Abstract
MicroRNA (miRNA) expression in fetal human retinal pigment epithelium (hfRPE), retina, and choroid were pairwise compared to determine those miRNAs that are enriched by 10-fold or more in each tissue compared with both of its neighbors. miRs-184, 187, 200a/200b, 204/211, and 221/222 are enriched in hfRPE by 10- to 754-fold compared with neuroretina or choroid (P<0.05). Five of these miRNAs are enriched in RPE compared with 20 tissues throughout the body and are 10- to 20,000-fold more highly expressed (P<0.005). miR-204 and 211 are the most highly expressed in the RPE. In addition, expression of miR-204/211 is significantly lower in the NCI60 tumor cell line panel compared with that in 13 normal tissues, suggesting the progressive disruption of epithelial barriers and increased proliferation. We demonstrated that TGF-β receptor 2 (TGF-βR2) and SNAIL2 are direct targets of miR-204 and that a reduction in miR-204 expression leads to reduced expression of claudins 10, 16, and 19 (message/protein) consistent with our observation that anti-miR-204/211 decreased transepithelial resistance by 80% and reduced cell membrane voltage and conductance. The anti-miR-204-induced decrease in Kir7.1 protein levels suggests a signaling pathway that connects TGF-βR2 and maintenance of potassium homeostasis. Overall, these data indicate a critical role for miR-204/211 in maintaining epithelial barrier function and cell physiology.—Wang, F. E., Zhang, C., Maminishkis, A., Dong, L., Zhi, C., Li, R., Zhao, J., Majerciak, V., Gaur, A. B., Chen, S., Miller, S. S. MicroRNA-204/211 alters epithelial physiology.
Keywords: tumorigenesis, transepithelial resistance, membrane potential, tight junctions, claudins, retinal pigment epithelium
Epithelia are fundamentally important for maintaining the health and integrity of organs and cavities throughout the body. They maintain an asymmetric distribution of proteins on their apical and basolateral membrane surfaces that carry out the selective and regulated flux of ions and metabolites via transcellular or paracellular pathways (1). In the paracellular pathway, tight junctions (TJs) form a continuous seal or barrier between the extracellular spaces that face the apical and basolateral membranes and are critically important in determining epithelial cell polarity (2). The molecular composition of the TJ determines its conductance and ion selectivity (3), and transport across the TJ is determined by a combination of conductance and transepithelial electrical and chemical driving forces (4).
In the distal retina, the apical membrane processes of the retinal pigment epithelium (RPE) have a close anatomical relationship with the photoreceptor outer segments (5). Because of this anatomic arrangement, the RPE plays a critical role in regulating and maintaining the microenvironments within the retina/RPE complex. This complex includes the subretinal (or extracellular) space facing the apical membrane and the choriocapillaris that faces the basal side of the epithelium. The choroidal blood supply is the main source of oxygen and nutrition for the photoreceptors. After light-dark transitions, the RPE participates with the photoreceptors in a wide range of mechanical, metabolic, biochemical, and electrical interactions that include photoreceptor outer segment renewal, vitamin A transport and metabolism, and regulation of subretinal space volume/chemical composition (6,7,8,9,10,11,12). Oxidative damage and immunological insult to the RPE monolayer are thought to be early events in age-related macular degeneration (AMD), a major cause of severe vision loss in people older than age 60 (13, 14). In addition, there are significant numbers of inherited retinal degenerative diseases (AMD included) that are initiated by gene mutations in the RPE (15,16,17,18).
MicroRNAs (miRNAs) are a class of evolutionarily conserved small noncoding RNAs that induce translational repression or mRNA degradation (19). More than 600 human miRNAs have been identified (20), and targets of miRNA have been predicted to cover ≥30% of human genes (21). Most miRNAs imperfectly base pair with the 3′ untranslated region (3′-UTR), and the 5′ proximal “seed” region of miRNAs provides most of the pairing specificity (22). On average, each miRNA regulates ∼200 target mRNAs (23). miRNA expression profiling with microarray or quantitative PCR has been used to associate changes in miRNA expression with different tissues or developmental, physiological, or pathological states (24, 25). Microarray studies have confirmed the existence of several tissue-specific miRNAs, and miRNA profiles for several human cancers suggest that these profiles could serve as tumor markers to help predict the efficacy of therapies (26, 27). The involvement of miRNAs in the development and function of ocular tissues is just beginning to be investigated (25, 28).
We profiled miRNA expression in human fetal RPE (hfRPE) and the adjacent neuroretina and choroid and identified 8 miRNAs (miR-184, 187, 200a, 200b, 204, 211, 221, and 222) that are relatively enriched in RPE. Two of these miRNAs, miR-204 and 211, are very highly expressed in RPE compared with other tissues throughout the body. They share the same seed-region sequence and only differ by two nucleotides in the 3′ region and have been classified as one family with the same set of predicted targets (TargetScan) (23). Tissue specificity of these two microRNAs in humans is possibly a result of differential expression of the miR host genes. In the absence of disease, human RPE is quiescent throughout the life of the organism. The present experiments describe a regulatory pathway by which miR-204/211 can maintain TJ integrity and therefore help ensure a stable and intact blood-retina barrier (1, 3, 29). It will be interesting to learn whether this regulatory pathway is used more widely throughout the body.
MATERIALS AND METHODS
Human tissues
Human fetal eyes [16–20 wk of gestation (WG)] were obtained from Advanced Bioscience Resources, Inc. (Alameda, CA, USA). The research followed the tenets of the Declaration of Helsinki and was reviewed and approved by the National Institutes of Health Institutional Review Board. The Calu-3 cell line was a generous gift of Dr. Terry Machen (University of California, Berkeley, CA, USA).
Primary cultures of hfRPE
All reagents were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA), unless otherwise indicated. Cells were prepared as described previously (10). The confluent cells were seeded onto clear extracellular matrix (ECM)-coated 12-well Transwells at 1–2 × 105 cells/well (Costar, Cambridge, MA, USA). Fully differentiated RPE monolayers were used in experiments unless otherwise indicated. