Keratan sulfate proteoglycans (KSPGs) are important for corneal stromal transparency. This study shows that JNK signaling downregulates KSPGs in TGF-β1– and FGF-2–activated corneal keratocytes, which is known to occur during corneal wound healing.
Abstract
Purpose.
Downregulation of lumican and keratocan expression is an undesirable phenotypic change that occurs during corneal wound healing. The present study was intended to determine whether the activation of Jun N-terminal kinase (JNK)-signaling pathway is involved in their downregulation in TGF-β1– and FGF-2–activated keratocytes.
Methods.
Keratocytes, isolated from rabbit corneal stroma, and cultured in a serum-free medium, pretreated or not treated with JNK inhibitor (SP600125), were activated with FGF-2/heparin sulfate (HS) or TGF-β1 in the presence or absence of SP600125. In another set of experiments, keratocytes were transfected with JNK1/2 Dicer-substrate RNA (DsiRNA) and then activated with TGF-β1 or FGF-2/HS. Specific phenotypic changes were analyzed immunocytochemically and correlated with Western blot analyses. The relative levels of specific mRNAs were estimated by quantitative RT-PCR using specific reagents.
Results.
The FGF-2/HS– or TGF-β–induced activation of corneal stromal keratocytes to fibroblast- or myofibroblast-phenotype, respectively, resulted in marked decreases in cell surfaceassociated and secreted keratan sulfate proteoglycans (KSPGs). Both keratocan and lumican proteins and their mRNAs were downregulated in the activated keratocytes. However, JNK inhibition during the activation of keratocytes, pretreated with the JNK inhibitor, suppressed the reduction in the cell–surface associated and secreted KSPGs (lumican and keratocan), and their mRNA transcripts. Downregulation of total KSPGs and their mRNAs was also inhibited by decreasing JNK1 and JNK2 levels via JNK1/2 DsiRNA transfection of keratocytes before their activation.
Conclusions.
Extrapolating from the present study, FGF-2– and TGF-β1–activation of JNK signaling pathway may be partly responsible for the downregulation of keratocan and lumican expression in activated corneal keratocytes observed during corneal stromal wound healing.
The cornea is a transparent avascular refractive structure consisting of three tissue layers, epithelium, stroma, and endothelium. A well-organized extracellular matrix (ECM) containing densely packed and regularly spaced thin collagen fibrils of uniform diameter, is largely responsible for transparency in the corneal stroma.1–3 Keratan sulfate proteoglycans (KSPGs) in the stromal ECM play a critical role in the development and maintenance of corneal transparency. Keratocan and lumican, the major KSPGs in the corneal stroma, regulate both fibril diameter and interfibrillar spacing as evident from the phenotype of lumican and keratocan knockout mice.4–9 Keratocan knockout mice have a thinner corneal stroma with irregular collagen fibril organization compared with the normal mice,8 and lumican knockout mice have increased collagen fibril diameter and develop opaque corneas.4,5
Corneal stromal cells (keratocytes), which synthesize keratocan and lumican during development, become quiescent in a fully-developed cornea. However, after an injury to the cornea, growth factors and cytokines originating from corneal epithelial cells, inflammatory cells, and tear fluid activate the keratocytes to fibroblast or myofibroblast phenotypes (reviews, see Refs. 10–14). Keratocan and lumican synthesis is downregulated in the activated keratocytes during wound healing.15–18 KSPG expression is also downregulated in vitro when cultured keratocytes are activated with growth factors including FGF-2 and TGF-β1.19–23 Therefore, an in vitro model of keratocyte activation is useful to study the signaling mechanisms that downregulate the expression of KSPGs. We had previously demonstrated that activation of the small GTPase Rho and its downstream target Rho kinase (ROCK) regulate several undesirable phenotypic changes including the downregulation of KSPGs in the activated keratocytes.23 Jun N-terminal kinase (JNK), a member of the mitogen activated protein kinase (MAPK) family, has been shown to mediate some of the Rho/ROCK regulated events.24–26 The present study was designed to investigate whether JNK activation is responsible for the observed TGF-β1– and FGF-2–induced downregulation of KSPGs in activated keratocytes.
Methods
Cell Culture
All procedures involving rabbits were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmology and Vision Research. Keratocytes were isolated from corneas excised from whole rabbit eyes obtained from Pel-Freez Biological (Rogers, AR) and cultured using a modified23 procedure of Jester et al.27 Isolated keratocytes were suspended in DMEM/F-12 containing 0.021% l-glutamine medium (glutaMAX; Invitrogen/Gibco, Carlsbad, CA) 0.011% pyruvate, 100 units /mL penicillin, and 100 μg/mL streptomycin (Invitrogen/Gibco) and the suspension was filtered through a cell strainer (70 μm BD, Falcon, Bedford, MA). Keratocytes were then centrifuged, resuspended, and plated at a density of 1.5 × 104 cells/cm2 into 60 mm dishes (Falcon Primaria; Becton Dickinson, Lincoln Park, NY), in the serum-free DMEM/F12 with 0.1 mM l-ascorbic acid 2-phosphate (SFM). After incubating the dishes at 37°C in a humidified 5% CO2/95% air incubator for 24 hours, the media were replaced with fresh SFM and the cells were incubated for an additional 48 hours. The cells were then incubated with SFM or without (controls) JNK inhibitor (20 μM, SP600125) for 3 hours. The cells were then activated by adding FGF-2 (40 ng/mL) and heparin sulfate (HS) (5 μg/mL) or TGF-β1 (2 ng/mL) to the media in the culture dishes. After 60 hours, the culture supernatants were removed and stored at 4°C after the addition of protease inhibitors (PMSF, 1 mM; NEM, 2mM, and pepstatin, 1 μg/mL) for the analyses of secreted KSPGs. Total RNAs and proteins were extracted from the cells in the respective buffers.
Immunostaining
For immunocytochemical analyses, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.25% Triton X-100 in PBS.28 After blocking with 10% heat-inactivated goat serum in PBS for 1 hour, the cells were treated with primary and secondary antibodies as described previously.28 Primary antibodies were ascites monoclonal mouse anti-KS (J19) at 1:100 dilution or rabbit anti–p-c-Jun (ser 63) (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:50 dilution. The secondary antibodies were goat anti-mouse IgG or anti-rabbit IgG conjugated to either Alexa Fluor 488 or Alexa Fluor 647 (Molecular Probes Inc./Invitrogen, San Diego, CA) at 1:2500 and 1:1500 dilutions, respectively. To stain actin filaments, Alexa 546 phalloidin (Molecular Probes) was included at 1:50 dilutions with the secondary antibody. Coverslips were mounted on the top of the cells (Immu-mount; Shandon, Pittsburgh, PA). Fluorescent images were captured with a scanning laser system attached to an inverted microscope (Olympus IX70; Olympus America Inc., Center Valley, PA) using the same settings for comparisons of the intensities of staining.
Western Blot Analyses
Cells were lysed in RIPA buffer (9.1 mM dibasic sodium phosphate, 1.7 mM monobasic sodium phosphate, pH 7.4; 150 mM NaCl, 1% Nonidet P-40; 0.5% sodium deoxycholate; 0.1% SDS; 0.03 TIU/mL aprotinin [Sigma-Aldrich, St. Louis, MO]; 1 mM PMSF and 1 mM sodium orthovanadate) for protein extraction. Because the final cell density depended on the treatment received, for the comparative Western blot analyses of secreted KSPGs from equal numbers of cells, the volume of culture supernatant in each sample was adjusted with PBS to 5 mL/100 mg of proteins in the corresponding cell extracts. Equal volumes of normalized samples were then treated with ¼ the volume of five times sample buffer for SDS-PAGE. For Western blotting of keratocan and lumican core proteins, the culture supernatants were concentrated 10-fold and then subjected to keratanase treatment.29 Protein bands on SDS-PAGE were electrophoretically transferred (Immobilon-P membrane; Millipore Corp, Bedford, MA), and the blots were reacted with anti-KS antibody or rabbit polyclonal anti-lumican or anti-keratocan antibodies (kind gift from John Hassell, University of South Florida, Tampa, FL) followed by HRP-conjugated secondary antibody as described previously.28 The immunoreactive bands were detected (Immobilon Western Chemiluminescent HRP Substrate; Millipore Corp.) following the manufacturer's protocols. The bands on x-ray film were scanned and analyzed using publicly available software (ImageJ, developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). In some experiments, the protein bands in the gel were blotted on transfer membranes (Millipore Immobilon-FL PVDF). The membranes were then blocked with blocking buffer (Odyssey; LI-COR, Lincoln, NE); and secondary antibodies (IRDye 680LT and 800CW, LI-COR) at 1:10,000 dilution were used to detect the primary antibody using an infrared imager (Odyssey; LI-COR). Densitometric analyses were performed using the infrared imager software (Odyssey; LI-COR).
Quantitative RT-PCR
Total RNA from the cells was isolated using a kit (RNeasy Mini; Qiagen, Valencia, CA) and RNA was isolated using quantitative RT-PCR. The quantification of specific mRNAs was carried out using RT-PCR reagents (SYBR Green; Fermentas; Thermo Scientific, Waltham, MA) according to the manufacturer's instructions. The reactions were carried out using a sequence detection system (ABI 7700; Applied Biosystems, Foster City, CA) as described previously.23 To design the primers used for quantitative RT-PCR (Table 1), partial sequences of the genes encoding rabbit keratocan, lumican, JNK1, and JNK2 were first determined from PCR of thermo-amplified cDNAs encoding these proteins. The primers used for PCR were designed from the conserved sequences of these genes in other animal species.
Table 1.
Primers Used in Quantitative RT-PCR
| Genes | Primer Sequence (5′ to 3′) |
|---|---|
| 18S (GenBank X 00640) | Forward: CTCAACACGGGAAACCTCAC |
| Reverse: ACCACCCACAGAATCGAGAA | |
| Lumican (GenBank AF020292) | Forward: TGCAGCTTACCCACA ACA AG |
| Reverse: TGAAGGTGAACGAAGGTCAA | |
| Keratocan (GenBank DQ239829) | Forward: CTCACGTGGCTTTGATGTGT |
| Reverse: GACCTTTGTGAGGCGATTGT | |
| JNK1 | Forward: AGCTCATGGATGCGAATCTT |
| Reverse: CGGAGTGAAGGTGCTTGATT | |
| JNK2 | Forward: GCCGGGATAATTCACAGAGA |
| Reverse: GAAATTGGTGCACGCTGTC |
DsiRNA Transfection
After plating, the keratocytes were incubated in SFM for 48 hours, and then the medium was replaced with fresh SFM. The cells were then transfected with 10 nM Dicer-substrate RNA (DsiRNA) for JNK1 and JNK2 or with nonspecific scrambled DsiRNA (Integrated DNA Technologies Inc., Coralville, IA) using lipid reagent (SilentFect; Bio-Rad, Hercules, CA) according to the manufacturer's instructions. The sequences of the DsiRNAs are shown in Table 2.
Table 2.
DsiRNA Sequences
| Gene | DsiRNA Sequence (5′ to 3′) |
|---|---|
| JNK1 | |
| Antisense | GCAAAGAUUCGCAUCCAUGAGCUCCAU |
| Sense | GGAGCUCAUGGAUGCGAAUCUUUGC |
| JNK2 | |
| Antisense | UUCAAAUUUGAUUCCAGGGUACUUCGG |
| Sense | GAAGUACCCUGGAAUCAAAUUUGAA |
| Scrambled | |
| Antisense | UCACAAGGGAGAGAAAGAGAGGAAGGA |
| Sense | 5phos/CUUCCUCUCUUU CUCUCCCUUGUGA |
Six hours after the transfection, the media were replaced with SFM or SFM containing TGF-β1 or FGF-2/HS. After 60 more hours of incubation, the cells were analyzed immunocytochemically or by Western blotting analysis for proteins, and by qRT-PCR for mRNAs.
Statistical Analysis
All data are presented as the mean ± SD. Statistical analyses of the data from three or more separate experiments were performed with repeated-measures ANOVA. The differences were considered significant at P ≤ 0.05.
Results
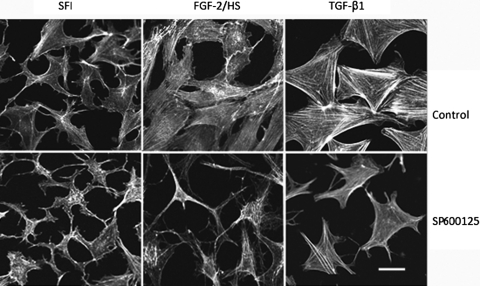
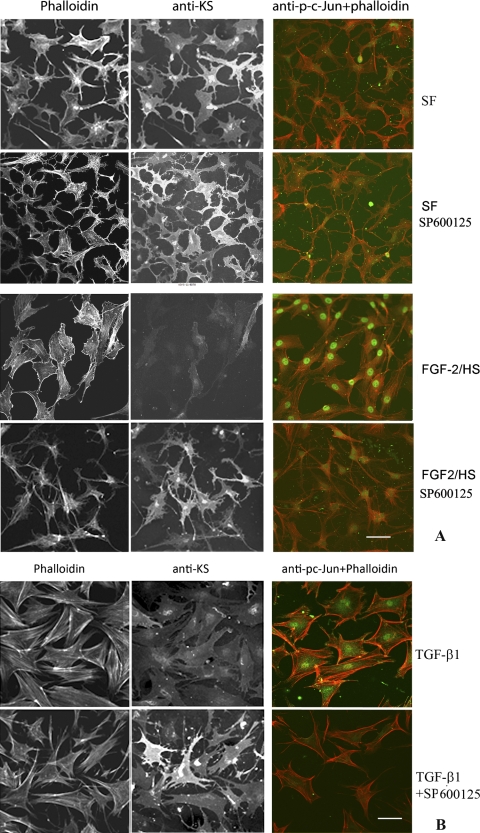
As expected30, keratocytes isolated from rabbit corneas, when cultured in SFM, exhibited dendritic morphology similar to that exhibited in vivo and also expressed KSPGs which were secreted in the culture media as well as associated with the cell surface (Figs. 1, 2). Activation of keratocytes with FGF-2 (40 ng/mL) and HS (5 μg/mL) or TGF-β1 (2 ng/mL) to fibroblast or myofibroblast phenotype, respectively (in the present study these cells will be referred to as FGF-2/HS– or TGF-β1–activated keratocytes), resulted in expected changes in the cell morphology, and assembly of actin stress fibers as seen by phalloidin staining (Fig. 1). The stress fiber-network was more robust in TGF-β1–activated keratocytes. By double staining with anti-KS antibody the cell surface–associated KSPG(s) was evident in nonactivated keratocytes, but was reduced on activation with FGF-2/HS or TGF-β1 (Figs. 2A, 2B). However, JNK inhibition with SP600125 during FGF-2– and TGF-β1–induced activation of keratocytes, pretreated with SP600125, resulted in the inhibition of the stress fiber assembly and changes in the cell morphology (Fig. 1). JNK inhibition also prevented the loss in cell-associated KSPG staining in FGF-2/HS– and TGF-β1–activated keratocytes (Figs. 2A and 2B, respectively). The inhibition of JNK activity by the JNK inhibitor was confirmed by a resulting decrease in its downstream target, nuclear p-c-Jun, as seen by immunostaining (Figs. 2A, 2B).
Figure 1.
JNK inhibition affects FGF-2/HS– and TGF-β1–induced assembly and organization of actin filaments in activated stromal keratocytes. Corneal stromal keratocytes, isolated from rabbit corneas, were cultured in DMEM/F12 without FBS (SFM). After pretreating with or without SP600125 for 3 hours in the SFM, the keratocytes were activated by supplementing the medium in the culture dishes with 40 ng/mL FGF-2 and 5μg/mL HS or 2 ng/mL TGF-β1. After 60 hours of incubation, the cells were reacted with Alexa Fluor 546-phalloidin. Bar, 50 μm.
Figure 2.
JNK inhibition occludes TGF-β1– and FGF-2–induced decreases in cell surface–associated KS in the activated corneal stromal keratocytes. Corneal stromal keratocytes, isolated from rabbit corneas, were cultured in DMEM/F12 without FBS (SFM). After pretreating with or without SP600125 for 3 hours in the SFM, the keratocytes were activated by supplementing the medium in the culture dishes with: (A) 40 ng/mL FGF-2 and 5 μg /mL HS; or (B) 2 ng/mL of TGF-β1. After 60 hours of incubation, cells were analyzed by double fluorescence staining using mouse anti-KS antibody or anti-p-c-Jun antibody followed by Alexa Fluor 488 anti-mouse IgG antibodies and Alexa Fluor 546 phalloidin. Bar, 50 μm.
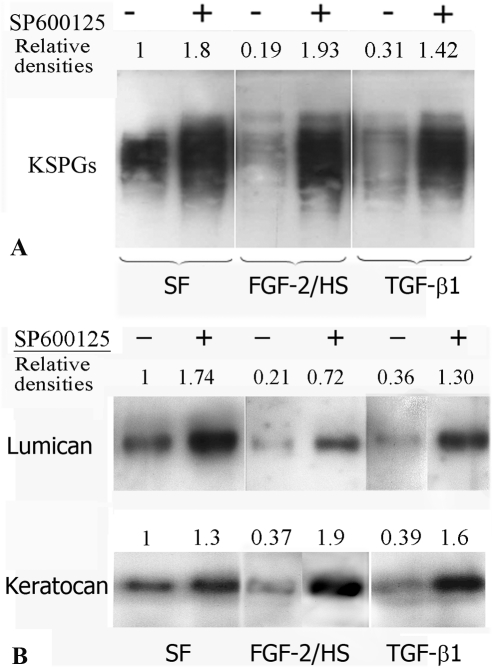
The relative levels of KSPGs in the culture supernatants, secreted by comparable numbers of cells, were analyzed by Western blotting. Based on the reduction in the densities of KSPGs bands (Fig. 3A), TGF-β1 and FGF-2/HS were found to inhibit KSPG synthesis. However, JNK inhibition suppressed the TGF-β1– and FGF-2/HS–induced decrease in the secreted KSPGs, and it also increased the levels of KSPG secreted by nonactivated keratocytes (Fig. 3A). To determine which of the KSPGs were regulated by JNK, keratanase- digested culture supernatants were analyzed for keratocan and lumican levels by Western blot analysis. Both keratocan and lumican were decreased in the culture supernatants of TGF-β1– as well as FGF-2/HS–activated keratocytes, which was evident from the decreases in the band densities in the Western blots (Fig. 3B). However, JNK inhibition occluded the loss in secreted lumican and keratocan. The increases in secreted lumican and keratocan on JNK inhibiton in FGF-2– and TGF-β1–activated cells were greater than those in the nonactivated cells.
Figure 3.
JNK inhibition increases KSPG secretion in nonactivated keratocytes and occludes TGF-β1– and FGF-2–induced decreases in KSPG secretion (A). The KSPGs, downregulated in the JNK-inhibited activated keratocytes, are characterized to be lumican and kertocan (B). Rabbit corneal keratocytes, cultured in DMEM/F12 without FBS (SFM), were incubated with or without SP600125 for 3 hours. The cells were then activated by supplementing the medium in the culture dishes with 40 ng/mL FGF-2 and 5μg/mL HS or 2 ng/mL TGF-β1 or not activated. After 60 hours, culture supernatants were analyzed by Western blotting using mouse anti-KS antibody (A). Aliquots of the supernatants were concentrated, digested with keratanase, and then analyzed by Western blotting using rabbit polyclonal anti-lumican or anti-keratocan antibodies (B).
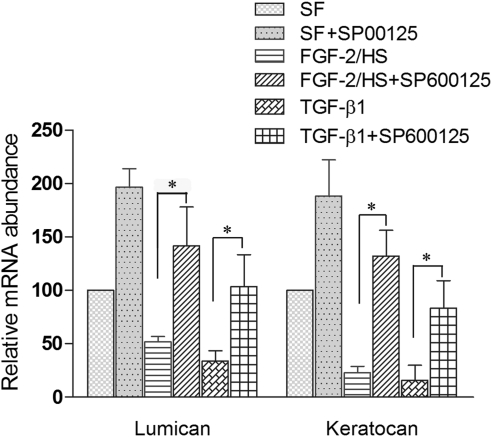
The levels of mRNA encoding lumican and keratocan were also decreased in keratocytes when activated with FGF-2/HS and TGF-β1 (Fig. 4). Activation of keratocytes with FGF-2/HS resulted in the reduction in lumican mRNA levels by 49.5% ± 5.2% and keratocan mRNA levels by 79.4% ± 6.5%. However, the inhibition of JNK activity during the FGF-2/HS–induced activation of keratocytes, pretreated with JNK inhibitor, resulted in a170% ± 70% increase in the levels of lumican mRNA and a 470% ± 50% increase in the levels of keratocan mRNA in the activated keratocytes (P < 0.05). The increased levels of expression were slightly higher than those in the nonactivated keratocytes (Fig. 4). Similarly, in TGF-β1–activated cells, lumican and keratocan mRNA levels were reduced by 77% ± 9% and 85% ± 14%, respectively. JNK inhibition during TGF-β1–induced activation of JNK-inhibited keratocytes resulted in a 240% ± 60% and a 511% ± 180% increase in the levels of lumican and keratocan mRNAs, respectively (P < 0.05). The increased levels of lumican and keratocan, resulting from the inhibition of JNK during the activation, were close to those in the nonactivated keratocytes. JNK inhibition in the nonactivated keratocytes also resulted in a 100% ± 30% and a 75% ± 30% increase in lumican and keratocan mRNA levels, respectively, indicating the presence of some JNK activity in the nonactivated keratocytes. SP600125-induced increases in the levels of keratocan and lumican mRNA levels were not significantly different in the growth factor-activated cells than those in the nonactivated keratocytes.
Figure 4.
JNK inhibition occludes decreases in the levels of lumican and keratocan mRNAs in TGF-β1– and FGF-2–activated keratocytes. For mRNA analysis rabbit corneal keratocytes cultured in DMEM/F12 without FBS (SFM) were either not activated (controls) or activated by supplementing the medium in the culture dishes with 40 ng/mL FGF-2 and 5 μg/mL HS or 2 ng/mL TGF-β1, without or with 20 μM JNK inhibitor (SP600125). After 60 hours in culture, the levels of specific mRNA in the cells were analyzed by real time RT-PCR using levels of 18S rRNA to normalize values in each sample. The values presented in the bar graph are the mean ± SD from three different experiments (*P < 0.05).
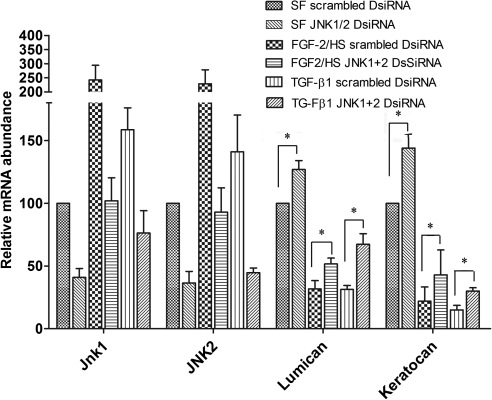
The results of the JNK inhibitor studies were verified using DsiRNA to knockdown JNK1 and JNK2 in the cells. FGF-2/HS–induced activation of keratocytes, transfected with scrambled DsiRNA (controls), resulted in a 140% ± 40% and a 130% ± 20% increase in JNK1 and JNK2 mRNA levels, respectively (Fig. 5). The levels of lumican and keratocan in these cells were reduced by 65% ± 3% and 45% ± 15%, respectively. When nonactivated keratocytes were transfected with JNK1/2 DsiRNA, the levels of JNK1 and JNK2 mRNAs were reduced by 53% ± 6% and 66% ± 7%, respectively. The levels of lumican and keratocan in these cells were 27% ± 5% and 44% ± 6% higher, respectively, than those in corresponding scrambled DsiRNA-transfected controls. Similarly, when the keratocytes, transfected with JNK1/2 DsiRNA, were activated with FGF-2/HS, the levels of JNK1 and JNK2 mRNAs were reduced by 75% ± 13% and 57% ± 8%, respectively. The levels of lumican and keratocan in these cells were 70% ± 20% and 100% ± 10% higher than in the corresponding scrambled DsiRNA controls (P < 0.05). Similarly, TGF-β1–induced activation of these keratocytes, transfected with scrambled DsiRNA, resulted in a 70% ± 20% and a 50% ± 16% increase in JNK-1 and JNK-2 mRNA levels, respectively, compared with those in nonactivated keratocytes. The levels of lumican and keratocan in TGF-β1–activated keratocytes were reduced by 68% ± 4% and 85% ± 5%, respectively. However, activation of JNK1/2 DsiRNA-transfected keratocytes with TGF-β1 resulted in a 55% ± 7% and a 69% ± 3% reduction in JNK1 and JNK2 mRNA levels, respectively, compared with those in the controls transfected with scrambled DsiRNA. The corresponding levels of lumican and keratocan in these cells were 100% ± 15% and 110% ± 30% higher, respectively, than those in the scrambled DsiRNA transfected controls. The above changes in the levels of JNK1, JNK2, lumican, and keratocan mRNAs, resulting from JNK1/2 DsiRNA transfection were statistically significant (P < 0.05). The increases in the levels of keratocan and lumican, resulting from JNK1/2 DsiRNA transfection, were not significantly different in nonactivated and activated keratocytes.
Figure 5.
Downegulation of JNK1/2 with JNK1/2 DsiRNA increases levels of lumican and keratocan mRNAs. Keratocytes cultured in DMEM/F12 without FBS (SFM) were first transfected with JNK1/2 DsiRNAs or scrambled DsiRNA, and then either activated with 40 ng/mL FGF-2 and 5 μg/mL HS or TGF-β1 (2 ng/mL) or not activated (SF transfected controls) and incubated for 60 hours. JNK1, JNK2, lumican, and keratocan mRNA levels were then analyzed by real time RT-PCR using levels of 18S rRNA to normalize values in each experiment. The values presented in the bar graph are the mean ± SD from three different experiments (*P < 0.05).
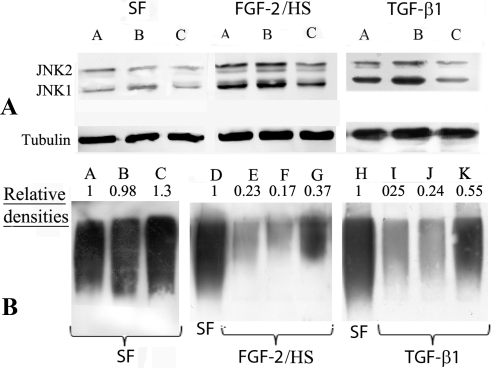
Western blot analyses of the cell extracts demonstrated that JNK1 and JNK2 were indeed present in nonactivated keratocytes, cultured in SFM (Fig. 6A; a representative Western blot). However, on activation with FGF-2/HS or TGF-β1 the densities of JNK1 and JNK2 bands increased by 380% and 350%, and 190% and 160%, respectively. As evident from the densities of the bands, JNK1 and JNK2 levels in JNK1/2 DsiRNA transfected nonactivated, FGF-2 activated, and TGF-β1 activated keratocytes, were less than those in the scrambled DsiRNA controls. In JNK1/2 DsiRNA transfected nonactivated keratocytes the band densities of JNK1 and JNK2 were 46.5% and 43.4% less, in FGF-2/HS-activated keratocytes they were 49.3% and 45.9% less, and in TGF-β1–activated keratocytes they 52% and 78% less, respectively, than those in the corresponding scrambled DsiRNA-transfected controls (Fig. 6A). The levels of secreted KSPGs in the JNK1/2 DsiRNA transfected cells were higher than those in the corresponding controls (Fig. 6B).
Figure 6.
TGF-β1– and FGF-2/HS–induced decrease in KSPGs is occluded by JNK1/2 DsiRNA transfection of keratocytes. Keratocytes, cultured in DMEM/F12 without FBS (SFM), were first transfected with JNK1/2 DsiRNAs or scrambled DsiRNA, then activated with 40 ng/mL FGF-2 and 5 μg/mL HS or 2 ng/mL of TGF-β1, or not activated (SF controls) for 60 hours. (A) Western blot analyses of JNK1 and JNK2 in the extracts of nonactivated (SF), and FGF-2/HS- and of TGF-β1–activated cells using secondary antibodies (IRDye 680) and an infrared imager (Odyssey; LI-COR). (A) Lanes A, control; B, transfected with scrambled DsiRNA, and C, transfected with JNK1/2 DsiRNA. Bottom panel shows tubulin bands (loading controls) in the corresponding lanes. (B) Western blot analyses of KSPGs in culture supernatants as determined using anti-KS antibody. Lanes A, D, and H, SFM; E and I, nontransfected controls; B, F, and J, transfected with scrambled DsiRNA, and C, G, and K, transfected with JNK1/2 DsiRNA.
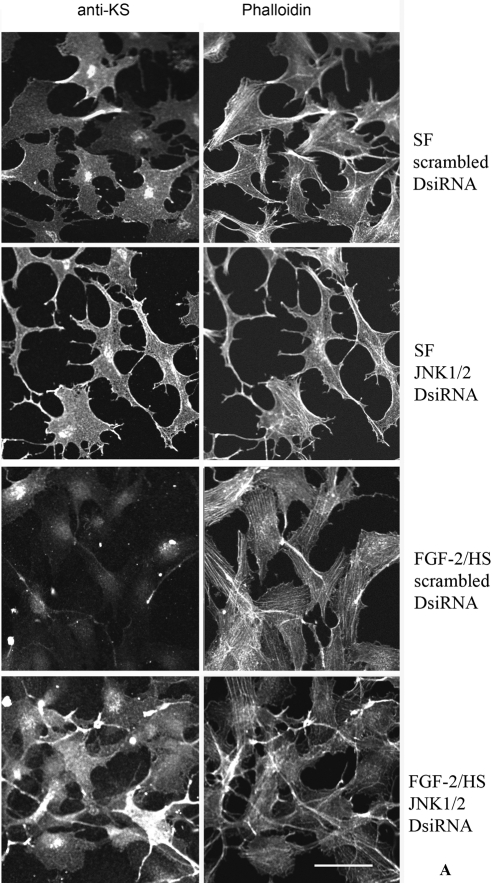
In the above experiments, where JNK1 and JNK2 were documented to be dowregulated by JNK1/2 DsiRNA transfection, a duplicate set of cells was analyzed immunocytochemically. The intensities of the fluorescent signal for cell-surface–associated KSPG(s) were diminished in the TGF-β1– or FGF-2/HS– activated keratocytes, which were transfected with scrambled DsiRNA (controls). However, the fluorescent staining of cell-surface–associated KS was evident in activated cells previously transfected with JNK1/2 DsiRNA (Fig. 7). These results indicated that JNK signaling pathway, at least in part, was responsible for the decreased KS staining in the FGF-2/HS– and TGF-β1–activated keratocytes.
Figure 7.
TGF-β1– and FGF-2/HS–induced decrease in the cell surface–associated KS is occluded by JNK1/2 transfection of keratocytes. A portion of DsiRNA transfected keratocytes, from the experiments described in Figure 5, activated with 40 ng/mL FGF-2 and 5 μg/mL HS (A), or with 2 ng/mL of TGF-β1 (B), were plated for immunocytochemical analyses. Double fluorescence staining was performed using mouse anti-KS antibody followed by Alexa Fluor 488-anti-mouse IgG antibodies and Alexa Fluor 546-phalloidin. Bar, 50 μm.
Discussion
An injury to the corneal stroma activates keratocytes to differentiate into fibroblasts and myofibroblasts which repair the wound. The phenotypic characteristics of the activated cells are influenced by extracellular components, including growth factors and cytokines. The activated keratocytes in the corneal wounds are different from those of keratocytes engaged in the embryonic and early postnatal development of corneal stroma. Notably, the ECM assembled during wound healing is not well organized and often results in the formation of nontransparent scar tissue. The growth factors, TGF-β1 and FGF-2, are responsible for many of the phenotypic changes in the activated keratocytes, including the downregulation or the loss of the expression of KSPGs which are detrimental to collagen fiber organization. These growth factors have been shown to induce downregulation of KSPGs in activated bovine21 and rabbit corneal keratocytes23 in culture. We have previously shown that Rho/ROCK inhibition suppressed FGF-2– and TGF-β1–mediated loss in the expression of keratocan and lumican.23 Therefore, we concluded that Rho-activation leads to the loss in KSPGs in the activated keratocytes. Several recent reports indicate that some of the downstream effects of Rho are regulated via the JNK signaling pathway.24,31 Therefore, in the present study, we examined whether JNK is involved in the downregulation of KSPGs in TGF-β1– and FGF-2–activated keratocytes.
