Abstract
Objective: This in vitro study evaluated the effectiveness of photodynamic therapy (PDT) for the inactivation of different species of Candida on maxillary complete dentures. Background data: The treatment of denture stomatitis requires the inactivation of Candida spp. on dentures. PDT has been reported as an effective method for Candida inactivation. Methods: Reference strains of C. albicans, C. glabrata, C. tropicalis, C. dubliniensis and C. krusei were tested. Thirty-four dentures were fabricated in a standardized procedure and subjected to ethylene oxide sterilization. The dentures were individually inoculated with one of the strains and incubated at 37°C for 24 h. Dentures submitted to PDT (P+L+) were individually sprayed with 50 mg/L of Photogem® (PS) and, after 30 min, illuminated by LED light for 26 min (37.5 J/cm2). Additional dentures were treated only with PS (P+L-) or light (P-L+) or neither (P-L-). Samples of serial dilutions were spread on Sabouraud dextrose agar and incubated at 37°C for 48 h. The colonies were counted and the values of log (cfu/mL) were analyzed by Kruskall-Wallis and Dunn tests (p<0.05). Results: For all species of Candida, PDT resulted in significant reduction (p<0.05) of cfu/mL values from dentures when compared with P-L- (reductions from 1.73 to 3.99 log10). Significant differences (p<0.05), but lower reductions, were also observed for P+L- and P-L+when compared with P-L- for some species of Candida. Conclusions: PDT was an effective method for reducing Candida spp. on dentures.
Introduction
Denture stomatitis (DS) is the most common form of oral candidosis, with overall incidence of 11–65% in complete denture wearers.1 This recurring disease is characterized by different degrees of inflammation of the mucosa under the upper denture.2 Although there may be systemic conditions related to DS, local factors also play an important role in the etiology of this disease.3 Etiological factors associated with the use of dentures include: increased age of denture, denture trauma, continuous denture wearing, and poor denture hygiene.4 Nonetheless, the denture–palatal interface offers a unique ecological niche for micro-organism colonization, because of the relatively anaerobic and acidic environment favoring yeast proliferation without any other predisposing factor present.5 Fungal species of Candida have high affinity for adhering to and colonizing acrylic surfaces,6,7 which is considered the first step in the pathogenesis of DS. Therefore, the presence of Candida spp. on dentures is considered a major factor in the development of this infection.8 C. albicans is the most prevalent and virulent species found in DS, but other species have been shown to cause infection, with C. glabrata, C. dubliniensis, C. krusei, and C. tropicalis being the most commonly described.9
Antifungal agents are commonly used to treat DS. Despite their effectiveness, the recurrence of infection after treatment is very common10 and the major problem associated with the use of antifungal agents is the development of resistant species.11 In addition, some Candida species, such as C. glabrata and C. krusei, are intrinsically more resistant to antifungals.12 Because these agents do not eradicate micro-organisms that colonize the denture,13 it is also necessary to improve denture hygiene, discontinue nocturnal denture wearing, and eventually re-line or replace the dentures. Nonetheless, the effective removal of denture plaque by brushing requires a certain degree of manual dexterity which is commonly compromised in the elderly. In addition, the irregularities and porosities present on the acrylic resin surface may also contribute to penetration of micro-organisms into the dentures, making it difficult to clean them by brushing.14,15 Moreover the use of denture disinfectants has been considered time consuming and inappropriate.
Photodynamic therapy (PDT) has been extensively investigated as an alternative antimicrobial modality. PDT combines a photosensitizing agent (PS) and an appropriate wavelength of light (the maximum absorption of the PS) in the presence of oxygen, producing cytotoxic reactive species.16,17 Because of the nonspecific oxidizing agents, organisms resistant to conventional antifungal agents could be successfully killed by PDT, and it seems to be unlikely that they will develop resistance to such therapy. The photoinactivation of Candida spp. has been widely demonstrated in vitro,18–23 including resistant strains,24,25 and also in vivo,26 suggesting that PDT could be an effective and alternative method for treating oral candidosis. A previous study has shown that the association of Photogem and blue LED light resulted in the inactivation of fluconazole-resistant strains of C. albicans and C. glabrata.25 Another in vivo study has demonstrated that this association was also effective in reducing C. albicans in a murine model of oral candidosis.26 However, for an effective treatment of DS, the inactivation of Candida spp. on dentures is also necessary because of the recurrent nature of this disease, as dentures may act as a reservoir of micro-organisms. Surface roughness and porosity of dentures facilitate retention and adhesion of micro-organisms. Thus, the aim of this study was to simulate the disinfection of dentures clinically using PDT. The effectiveness of PDT, using Photogem and blue LED light, was evaluated as an antifungal method for dentures colonized with different species of Candida commonly found in DS.
Methods
Photosensitizer and light source
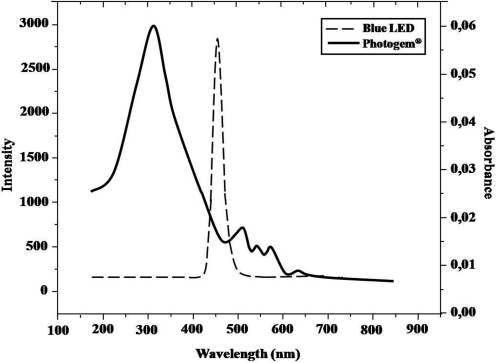
The PS used in this study was a hematoporphyrin derivative produced in Moscow, Russia (Photogem®; Limited Liability Company Photogem, Moscow, Russia). Solutions of 50 mg/L of Photogem (pH 6.6) were prepared instantly before use by dissolving the powder in sterile saline. This solution was stored in a sterile spray bottle and kept in the dark. The absorption bands of Photogem are shown in Figure 1.
FIG. 1.
Absorption bands of Photogem® and intensity of blue (455 nm) LED light.
A light-emitting diode device (LEDs, LXHL-PR09, Luxeon® III Emitter, Lumileds Lighting, San Jose, CA) was designed by the Instituto de Física de São Carlos (Physics Institute, University of São Paulo, São Carlos, SP, Brazil). It covered the wavelength range from 440 to 460 nm, with maximum emission at 455 nm (royal blue). This device was composed of 24 LEDs uniformly distributed throughout the device, resulting in a light intensity of 24 mW/cm2, and three air coolers to prevent the denture from heating.
Microorganisms and culture condition
Reference strains (ATCC; Rockville, MD) of C. albicans (ATCC 90028), C. glabrata (ATCC 2001), C. tropicalis (ATCC 4563), C. krusei (ATCC 6258) and C. dubliniensis (ATCC 7987) were used to contaminate the dentures. These strains were individually maintained in yeast-peptone-glucose (YEPD, 1.0% yeast extract, 2.0% peptone, 2.0% glucose, 0.1M citrate-phosphate buffer pH 5.0) and glycerol medium at −70°C. Each strain was reactivated by cultivation in Sabouraud dextrose agar (SDA, Acumedia Manufactures Inc., Baltimore, MD) containing 5 μg/mL gentamicin at 37°C for 48 h before each experiment.
Simulated denture base production
A total of 34 dentures were made for this study, according to the method described by Sanitá et al.27 A stainless steel master die simulating an edentulous maxilla was duplicated by using a high-viscosity silicone mould (RTV 3120, Daltomare, Santo Amaro, SP, Brazil) to produce 34 dental stone casts (Herodent, Vigodent, Bonsucesso, RJ, Brazil). On one of prepared casts, a simulated maxillary complete denture base was waxed and acrylic resin denture teeth were arranged on it. This waxed-up denture was duplicated using the high-viscosity silicone, and 34 identical simulated maxillary dentures were obtained. This was accomplished by first placing the acrylic artificial teeth (Dental Vip Ltd, Pirassununga, SP, Brazil) in the silicone mold, pouring the melted wax into it, and fully seating a duplicate cast in the mold. After bench cooling at room temperature for 30 min, the wax-simulated dentures were removed from the silicone mold and conventionally invested in metal dental flasks (Jon 5.5, Jon Produtos Odontológicos, São Paulo, SP, Brazil) with dental stone. After the stone was set, the flasks were placed in boiling water to soften the base plate wax. The flasks were separated, the wax was removed, and the stone and teeth were cleaned with boiling water and liquid detergent (ODD, Bombril-Cirio, São Paulo, SP, Brazil). Two coats of sodium alginate (Isolak, Clássico Dental Products, São Paulo, SP, Brazil) were used as mold separator. Poly (methyl methacrylate) dental base resin (Lucitone 550-Dentsply International Inc, York, PA) was prepared according to the manufacturer's directions by mixing 21 g polymer powder with 10 mL monomer liquid. The denture base resin at dough stage was packed into the molds and the flasks closed under pressure using a hydraulic press (Dental Vip Ltd, Pirassununga, SP, Brazil). The flasks were placed in an automatic polymerization tank (Termotron P-100, Termotron Equipamentos, Piracicaba, SP, Brazil) at 73°C for 90 min followed by 30 min in boiling water at 100°C. After polymerization, the flasks were bench cooled for 30 min and placed under running tap water for 15 min. The flasks were opened, and the dentures carefully removed and trimmed using a metal bur (Maxi-Cut, Dentsply-Malleifer, Ballaigues, Switzerland). After this they were finished with a handheld micromotor (Kavo, Biberach/Riss, Germany) using 360, 400, 600, and 1200-grit abrasive papers (Norton, Saint-Gobain Abrasivos Ltd, Guarulhos, SP, Brazil). Finally, the dentures were polished on a wet rag wheel with a slurry of coarse pumice followed by tin oxide. After polishing, the dentures were stored, being individually placed in a 200 mL beaker of distilled water at 37°C±1°C for 48±2 h.27,28
Denture sterilization
After 48±2 h of storage in water, all dentures were sterilized with ethylene oxide (ACECIL – Comércio e Esterilização a Óxido de Etileno Ltd, Campinas, SP, Brazil). To confirm the effectiveness of this procedure, two additional dentures were tested as negative controls as follows. Fifteen days after sterilization, each of the dentures was individually placed in 200 mL of Tryptic Soy Broth (TSB) (Acumedia Manufactures, Inc. Baltimore, MD) in a 600-mL sterile beaker that was sealed with foil. The beakers were then incubated at 37°C for 7 days. At 48 h and 7 days, the broths were evaluated for microbial growth (turbidity). No turbidity was observed in the broth beakers at 48 h and 7 days.
Contamination of dentures and PDT
The protocol used in this study to contaminate the dentures was based on a method described by Sanitá et al.,27 Silva et al.,29 and Dovigo et al.30 On day one, yeasts were individually inoculated with 0.5 McFarland turbidity standard corresponding to 107 organisms/mL in 5 mL of TSB and grown aerobically at 37°C for 24 h. The following day, 15 μL of inoculated TSB was transferred to each 600-mL sterile beaker containing the 200 mL of sterile TSB. Each sterile denture was aseptically placed into the beaker, sealed with foil, and incubated for 24 h at 37°C.
After incubation, each denture was carefully transferred into a 600-mL beaker and washed once with 200 mL of sterile saline to remove excess medium and nonadherent cells. Dentures submitted to PDT (P+L+ group) were then aseptically removed from the saline solution and individually sprayed with PS. For this, ∼ 5 mL of PS was sprayed on the inner and outer surfaces of each denture. Then, the denture was placed in a transparent plastic bag and left in the dark for 30 min (pre-irradiation time). For illumination, the denture was placed inside the LED device and irradiated for 26 min (37.5 J/cm2). The denture was centered within the device and surrounded by LEDs during illumination. Thus, inner and outer surfaces of denture were illuminated. To determine whether PS alone had any effect on cell viability, additional inoculated dentures were individually exposed to PS under identical conditions to those described previously, but not to LED light (P+L- group). The effect of LED light alone was determined by exposing contaminated dentures to light without their being previously exposed to PS (P-L+ group). Inoculated dentures exposed to neither PS nor LED light acted as an overall control (P-L- group). Thirty-two dentures were used for each Candida species (eight for each group). Before reusing, each denture was autoclaved, cleaned, polished, and sterilized with ethylene oxide.
Next each denture was individually placed in sterile beakers containing 200 mL of saline solution. These beakers were vortexed vigorously in a shaker incubator (Model MA-562, Marconi Equipamentos Laboratoriais Ltda, Piracicaba, SP, Brazil) for 1 min and allowed to stand for 9 min, followed by a short vortex to re-suspend any organisms present. To determine the number of micro-organisms in the 10−1, 10−2, 10−3, and 10−4 dilutions, duplicate specimens (25 μL) of the suspension were transferred to SDA plates. These plates were incubated at 37°C for 48 h. After this 48-h incubation period, yeast colony counts of each plated denture were quantified using a digital colony counter (Phoenix CP 600 Plus, Phoenix Ind. e Com. de Equipamentos Científicos Ltd, Araraquara, SP, Brazil). Only dilutions on plates showing a good separation of colonies (between 30 and 300 colonies per dilution) were considered. The average number of colonies for each denture was calculated after counting duplicate plates.
Statistical analysis
The logarithm of colony-forming units per milliliter (log cfu/mL) was calculated. Adherence to the assumptions of normality and homoscedasticity was verified using the Shapiro–Wilk and Bartlett's tests (α=0.05). Because the data did not conform to the requirements of a normal distribution and homoscedasticity, a Kruskal Wallis one-way analysis of variance, at 95% confidence level (α=0.05) on ranks was used. If significant differences (p<0.05) in the log cfu/mL numbers were found, pairwise multiple comparison procedures (Dunn test) were performed to analyze the data (α=0.05).
