Abstract
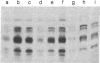
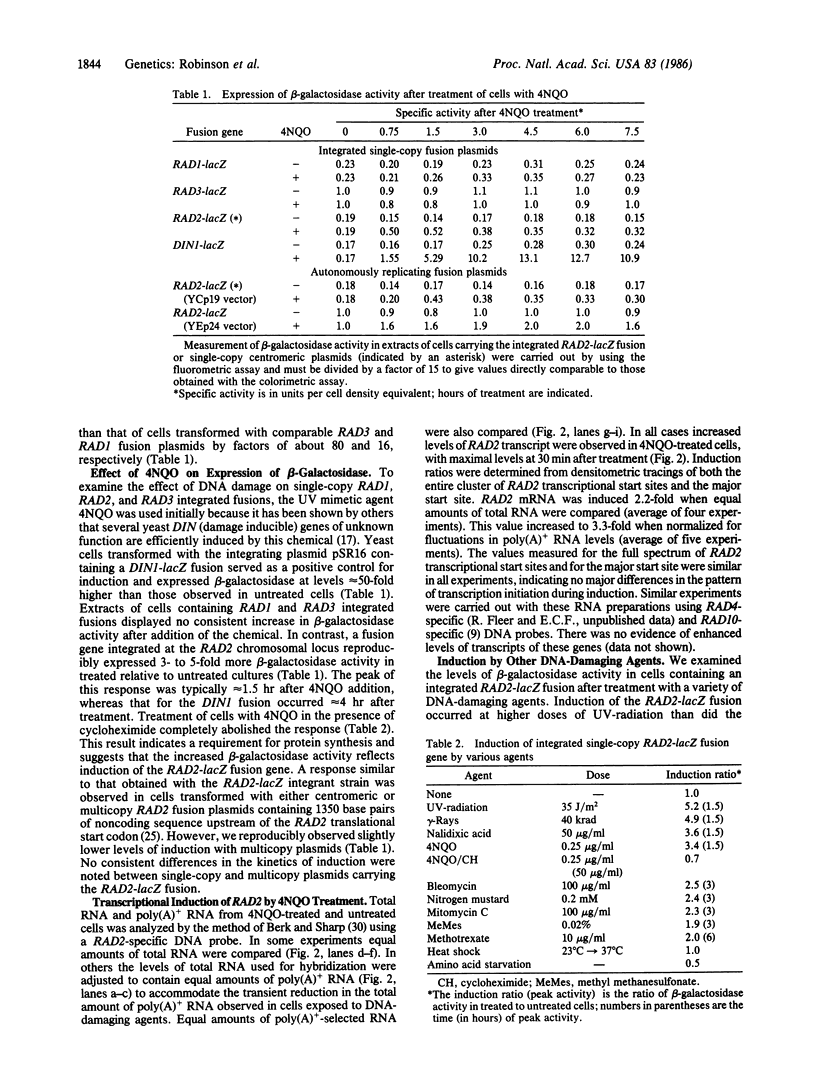
Plasmids containing various RAD-lacZ gene fusions were integrated into the chromosome of haploid yeast cells. These integrant strains were tested for expression of Escherichia coli beta-galactosidase after treatment with agents that damage DNA or interfere with normal DNA replication. We did not observe induction of single-copy RAD1-lacZ or RAD3-lacZ fusion genes under the experimental conditions used. However, exposure of cells containing an integrated RAD2-lacZ fusion gene to UV-radiation, gamma-radiation, 4-nitroquinoline 1-oxide, or nalidixic acid resulted in 4- to 6-fold enhanced expression of beta-galactosidase. Induction of the RAD2 gene after treatment of untransformed cells with 4-nitroquinoline 1-oxide was confirmed by direct examination of RAD2 mRNA. Lower levels of induction (approximately equal to 50%) were observed after treatment of cells with other chemicals. Induction of the RAD2-lacZ fusion gene was also observed in cells transformed with single-copy and multicopy autonomously replicating plasmids.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Polynucleotide sequences in eukaryotic DNA and RNA that form ribonuclease-resistant complexes with polyuridylic acid. J Mol Biol. 1974 May 5;85(1):75–86. doi: 10.1016/0022-2836(74)90130-2. [DOI] [PubMed] [Google Scholar]
- Brazzell C., Ingolia T. D. Stimuli that induce a yeast heat shock gene fused to beta-galactosidase. Mol Cell Biol. 1984 Dec;4(12):2573–2579. doi: 10.1128/mcb.4.12.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Forster J. W., Strike P. Organisation and control of the Escherichia coli uvrC gene. Gene. 1985;35(1-2):71–82. doi: 10.1016/0378-1119(85)90159-3. [DOI] [PubMed] [Google Scholar]
- Goto T., Wang J. C. Yeast DNA topoisomerase II. An ATP-dependent type II topoisomerase that catalyzes the catenation, decatenation, unknotting, and relaxation of double-stranded DNA rings. J Biol Chem. 1982 May 25;257(10):5866–5872. [PubMed] [Google Scholar]
- Herrlich P., Mallick U., Ponta H., Rahmsdorf H. J. Genetic changes in mammalian cells reminiscent of an SOS response. Hum Genet. 1984;67(4):360–368. doi: 10.1007/BF00291392. [DOI] [PubMed] [Google Scholar]
- Higgins D. R., Prakash L., Reynolds P., Prakash S. Isolation and characterization of the RAD2 gene of Saccharomyces cerevisiae. Gene. 1984 Oct;30(1-3):121–128. doi: 10.1016/0378-1119(84)90112-4. [DOI] [PubMed] [Google Scholar]