Taken together, our results demonstrate that JNK activation downregulates expression of keratocan and lumican in cultured nonactivated as well as in TGF-β1– and FGF-2–activated keratocytes. Here we show that JNK inhibition increased KSPG expression in keratocytes cultured in SFM and also partially occluded the growth factor–induced decrease in the secreted and cell-associated KSPG proteins in the activated keratocytes. While total increases in the amounts of secreted keratocan and lumican, resulting from JNK inhibition with SP600125, were slightly higher in the activated than in the nonactivated keratocytes, those resulting from DSiRNA transfection were not significantly different. The increases in lumican and keratocan mRNA levels in nonactivated or activated cells, resulting from SP600125 treatments or JNK1/2 transfection, were not significantly different. Low levels of JNK1 and JNK2 were present in the nonactivated keratocytes, and they significantly increased on TGF-β1– or FGF-2–induced activation. Because the increases in the levels of expression of KSPGs, resulting from JNK inhibition, were not significantly different between activated and nonactivated keratocytes, and JNK inhibition did not completely prevent TGF-β1– or FGF-2–induced decrease in either keratocan or lumican, we speculate that activation or inhibition of other signaling pathway(s) contributes to further loss in expression of these KSPGs, independently of the JNK signaling pathway.
The occlusion of TGF-β1– or FGF-2–induced decreases in the KSPGs was relatively less when JNK1/2 expression was inhibited with JNK1/2 DsiRNA, than when JNK1/2 activity was inhibited using the chemical inhibitor SP600125. These differences were possibly due to incomplete or nonsustained downregulation of JNK1/2 activity during the activation, using the DsiRNA method. The possibility also exists that SP600125 may have some nonspecific effects which also block the loss in KSPGs. Nonetheless, the changes in KSPG protein and mRNA levels correlated with corresponding inverse changes in the JNK1/2 proteins and mRNAs. Therefore, we conclude that JNK acts, at least in part, to downregulate the transcription of lumican and keratocan during the growth factor–induced activation of keratocytes.
Zhang et al.32 reported that MAPK kinase kinase (MEKK)1-deficient mouse fetuses had normal corneal morphology and thickness, but reduced transcription of keratocan, lumican, and collagen I. Contrary to our findings, their observations suggest that the JNK pathway induces keratocan and lumican expression because MEKK1 preferentially regulates the JNK pathway. This discrepancy indicates that the downstream effects of JNK in the corneal stromal keratocytes of the developing cornea may be different from those in the reactivated ketatocytes during wound healing. JNK has been reported to regulate TGF-β1–mediated expression of connective tissue growth factor (CTGF) and fibronectin; thus, it regulates other phenotypic changes which contribute to scar tissue formation.33,34 JNK activation also regulates thromspondin- induced corneal neovascularization,35 and Toll-like receptor 2–induced corneal inflammation.36 Based on our present findings that JNK inhibition increases KSPG expression in activated keratocytes and the reported observations described above, JNK inhibition may potentially be a valuable approach to inhibit scar tissue formation following corneal stromal injury or other diseased states.
Footnotes
Supported by NIH Grant EY03263 (NS) and Core Grant EY008098; Research to Prevent Blindness; and The Eye and Ear Foundation (Pittsburgh, Pennsylvania).
Disclosure: J. Chen, None; J. Wong-Chong, None; N. SundarRaj, None
References
- 1. Benedek GB. Theory of transparency of the eye. Appl Opt. 1971;10:459–473 [DOI] [PubMed] [Google Scholar]
- 2. Meek KM, Boote C. The organization of collagen in the corneal stroma. Exp Eye Res. 2004;78:503–512 [DOI] [PubMed] [Google Scholar]
- 3. Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chakravarti S, Petroll WM, Hassell JR, et al. Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci. 2000;41:3365–3373 [PMC free article] [PubMed] [Google Scholar]
- 6. Chakravarti S. Functions of lumican and fibromodulin: lessons from knockout mice 1. Glycoconj J. 2002;19:287–293 [DOI] [PubMed] [Google Scholar]
- 7. Kao WW, Liu CY. Roles of lumican and keratocan on corneal transparency 1. Glycoconj J. 2002;19:275–285 [DOI] [PubMed] [Google Scholar]
- 8. Liu CY, Birk DE, Hassell JR, Kane B, Kao WW. Keratocan-deficient mice display alterations in corneal structure. J Biol Chem. 2003;278:21672–21677 [DOI] [PubMed] [Google Scholar]
- 9. Chakravarti S, Zhang G, Chervoneva I, Roberts L, Birk DE. Collagen fibril assembly during postnatal development and dysfunctional regulation in the lumican-deficient murine cornea 1. Dev Dyn. 2006;235:2493–2506 [DOI] [PubMed] [Google Scholar]