Results
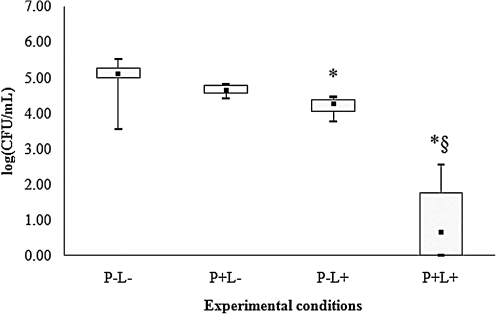
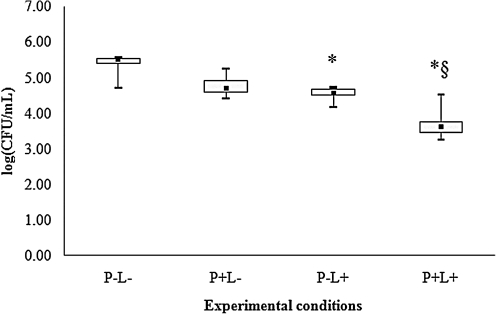
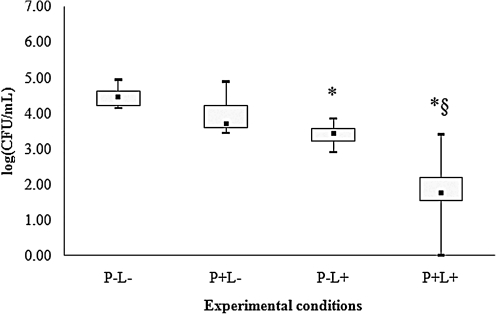
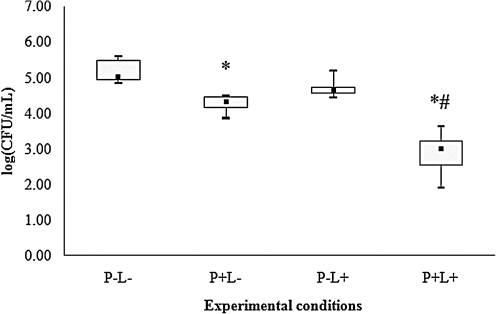
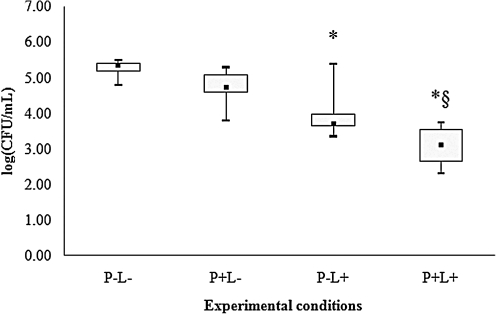
For all species of Candida evaluated, control group (P-L-) showed the highest cfu/mL values (Figs. 2–6), and dentures submitted to PDT (P+L) showed significant reduction in cfu/mL values (p<0.05) when compared with the control (P-L-). C. tropicalis showed the highest reduction (3.99 log10, Fig. 2) in cfu/mL values after PDT, whereas reductions of 2.67, 2.29, 2.17, and 1.73 log10 were observed for C. krusei, C. albicans, C. glabrata and C. dubliniensis, respectively (Figs. 3–6).
FIG. 2.
Values of log (cfu/mL) obtained from dentures contaminated with C. tropicalis. *Significant difference (p<0.05) when compared with control group (P-L-). §Significant difference (p<0.05) when compared with P+L- group.
FIG. 6.
Values of log (cfu/mL) obtained from dentures contaminated with C. dubliniensis. *Significant difference (p<0.05) when compared with control group (P-L-). §Significant difference (p<0.05) when compared with P+L- group.
FIG. 3.
Values of log (cfu/mL) obtained from dentures contaminated with C. krusei. *Significant difference (p<0.05) when compared with control group (P-L-). §Significant difference (p<0.05) when compared with P+L- group.