- Higgins D. R., Prakash S., Reynolds P., Polakowska R., Weber S., Prakash L. Isolation and characterization of the RAD3 gene of Saccharomyces cerevisiae and inviability of rad3 deletion mutants. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5680–5684. doi: 10.1073/pnas.80.18.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. R., Prakash S., Reynolds P., Prakash L. Molecular cloning and characterization of the RAD1 gene of Saccharomyces cerevisiae. Gene. 1983 Dec;26(2-3):119–126. doi: 10.1016/0378-1119(83)90181-6. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse H., Guesdon J. L., Ragimbeau J., Avrameas S. Miniaturization of beta-galactosidase immunoassays using chromogenic and fluorogenic substrates. J Immunol Methods. 1982;48(2):133–147. doi: 10.1016/0022-1759(82)90188-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McClanahan T., McEntee K. Specific transcripts are elevated in Saccharomyces cerevisiae in response to DNA damage. Mol Cell Biol. 1984 Nov;4(11):2356–2363. doi: 10.1128/mcb.4.11.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal M. L., Higgins D. R., Prakash S. Expression of the RAD1 and RAD3 genes of Saccharomyces cerevisiae is not affected by DNA damage or during the cell division cycle. Mol Gen Genet. 1985;199(1):59–63. doi: 10.1007/BF00327510. [DOI] [PubMed] [Google Scholar]
- Naumovski L., Chu G., Berg P., Friedberg E. C. RAD3 gene of Saccharomyces cerevisiae: nucleotide sequence of wild-type and mutant alleles, transcript mapping, and aspects of gene regulation. Mol Cell Biol. 1985 Jan;5(1):17–26. doi: 10.1128/mcb.5.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumovski L., Friedberg E. C. Molecular cloning of eucaryotic genes required for excision repair of UV-irradiated DNA: isolation and partial characterization of the RAD3 gene of Saccharomyces cerevisiae. J Bacteriol. 1982 Oct;152(1):323–331. doi: 10.1128/jb.152.1.323-331.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumovski L., Friedberg E. C. Saccharomyces cerevisiae RAD2 gene: isolation, subcloning, and partial characterization. Mol Cell Biol. 1984 Feb;4(2):290–295. doi: 10.1128/mcb.4.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolet C. M., Chenevert J. M., Friedberg E. C. The RAD2 gene of Saccharomyces cerevisiae: nucleotide sequence and transcript mapping. Gene. 1985;36(3):225–234. doi: 10.1016/0378-1119(85)90177-5. [DOI] [PubMed] [Google Scholar]
- Oishi M., Irbe R. M., Morin L. M. Molecular mechanism for the induction of "SOS" functions. Prog Nucleic Acid Res Mol Biol. 1981;26:281–301. doi: 10.1016/s0079-6603(08)60412-2. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Genetic applications of yeast transformation with linear and gapped plasmids. Methods Enzymol. 1983;101:228–245. doi: 10.1016/0076-6879(83)01017-4. [DOI] [PubMed] [Google Scholar]