- 10. Ahmadi AJ, Jakobiec FA. Corneal wound healing: cytokines and extracellular matrix proteins. Int Ophthalmol Clin. 2002;42:13–22 [DOI] [PubMed] [Google Scholar]
- 11. Baldwin HC, Marshall J. Growth factors in corneal wound healing following refractive surgery: a review. Acta Ophthalmol Scand. 2002;80:238–247 [DOI] [PubMed] [Google Scholar]
- 12. Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19:113–129 [DOI] [PubMed] [Google Scholar]
- 13. Nishida T, Tanaka T. Extracellular matrix and growth factors in corneal wound healing. Curr Opin Ophthalmol. 1996;7:2–11 [DOI] [PubMed] [Google Scholar]
- 14. Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18:529–551 [DOI] [PubMed] [Google Scholar]
- 15. Funderburgh JL, Cintron C, Covington HI, Conrad GW. Immunoanalysis of keratan sulfate proteoglycan from corneal scars. Invest Ophthalmol Vis Sci. 1988;29:1116–1124 [PubMed] [Google Scholar]
- 16. SundarRaj N, Fite D, Belak R, et al. Proteoglycan distribution during healing of corneal stromal wounds in chick 266. Exp Eye Res. 1998;67:433–442 [DOI] [PubMed] [Google Scholar]
- 17. Brown CT, Applebaum E, Banwatt R, Trinkaus-Randall V. Synthesis of stromal glycosaminoglycans in response to injury. J Cell Biochem. 1995;59:57–68 [DOI] [PubMed] [Google Scholar]
- 18. Carlson EC, Wang IJ, Liu CY, Brannan P, Kao CW, Kao WW. Altered KSPG expression by keratocytes following corneal injury. Mol Vis. 2003;9:615–623 [PubMed] [Google Scholar]
- 19. Funderburgh JL, Funderburgh ML, Mann MM, Prakash S, Conrad GW. Synthesis of corneal keratan sulfate proteoglycans by bovine keratocytes in vitro. J Biol Chem. 1996;271:31431–31436 [DOI] [PubMed] [Google Scholar]
- 20. Beales MP, Funderburgh JL, Jester JV, Hassell JR. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Invest Ophthalmol Vis Sci. 1999;40:1658–1663 [PubMed] [Google Scholar]
- 21. Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta-induced keratocyte-myofibroblast transdifferentiation 9. J Biol Chem. 2001;276:44173–44178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis 6. J Biol Chem. 2003;278:45629–45637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen J, Guerriero E, Sado Y, SundarRaj N. Rho-mediated regulation of TGF-beta1- and FGF-2-induced activation of corneal stromal keratocytes. Invest Ophthalmol Vis Sci. 2009;50:3662–3670 [DOI] [PubMed] [Google Scholar]
- 24. Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895 [DOI] [PubMed] [Google Scholar]
- 25. Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor beta-mediated signaling. J Biol Chem. 1997;272:1429–1432 [DOI] [PubMed] [Google Scholar]
- 26. Shimada H, Rajagopalan LE. Rho-kinase mediates lysophosphatidic acid-induced IL-8 and MCP-1 production via p38 and JNK pathways in human endothelial cells. FEBS Lett. 2010;584:2827–2832 [DOI] [PubMed] [Google Scholar]
- 27. Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505–516 [PubMed] [Google Scholar]
- 28. Guerriero E, Chen J, Sado Y, et al. Loss of alpha3(IV) collagen expression associated with corneal keratocyte activation 3. Invest Ophthalmol Vis Sci. 2007;48:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oike Y, Kimata K, Shinomura T, Nakazawa K, Suzuki S. Structural analysis of chick-embryo cartilage proteoglycan by selective degradation with chondroitin lyases (chondroitinases) and endo-beta-D-galactosidase (keratanase). Biochem J. 1980;191:193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen J, Guerriero E, Lathrop K, SundarRaj N. Rho/ROCK signaling in regulation of corneal epithelial cell cycle progression 1. Invest Ophthalmol Vis Sci. 2008;49:175–183 [DOI] [PubMed] [Google Scholar]
- 31. Shirai H, Autieri M, Eguchi S. Small GTP-binding proteins and mitogen-activated protein kinases as promising therapeutic targets of vascular remodeling. Curr Opin Nephrol Hypertens. 2007;16:111–115 [DOI] [PubMed] [Google Scholar]
- 32. Zhang L, Deng M, Kao CW, Kao WW, Xia Y. MEK kinase 1 regulates c-Jun phosphorylation in the control of corneal morphogenesis. Mol Vis. 2003;9:584–593 [PubMed] [Google Scholar]
- 33. Chang Y, Wu XY. The role of c-Jun N-terminal kinases 1/2 in transforming growth factor beta(1)-induced expression of connective tissue growth factor and scar formation in the cornea. J Int Med Res. 2009;37:727–736 [DOI] [PubMed] [Google Scholar]
- 34. Chang Y, Wu XY. JNK1/2 siRNA inhibits transforming-growth factor-beta1-induced connective tissue growth factor expression and fibrotic function in THSFs. Mol Cell Biochem. 2010;335:83–89 [DOI] [PubMed] [Google Scholar]
- 35. Jimenez B, Volpert OV, Reiher F, et al. c-Jun N-terminal kinase activation is required for the inhibition of neovascularization by thrombospondin-1. Oncogene. 2001;20:3443–3448 [DOI] [PubMed] [Google Scholar]
- 36. Adhikary G, Sun Y, Pearlman E. C-Jun NH2 terminal kinase (JNK) is an essential mediator of Toll-like receptor 2-induced corneal inflammation. J Leukoc Biol. 2008;83:991–997 [DOI] [PMC free article] [PubMed] [Google Scholar]