When dentures were treated with PS or LED light only, significant differences were observed for some species of Candida. For C. albicans (Fig. 4), spraying PS on dentures without light (P+L-) promoted significant reduction in cfu/mL values (p<0.05) when compared with the control (P-L-). When dentures were only illuminated by LED light (P-L+), a significant reduction in cfu/mL values (p<0.05) was verified for C. tropicalis (Fig. 2), C. krusei (Fig. 3), C. glabrata (Fig. 5) and C. dubliniensis (Fig. 6) in comparison with the control (P-L-). Dentures subjected to PDT (P+L+) also showed significant reduction (p<0.05) for C. albicans (Fig. 4) when compared with dentures treated with LED light alone (P-L+), and for C. tropicalis (Fig. 2), C. krusei (Fig. 3), C. glabrata (Fig. 5), and C. dubliniensis (Fig. 6) when compared with dentures treated with PS alone (P+L-). Nonetheless, no significant difference was observed between dentures treated with PS alone (P+L-) and those treated with light alone (P-L+) for any species of Candida evaluated.
FIG. 4.
Values of log (cfu/mL) obtained from dentures contaminated with C. albicans. *Significant difference (p<0.05) when compared with control group (P-L-). #Significant difference (p<0.05) when compared with P-L+ group.
FIG. 5.
Values of log (cfu/mL) obtained from dentures contaminated with C. glabrata. *Significant difference (p<0.05) when compared with control group (P-L-). §Significant difference (p<0.05) when compared with P+L- group.
Discussion
This investigation demonstrated that PDT applied to dentures promoted a significant reduction in the viability of different species of Candida commonly found in DS. Topical application of Photogem followed by illumination of dentures with blue LED light resulted in reduction ranging from 1.73 to 3.99 log10 in the viability of Candida spp. This outcome is in agreement with previous studies that verified the photoinactivation of Candida spp. with Photogem and LED light (455 nm) in vitro and in vivo.25,26 However, complete yeast inactivation was not achieved in the present investigation, which evaluated only one concentration of the PS and light fluence. Dovigo et al.25 observed dose-dependent photoinactivation of C. albicans and C. glabrata strains using several concentrations of Photogem and blue (455 nm) LED light fluences. According to these authors, complete killing of planktonic cultures of all strains was observed when 50 mg/L of Photogem was associated with 37.5 J/cm2 of LED light.25 As these parameters were the same as those used in the present study for PDT application, it may be suggested that the dentures could have contributed to the yeast survival. Porosities, roughness, free energy and surface characteristics of the denture resin as well as cell surface mannoprotein and hydrophobicity are factors responsible for Candida adherence,31 and the complex interaction between yeast and acrylic may contribute to yeast survival. In this investigation, complete inactivation of Candida spp. after PDT (P+L+ groups) could possibly have been achieved if higher concentrations of PS and light fluences had been assessed, as a dose-dependent photoinactivation of Candida spp. has previously been verified.25 On the other hand, even a Photogem concentration 10 times higher (500 mg/L) than that used in this study, associated with a blue (455 nm) LED light fluence of 305 J/cm2, did not achieve complete inactivation of C. albicans in a murine model of oral candidosis.26 At high concentrations, PS may suffer a self-aggregation process in solution reducing the singlet oxygen yield.32 Furthermore, other LED parameters, such as higher light fluences, were not evaluated in this investigation, because longer periods of illumination would be necessary, which could make treatment not feasible clinically. It is possible that complete yeast killing would be achieved if other sensitizers had been combined with LED light. However, only microbial reduction may be sufficient for treatment, as Candida spp. is a commensal yeast commonly found on oral mucosa. In addition, some antifungal agents, such as the azoles, have a fungistatic activity, which does not result in complete yeast killing either. Furthermore, antifungal agents may lead to the development of resistant strains. Hence, PDT may be clinically more effective on denture disinfection since the development of microbial resistance to PDT may be improbable because of its mechanism of action.
As described previously, the PDT parameters used in the present investigation were the same as those used previously by Dovigo et al.25 when complete inactivation of Candida spp. was observed (i.e., 50 mg/L of Photogem, 30 min of pre-irradiation time, and 37.5 J/cm2 of blue LED light). Clinically, shorter pre-irradiation times would be more feasible. However, shorter times should be previously evaluated in vitro using planktonic cultures of Candida spp. in order to verify whether candidal cells would be sensitized.
