infected with a variant strain (Ap-V1) of Ap (Massung et al. 2005). The natural history of this variant is unknown. It is present in Is (Courtney et al. 2003, Massung et al. 2007), but it is not infectious for rodents and humans (Massung et al. 2003). Thus, we have two biologically distinct strains of Ap circulating in the Is population at Camp Ripley. Although deer are incompetent reservoirs of the LD spirochete (Telford et al. 1988), they do generate an antibody response to the spirochete. Seropositivity levels of deer sera from Camp Ripley were greater than 80%, but fewer than 3% had B. burgdorferi DNA in their blood. However, since Camp Ripley has a very large population of deer and they have high burdens of all three developmental stages of Is, they may play a minor role in the transmission of Bb. In addition, the level of Bb infection may be at a higher level, as our blood specimens are obtained in late fall when Is activity is minimal.
Black bears are another potential maintenance host for the adult stage of Is and a possible reservoir host for Ap and Bb. Although our blood specimens from black bears are not obtained at the time of peak tick activity, the results suggest that the black bear is not a reservoir host for Ap or Bb.
Acknowledgments
We appreciate the assistance of the Metropolitan Mosquito Control District for small mammal trapping, tick collection and identification. We thank Jay Brezinka, the staff of the Training Site-Environmental Office of Camp Ripley, and the MN DNR Wildlife Management personnel for their assistance in the collection of animal specimens. We are grateful for the expertise and advice of Durland Fish (Yale School of Medicine), Joe Piesman (CDC, Fort Collins), and Tom Schwan (NIH, Rocky Mountain Laboratories).
This research was supported in part by Public Health Service grant R01 AR 34744 (to R.C.J.).
Disclosure Statement
No competing financial interests exist.
References
- Bakken JS. Dumler S. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2008;22:433–448. doi: 10.1016/j.idc.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- Barthold SW. de Souza MS. Jantka JL. Smith AL, et al. Animal model. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:951–971. [PMC free article] [PubMed] [Google Scholar]
- Bunikis J. Tsao J. Luke CJ. Luna MG, et al. Borrelia burgdorferi infection in a natural population of Peromyscus leucopus mice: a longitudinal study in an area where Lyme disease is highly endemic. J Infect Dis. 2004;189:1515–1523. doi: 10.1086/382594. [DOI] [PubMed] [Google Scholar]
- Carter SE. Ravin MD. Xu Y. Johnson RC. Molecular typing of the etiological agent of human granulocytic ehrlichiosis. J Clin Microbiol. 2001;39:3398–3401. doi: 10.1128/JCM.39.9.3398-3401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney JW. Dryden RL. Montgomery J. Schneider BS, et al. Molecular characterization of Anaplasma phagocytophilum and Borrelia burgdorferi in Ixodes scapularis ticks from Pennsylvania. J Clin Microbiol. 2003;41:1569–1573. doi: 10.1128/JCM.41.4.1569-1573.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidar P. Wilson M. Ribeiro JM. Differential distribution of immature Ixodes dammini (Acari:Ixodidae) on rodent hosts. J Parasitol. 1989;75:898–904. [PubMed] [Google Scholar]
- Dumler JS. Choi KS. Warner CK. Cookson K, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11:1828–1834. doi: 10.3201/eid1112.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom SM. Shoop E. Johnson RC. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood AG. Lieban KA. Your'h G. Bunikis J, et al. Climate and tick seasonality core predictors of Borrelia burgdorferi genotype distributions. Appl Environ Microbiol. 2009;75:2476–2483. doi: 10.1128/AEM.02633-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic E. Feng S. Freet KJ. Barthold SW. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect Immun. 2003;71:5042–5055. doi: 10.1128/IAI.71.9.5042-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeister EK. Ellis BA. Glass GE. Childs JE. Longitudinal study of infection with Borrelia burgdorferi in a population of Peromyscus leucopus at a Lyme disease-enzootic site in Maryland. Am J Trop Med Hyg. 1999;60:598–609. doi: 10.4269/ajtmh.1999.60.598. [DOI] [PubMed] [Google Scholar]
- Johnson RC. Kodner C. Russell M. Passive immunization of hamsters against experimental infection with the Lyme disease spirochete. Infect Immun. 1986;53:713–714. doi: 10.1128/iai.53.3.713-714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CJ. Kitron UD. Populations of Ixodes scapularis (Acari: Ixodidae) are modulated by drought at a Lyme disease focus in Illinois. J Med Entomol. 2000;37:408–415. doi: 10.1603/0022-2585(2000)037[0408:POISAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kamal KA. Shaheen HI. El-Said AA. Applicability of ELISA on buffer-eluates of capillary blood spotted on filter papers for the diagnosis and clinical staging of human schistosomiasis. Trop Geograph Med. 1994;46:138–141. [PubMed] [Google Scholar]
- Levine JF. Wilson ML. Spielman A. Mice as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg. 1985;34:355–360. doi: 10.4269/ajtmh.1985.34.355. [DOI] [PubMed] [Google Scholar]
