Abstract
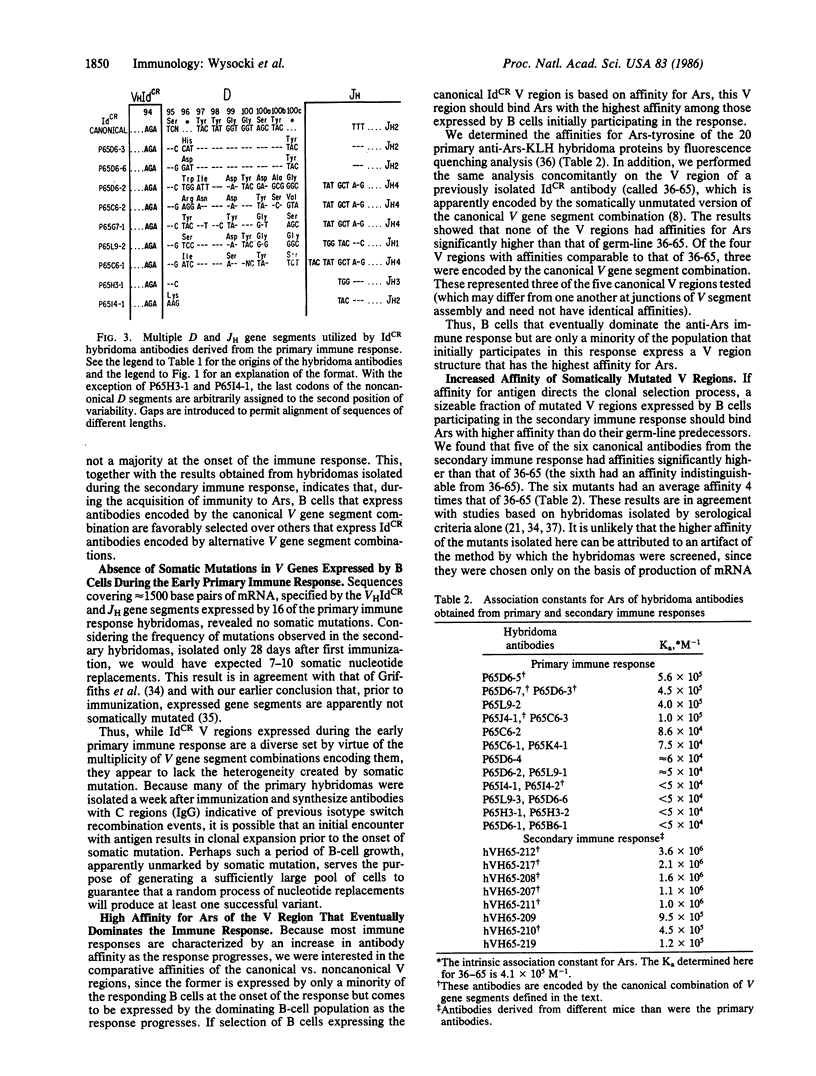
Immunization of strain A mice with p-azophenylarsonate-conjugated protein stimulates B cells that synthesize anti-p-azophenylarsonate antibodies. A large fraction of these cells produce antibodies with variable (V) regions encoded by a single heavy chain V gene segment together with multiple combinations of diversity, heavy chain joining, light chain variable, and light chain joining gene segments. Early in the immune response, these V regions are not somatically mutated. One of these V regions is initially expressed by only a minority of the responding B cells but binds p-azophenylarsonate with the highest affinity. After a secondary immunization, B cells synthesizing mutated derivatives of this single V region dominate the response and bind p-azophenylarsonate with even higher affinity than does the unmutated V region. These results suggest that antigen directs both the expression of the immune repertoire and the amplification of V region diversity by a sequential process of clonal selection of B cells expressing receptor antibodies encoded by unmutated V genes, induction of mutation in the V genes expressed by the selected cells, and reselection of B cells expressing antibodies with mutated V regions of higher affinity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan S. S., Ball R. K., Chang J. Y., Braun D. G. Heterogeneity of cross-reactive idiotypes. Serological and structural analysis of monoclonal anti-p-azobenzene-arsonate antibodies expressing major and minor idiotypes. Mol Immunol. 1983 Feb;20(2):203–211. doi: 10.1016/0161-5890(83)90132-3. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball R. K., Chang J. Y., Alkan S. S., Braun D. G. The complete amino acid sequence of the light chain variable region of two monoclonal anti-p-azobenzene-arsonate antibodies bearing the cross-reactive idiotype. Mol Immunol. 1983 Feb;20(2):197–201. doi: 10.1016/0161-5890(83)90131-1. [DOI] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Goidl E. A., Paul W. E., Siskind G. W., Benacerraf B. The effect of antigen dose and time after immunization on the amount and affinity of anti-hapten antibody. J Immunol. 1968 Feb;100(2):371–375. [PubMed] [Google Scholar]
- Gridley T., Margolies M. N., Gefter M. L. The association of various D elements with a single-immunoglobulin VH gene segment: influence on the expression of a major cross-reactive idiotype. J Immunol. 1985 Feb;134(2):1236–1244. [PubMed] [Google Scholar]
- Griffiths G. M., Berek C., Kaartinen M., Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984 Nov 15;312(5991):271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- Henry C., Kimura J., Wofsy L. Cell separation on affinity columns: the isolation of immunospecific precursor cells from unimmunized mice (lactoside hapten-lymphocyte receptors-immunology). Proc Natl Acad Sci U S A. 1972 Jan;69(1):34–36. doi: 10.1073/pnas.69.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T. Immunoglobulin genes. Annu Rev Immunol. 1983;1:499–528. doi: 10.1146/annurev.iy.01.040183.002435. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Herzenberg L. A. Isolation of antigen-binding cells from unprimed mice: demonstration of antibody-forming cell precursor activity and correlation between precursor and secreted antibody avidities. J Exp Med. 1974 Oct 1;140(4):904–920. doi: 10.1084/jem.140.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen M., Griffiths G. M., Hamlyn P. H., Markham A. F., Karjalainen K., Pelkonen J. L., Mäkelä O., Milstein C. Anti-oxazolone hybridomas and the structure of the oxazolone idiotype. J Immunol. 1983 Feb;130(2):937–945. [PubMed] [Google Scholar]
- Kim S., Davis M., Sinn E., Patten P., Hood L. Antibody diversity: somatic hypermutation of rearranged VH genes. Cell. 1981 Dec;27(3 Pt 2):573–581. doi: 10.1016/0092-8674(81)90399-8. [DOI] [PubMed] [Google Scholar]
- Kuettner M. G., Wang A. L., Nisonoff A. Quantitative investigations of idiotypic antibodies. VI. Idiotypic specificity as a potential genetic marker for the variable regions of mouse immunoglobulin polypeptide chains. J Exp Med. 1972 Mar 1;135(3):579–595. doi: 10.1084/jem.135.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LIND P. E., BURNET F. M. Recombination between virulent and non-virulent strains of influenza virus. II. The behaviour of virulence markers on recombination. Aust J Exp Biol Med Sci. 1957 Feb;35(1):67–78. doi: 10.1038/icb.1957.8. [DOI] [PubMed] [Google Scholar]