In the present study, C. tropicalis was the species most susceptible to PDT (reduction of ∼4 log10) and C. dubliniensis was the least susceptible (reduction of 1.73 log10). This result shows that susceptibility of Candida spp. to PDT varies according to the strains and differs from its susceptibility to antifungal agents. Although C. glabrata and C. krusei are intrinsically resistant to antifungal agents,12 in this investigation C. krusei was the second most susceptible species to PDT, and C. glabrata was the second most resistant. Therefore, this difference in the susceptibility of Candida spp. to PDT and antifungal drugs can be explained by the distinct mechanisms of yeast inactivation of the two therapies. Whereas most antifungal agents inhibit the biosynthesis of ergosterol, the main sterol in membranes of fungi,33 the reactive oxygen species yielded by PDT promote perforation of the cell wall and membrane, thereby permitting the PS to translocate into the cell. Once inside the cell, oxidizing species generated by light excitation induce photodamage to internal cell organelles and cell death.17,18 Other investigations have shown differences in the susceptibility of Candida spp. to PDT. Wilson and Mia19 verified that C. tropicalis was less susceptible to PDT than was C. albicans, when toluidine blue was associated with He-Ne laser. Using Photofrin® and a mercury arc lamp, Bliss et al.20 demonstrated similar susceptibility of C. krusei and C. albicans to PDT, with C. krusei being only slightly more resistant, and C. glabrata being the most resistant. When methylene blue was used in conjunction with a diode laser, de Souza et al.21 observed that C. krusei was the species that presented a higher percentage of cfu/mL reduction (91.6%), followed by C. albicans (88.6%), C. dubliniensis (84.8%) and C. tropicalis (82.3%), respectively. On the other hand, Soares et al.22 verified similar susceptibility to PDT among isolates of C. albicans and C. tropicalis sensitized with toluidine blue and illuminated with LED light. Gasparetto et al.23 observed that strains of C. dubliniensis showed a reduction of >98% after sensitization with extracts of A. maritime and irradiation with diode laser. The outcomes of Mang et al.24 demonstrated that strains of C. albicans, C. glabrata, and C. krusei showed significant sensitivity to Photofrin and laser light, with C. krusei being less sensitive. The results of these studies and those of the present investigation suggest that the susceptibility of different species of Candida to PDT may vary according to the type of PS and light source. Moreover, Dovigo et al.25 reported that the fungicidal effect of PDT was strain dependent, as the response to PDT was not homogenous among strains of the same species.
Although significant differences were observed when dentures were treated with PS or light alone when compared with the control in this investigation, the reductions obtained ranged from 0.76 to 1.28 log10 depending upon the species evaluated. These values were lower than those obtained after PDT (1.73 to 3.99 log10 when compared with control), confirming that there is a greater antimicrobial effect when PS is combined with light. Nonetheless, the reduction obtained for C. albicans after spraying PS on dentures (P+L-) may be explained by the mechanical removal of cells, as no cytotoxic effect of Photogem on C. albicans cells has previously been verified,25 even at higher concentrations.26 However this effect was not seen in other species. Probably, the complex interactions between non-albicans species and acrylic resin, such as superior adherence and hydrophobicity,27,34 may justify this effect. On the other hand, the significant reduction observed for most Candida species evaluated when dentures were irradiated with blue LED light only (P-L+), may be justified by the conversion of light energy into heat energy inside the LED device, thus generating heat in the acrylic resin dentures. Although the LED device used in this study was equipped with three air coolers, after the 26 min of irradiation (37.5 J/cm2) the dentures were heated. This increase in the temperature of the dentures was not measured in the present investigation, but the light fluence used showed no cytotoxic effect on Candida spp. previously when cell suspensions were irradiated for 50 min by the same blue LED light.25 In this study, dentures were heated after illumination, but this increase in temperature did not prevent manipulation of dentures. Denture distortion may be the result of high temperatures. Nonetheless, in a clinical study, Basso et al. observed that microwaving dentures (3 min at 650 W) three times a week resulted in a significantly greater shrinkage of dentures, but no significant clinical finding was observed.35 Despite the fact that the temperature of dentures was not measured in this investigation, we believe that the temperature rise was not so high as to cause distortion of dentures. However, dimensional stability of dentures after illumination during PDT procedure should be investigated in future studies.
Conclusions
PDT resulted in significant reduction in the viability of different species of Candida on dentures. This promising outcome suggests that PDT may be used for reducing the fungal load in dentures as an adjuvant treatment of DS. However, in vivo conditions were not simulated in this investigation, and clinical trials are indispensible in order to evaluate the effectiveness of this treatment.