- Peterson T. A., Prakash L., Prakash S., Osley M. A., Reed S. I. Regulation of CDC9, the Saccharomyces cerevisiae gene that encodes DNA ligase. Mol Cell Biol. 1985 Jan;5(1):226–235. doi: 10.1128/mcb.5.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L., Dumais D., Polakowska R., Perozzi G., Prakash S. Molecular cloning of the RAD10 gene of Saccharomyces cerevisiae. Gene. 1985;34(1):55–61. doi: 10.1016/0378-1119(85)90294-x. [DOI] [PubMed] [Google Scholar]
- Reynolds R. J., Friedberg E. C. Molecular mechanisms of pyrimidine dimer excision in Saccharomyces cerevisiae: incision of ultraviolet-irradiated deoxyribonucleic acid in vivo. J Bacteriol. 1981 May;146(2):692–704. doi: 10.1128/jb.146.2.692-704.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M., Botstein D. Structure and function of the yeast URA3 gene. Differentially regulated expression of hybrid beta-galactosidase from overlapping coding sequences in yeast. J Mol Biol. 1983 Nov 15;170(4):883–904. doi: 10.1016/s0022-2836(83)80193-4. [DOI] [PubMed] [Google Scholar]
- Ruby S. W., Szostak J. W. Specific Saccharomyces cerevisiae genes are expressed in response to DNA-damaging agents. Mol Cell Biol. 1985 Jan;5(1):75–84. doi: 10.1128/mcb.5.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin A. SOS response in mammalian cells. Cancer Invest. 1985;3(2):163–174. doi: 10.3109/07357908509017499. [DOI] [PubMed] [Google Scholar]
- Shen L. L., Pernet A. G. Mechanism of inhibition of DNA gyrase by analogues of nalidixic acid: the target of the drugs is DNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):307–311. doi: 10.1073/pnas.82.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siede W., Eckardt F. Inducibility of error-prone DNA repair in yeast? Mutat Res. 1984 Oct;129(1):3–11. doi: 10.1016/0027-5107(84)90116-7. [DOI] [PubMed] [Google Scholar]
- Siede W., Obermaier S., Eckardt F. Influence of different inhibitors on the activity of the RAD54 dependent step of DNA repair in Saccharomyces cerevisiae. Radiat Environ Biophys. 1985;24(1):1–7. doi: 10.1007/BF01212648. [DOI] [PubMed] [Google Scholar]
- Weiss W. A., Friedberg E. C. Molecular cloning and characterization of the yeast RAD10 gene and expression of RAD10 protein in E. coli. EMBO J. 1985 Jun;4(6):1575–1582. doi: 10.1002/j.1460-2075.1985.tb03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox D. R., Prakash L. Incision and postincision steps of pyrimidine dimer removal in excision-defective mutants of Saccharomyces cerevisiae. J Bacteriol. 1981 Nov;148(2):618–623. doi: 10.1128/jb.148.2.618-623.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E., Friedberg E. C. Molecular cloning and nucleotide sequence analysis of the Saccharomyces cerevisiae RAD1 gene. Mol Cell Biol. 1984 Oct;4(10):2161–2169. doi: 10.1128/mcb.4.10.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]