- LoGiudice K. Ostfeld RS. Schmidt KA. Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100:567–671. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main AJ. Carey AB. Carey MG. Goodwin RH. Immature Ixodes dammini (Acari: Ixodidae) on small animals in Connecticut, USA. J Med Entomol. 1982;19:655–659. doi: 10.1093/jmedent/19.6.655. [DOI] [PubMed] [Google Scholar]
- Mannelli A. Kitron U. Jones CJ. Slajchert TL. Role of the eastern chipmunk as a host for immature Ixodes dammini (Acari: Ixodidae) in northwestern Illinois. J Med Entomol. 1993;30:87–93. doi: 10.1093/jmedent/30.1.87. [DOI] [PubMed] [Google Scholar]
- Massung RF. Courtney JW. Hiratzka SL. Pitzer VE, et al. Anaplasma phagocytophilum in white-tailed deer. Emerg Infect Dis. 2005;11:1604–1606. doi: 10.3201/eid1110.041329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massung RF. Levin ML. Munderloh UG. Silverman DJ, et al. Isolation and propagation of the AP-Variant 1 strain of Anaplasma phagocytophilum in a tick cell line. J Clin Microbiol. 2007;45:2138–2143. doi: 10.1128/JCM.00478-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massung RF. Priestley RA. Levin ML. Transmission route efficacy and kinetics of Anaplasma phagocytophilum infection in white-footed mouse, Peromyscus leucopus. Vector Borne Zoonotic Dis. 2004;4:310–318. doi: 10.1089/vbz.2004.4.310. [DOI] [PubMed] [Google Scholar]
- Massung RF. Priestley RA. Miller NJ. Mather TN, et al. Inability of a variant strain of Anaplasma phagocytophilum to infect mice. J Infect Dis. 2003;188:1757–1763. doi: 10.1086/379725. [DOI] [PubMed] [Google Scholar]
- Mather TN. Wilson ML. Moore SI. Ribeiro JM, et al. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi) Am J Epidemiol. 1989;130:143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- McLean RG. Ubico SR. Cooksey LM. Experimental infection of the eastern chipmunk (Tamias striatus) with the Lyme disease spirochete (Borrelia burgdorferi) J Wildl Dis. 1993;29:527–532. doi: 10.7589/0090-3558-29.4.527. [DOI] [PubMed] [Google Scholar]
- Nieto NC. Foley JE. Meta-analysis of coinfection and coexposure with Borrelia burgdorferi and Anaplasma phagocytophilum in humans, domestic animals, wildlife, and Ixodes ricinus–complex ticks. Vector Borne Zoonotic Dis. 2009a;9:93–1001. doi: 10.1089/vbz.2008.0072. [DOI] [PubMed] [Google Scholar]
- Nieto NC. Foley JE. Reservoir competence of the redwood chipmunk (Tamias ochrogenys) for anaplasma phagocytophilum. Vector Borne Zoonotic Dis. 2009b;9:573–577. doi: 10.1089/vbz.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picken MM. Picken RN. Han D. Cheng Y, et al. Single-tube nested polymerase chain reaction assay based on flagellin gene sequences for detection of Borrelia burgdorferi sensu lato. Eur J Clin Microbiol Infect Dis. 1996;15:489–498. doi: 10.1007/BF01691317. [DOI] [PubMed] [Google Scholar]
- Piesman J. Ecology of Borrelia burgdorferi sensu lato in North America. In: Gray JS, editor; Kahl O, editor; Lane RS, editor; Stanek G, editor. Lyme borreliosis: biology, epidemiology and control. CABI Publishing: New York; 2002. pp. 223–249. [Google Scholar]
- Ravyn MD. Goodman JL. Kodner CB. Westad DK, et al. Immunodiagnosis of human granulocytic ehrlichiosis using culture derived human isolates. J Clin Microbiol. 1998;36:1480–1488. doi: 10.1128/jcm.36.6.1480-1488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravyn MD. Kodner CB. Carter SE, et al. Isolation of the etiologic agent of human granulocytic ehrlichiosis from the white-footed mouse (Peromyscus leucopus) J Clin Microbiol. 2001;39:335–338. doi: 10.1128/JCM.39.1.335-338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze TL. Jordan RA. Schulze CJ. Host associations of Ixodes scapularis (Acari: Ixodidae) in residential and natural settings in a Lyme disease-endemic area in New Jersey. J Med Entomol. 2005;42:966–973. doi: 10.1093/jmedent/42.6.966. [DOI] [PubMed] [Google Scholar]
- Slajchert T. Kitron UD. Jones CJ. Mannelli A. Role of the eastern chipmunk (Tamias striatus) in the epizootiology of Lyme borreliosis in northwestern Illinois, USA. J Wildl Dis. 1997;33:40–46. doi: 10.7589/0090-3558-33.1.40. [DOI] [PubMed] [Google Scholar]
- Stafford KC., III . Tick management handbook. Connecticut Agricultural Experimental Station; New Haven, CT: 2004. [Google Scholar]
- Telford SR. Dawson JE. Katavolos P, et al. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR. Mather TN. Moore SI. Wilson ML, et al. Incompetence of deer as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg. 1988;39:105–109. doi: 10.4269/ajtmh.1988.39.105. [DOI] [PubMed] [Google Scholar]
- Tsao JI. Wootton JT. Bunikis J. Luna MG, et al. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci USA. 2004;101:18159–18164. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. Healthy People 2010. US Department of Health and Human Services; Washington, DC: 2000. [Google Scholar]
- Walls J. Grieg B. Neitzel D. Dumler J. Natural infection of small mammal species in Minnesota with the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:853–855. doi: 10.1128/jcm.35.4.853-855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ML. Adler GH. Spielman A. Correlation between abundance of deer and that of the deer tick, Ixodes dammini (Acari: Ixodidae) Ann Entomol Soc Am. 1985;78:172–176. [Google Scholar]