Acknowledgments
The authors are grateful to ACECIL – Comércio e Esterilização a Óxido de Etileno Ltda. – for kindly providing sterilization of the dentures with ethylene oxide. This research was supported by São Paulo Council of Research (FAPESP – Grant No. 2005/02193-4 and 2005/03226-3, and Center for Research in Optics and Photonics—CePOF – CEPID Program, Center for Research, Innovation, and Diffusion).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Jeganathan S. Lin C.C. Denture stomatitis—a review of the aetiology, diagnosis and management. Aust. Dent. J. 1992;37:107–114. doi: 10.1111/j.1834-7819.1992.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 2.Newton A.V. Denture sore mouth as possible etiology. Br. Dent. J. 1962;112:357–360. [Google Scholar]
- 3.Arendorf T.M. Walker D.M. Denture stomatitis: a review. J. Oral Rehabil. 1987;14:217–227. doi: 10.1111/j.1365-2842.1987.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 4.Budtz–Jorgensen E. Oral mucosal lesions associated with the wearing of removable dentures. J. Oral Pathol. 1981;10:65–80. doi: 10.1111/j.1600-0714.1981.tb01251.x. [DOI] [PubMed] [Google Scholar]
- 5.Budtz–Jorgensen E. The significance of Candida albicans in denture stomatitis. Scand. J. Dent. Res. 1974;82:151–190. doi: 10.1111/j.1600-0722.1974.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 6.Budtz–Jorgensen E. Bertram U. Denture stomatitis. II. The effect of antifungal and prosthetic treatment. Acta Odontol. Scand. 1970;28:283–304. doi: 10.3109/00016357009032036. [DOI] [PubMed] [Google Scholar]
- 7.McCourtie J. Douglas L.J. Extracellular polymer of Candida albicans: isolation, analysis and role in adhesion. J. Gen. Microbiol. 1985;131:495–503. doi: 10.1099/00221287-131-3-495. [DOI] [PubMed] [Google Scholar]
- 8.Theilade E. Budtz–Jorgensen E. Predominant cultivable microflora of plaque on removable dentures in patients with denture induced stomatitis. Oral Microbiol. Immunol. 1988;3:8–13. doi: 10.1111/j.1399-302x.1988.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 9.Lyon J.P. da Costa S.C. Totti V.M. Munhoz M.F. de Resende M.A. Predisposing conditions for Candida spp. carriage in the oral cavity of denture wearers and individuals with natural teeth. Can. J. Microbiol. 2006;52:462–467. doi: 10.1139/w05-148. [DOI] [PubMed] [Google Scholar]
- 10.Bissell V. Felix D.H. Wray D. Comparative trial of fluconazole and amphotericin in the treatment of denture stomatitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1993;76:35–39. doi: 10.1016/0030-4220(93)90290-k. [DOI] [PubMed] [Google Scholar]
- 11.White T.C. Marr K.A. Bowden R.A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter K.D. Gibson J. Lockhart P. Pithie A. Bagg J. Fluconazole-resistant Candida species in the oral flora of fluconazole-exposed HIV-positive patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998;85:558–564. doi: 10.1016/s1079-2104(98)90291-8. [DOI] [PubMed] [Google Scholar]
- 13.Banting D.W. Greenhorn P.A. McMinn J.G. Effectiveness of a topical antifungal regimen for the treatment of oral candidiasis in older, chronically ill, institutionalized, adults. J. Can. Dent. Assoc. 1995;61:199–200. 203–205. [PubMed] [Google Scholar]
- 14.Kulak Y. Arikan A. Kazazoglu E. Existence of Candida albicans and microorganisms in denture stomatitis patients. J. Oral Rehabil. 1997;24:788–790. doi: 10.1046/j.1365-2842.1997.00550.x. [DOI] [PubMed] [Google Scholar]
- 15.Davenport J.C. The denture surface. Br. Dent. J. 1972;133:101–105. doi: 10.1038/sj.bdj.4802881. [DOI] [PubMed] [Google Scholar]
- 16.Konopka K. Goslinski T. Photodynamic therapy in dentistry. J. Dent. Res. 2007;86:694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- 17.Donnelly R.F. McCarron P.A. Tunney M.M. Antifungal photodynamic therapy. Microbiol. Res. 2008;163:1–12. doi: 10.1016/j.micres.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Bertoloni G. Reddi E. Gatta M. Burlini C. Jori G. Factors influencing the haematoporphyrin-sensitized photoinactivation of Candida albicans. J. Gen. Microbiol. 1989;135:957–966. doi: 10.1099/00221287-135-4-957. [DOI] [PubMed] [Google Scholar]