- Manser T., Gefter M. L. Isolation of hybridomas expressing a specific heavy chain variable region gene segment by using a screening technique that detects mRNA sequences in whole cell lysates. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2470–2474. doi: 10.1073/pnas.81.8.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser T., Huang S. Y., Gefter M. L. Influence of clonal selection on the expression of immunoglobulin variable region genes. Science. 1984 Dec 14;226(4680):1283–1288. doi: 10.1126/science.6334361. [DOI] [PubMed] [Google Scholar]
- Margolies M. N., Marshak-Rothstein A., Gefter M. L. Structural diversity among anti-p-azophenylarsonate monoclonal antibodies from A/J mice; comparison of Id- and Id+ sequences. Mol Immunol. 1981 Dec;18(12):1065–1077. doi: 10.1016/0161-5890(81)90022-5. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Siekevitz M., Margolies M. N., Mudgett-Hunter M., Gefter M. L. Hybridoma proteins expressing the predominant idiotype of the antiazophenylarsonate response of A/J mice. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1120–1124. doi: 10.1073/pnas.77.2.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner E. C., Capra J. D. VH families in the antibody response to p-azophenylarsonate: correlation between serology and amino acid sequence. J Immunol. 1982 Jul;129(1):193–199. [PubMed] [Google Scholar]
- Nussenzweig V., Benacerraf B. Antihapten antibody specificity and L chain type. J Exp Med. 1967 Oct 1;126(4):727–743. doi: 10.1084/jem.126.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell J. D., Gearhart P. J., Karush F. Restriction in IgM expression. IV. Affinity analysis of monoclonal anti-phosphorylcholine antibodies. J Immunol. 1983 Jan;130(1):313–316. [PubMed] [Google Scholar]
- Rothstein T. L., Gefter M. L. Affinity analysis of idiotype-positive and idiotype-negative Ars-binding hybridoma proteins and Ars-immune sera. Mol Immunol. 1983 Feb;20(2):161–168. doi: 10.1016/0161-5890(83)90127-x. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Selsing E., Storb U. Somatic mutation of immunoglobulin light-chain variable-region genes. Cell. 1981 Jul;25(1):47–58. doi: 10.1016/0092-8674(81)90230-0. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Siegelman M., Capra J. D. Complete amino acid sequence of light chain variable regions derived from five monoclonal anti-p-azophenylarsonate antibodies differing with respect to a crossreactive idiotype. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7679–7683. doi: 10.1073/pnas.78.12.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Huang S. Y., Gefter M. L. The genetic basis of antibody production: a single heavy chain variable region gene encodes all molecules bearing the dominant anti-arsonate idiotype in the strain A mouse. Eur J Immunol. 1983 Feb;13(2):123–132. doi: 10.1002/eji.1830130207. [DOI] [PubMed] [Google Scholar]
- Slaughter C. A., Capra J. D. Amino acid sequence diversity within the family of antibodies bearing the major antiarsonate cross-reactive idiotype of the A strain mouse. J Exp Med. 1983 Nov 1;158(5):1615–1634. doi: 10.1084/jem.158.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velick S. F., Parker C. W., Eisen H. N. EXCITATION ENERGY TRANSFER AND THE QUANTITATIVE STUDY OF THE ANTIBODY HAPTEN REACTION. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1470–1482. doi: 10.1073/pnas.46.11.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M., Perry R., Kelley D., Hunkapiller T., Schilling J., Hood L. The joining of V and J gene segments creates antibody diversity. Nature. 1980 Jan 31;283(5746):497–499. doi: 10.1038/283497a0. [DOI] [PubMed] [Google Scholar]
- Woodland R., Cantor H. Idiotype-specific T helper cells are required to induce idiotype-positive B memory cells to secrete antibody. Eur J Immunol. 1978 Aug;8(8):600–606. doi: 10.1002/eji.1830080812. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Margolies M. N., Huang B., Nemazee D. A., Wechsler D. S., Sato V. L., Smith J. A., Gefter M. L. Combinational diversity within variable regions bearing the predominant anti-p-azophenylarsonate idiotype of strain A mice. J Immunol. 1985 Apr;134(4):2740–2747. [PubMed] [Google Scholar]