- 19.Wilson M. Mia N. Sensitisation of Candida albicans to killing by low-power laser light. J. Oral Pathol. Med. 1993;22:354–357. doi: 10.1111/j.1600-0714.1993.tb01088.x. [DOI] [PubMed] [Google Scholar]
- 20.Bliss J.M. Bigelow C.E. Foster T.H. Haidaris C.G. Susceptibility of Candida species to photodynamic effects of photofrin. Antimicrob. Agents Chemother. 2004;48:2000–2006. doi: 10.1128/AAC.48.6.2000-2006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Souza S.C. Junqueira J.C. Balducci I. Koga–Ito C.Y. Munin E. Jorge A.O.C. Photosensitization of different Candida species by low power laser light. J. Photochem. Photobiol. B. 2006;83:34–38. doi: 10.1016/j.jphotobiol.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Soares B.M. da Silva D.L. Sousa G.R., et al. In vitro photodynamic inactivation of Candida spp. growth and adhesion to buccal epithelial cells. J. Photochem. Photobiol. B. 2009;94:65–70. doi: 10.1016/j.jphotobiol.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Gasparetto A. Lapinski T.F. Zamuner S.R., et al. Extracts from Alternanthera maritima as natural photosensitizers in photodynamic antimicrobial chemotherapy (PACT) J. Photochem. Photobiol. B. 2010;99:15–20. doi: 10.1016/j.jphotobiol.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Mang T.S. Mikulski L. Hall R.E. Photodynamic inactivation of normal and antifungal resistant Candida species. Photodiagnosis Photodyn. Ther. 2010;7:98–105. doi: 10.1016/j.pdpdt.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Dovigo L.N. Pavarina A.C. Mima E.G. Giampaolo E.T. Vergani C.E. Bagnato V.S. Fungicidal effect of photodynamic therapy against fluconazole-resistant Candida albicans and Candida glabrata. Mycoses. 2011;54:123–130. doi: 10.1111/j.1439-0507.2009.01769.x. [DOI] [PubMed] [Google Scholar]
- 26.Mima E.G. Pavarina A.C. Dovigo L.N., et al. Susceptibility of Candida albicans to photodynamic therapy in a murine model of oral candidosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010;109:392–401. doi: 10.1016/j.tripleo.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Sanitá P.V. Vergani C.E. Giampaolo E.T. Pavarina A.C. Machado A.L. Growth of Candida species on complete dentures: effect of microwave disinfection. Mycoses. 2009;52:154–160. doi: 10.1111/j.1439-0507.2008.01558.x. [DOI] [PubMed] [Google Scholar]
- 28.ISO 1567 for denture base polymers. International Standards Organization. 1998. http://www.iso.org/iso/prods-service/ISOstore/store.html. [Jul 15;2009 ]. http://www.iso.org/iso/prods-service/ISOstore/store.html
- 29.Silva M.M. Vergani C.E. Giampaolo E.T. Neppelenbroek K.H. Spolidorio D.M. Machado A.L. Effectiveness of microwave irradiation on the disinfection of complete dentures. Int. J. Prosthodont. 2006;19:288–293. [PubMed] [Google Scholar]
- 30.Dovigo L.N. Pavarina A.C. Ribeiro D.G. de Oliveira J.A. Vergani C.E. Machado A.L. Microwave disinfection of complete dentures contaminated in vitro with selected bacteria. J. Prosthodont. 2009;18:611–617. doi: 10.1111/j.1532-849X.2009.00489.x. [DOI] [PubMed] [Google Scholar]
- 31.Radford D.R. Challacombe S.J. Walter J.D. Denture plaque and adherence of Candida albicans to denture-base materials in vivo and in vitro. Crit. Rev. Oral Biol. Med. 1999;10:99–116. doi: 10.1177/10454411990100010501. [DOI] [PubMed] [Google Scholar]
- 32.Sternberg E.D. Dolphin D. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron. 1998;54:4151–4202. [Google Scholar]
- 33.Borgers M. Mechanism of action of antifungal drugs, with special reference to the imidazole derivatives. Rev. Infect. Dis. 1980;2:520–534. doi: 10.1093/clinids/2.4.520. [DOI] [PubMed] [Google Scholar]
- 34.Luo G. Samaranayake L.P. Candida glabrata, an emerging fungal pathogen, exhibits superior relative cell surface hydrophobicity and adhesion to denture acrylic surfaces compared with Candida albicans. APMIS. 2002;110:601–610. doi: 10.1034/j.1600-0463.2002.1100902.x. [DOI] [PubMed] [Google Scholar]
- 35.Basso M.F. Giampaolo E.T. Vergani C.E. Machado A.L. Pavarina A.C. Compagnoni M.A. Influence of microwave disinfection on the linear dimensional stability of complete dentures: a clinical study. Int. J. Prosthodont. 2010;23:318–320. [PubMed] [Google Scholar]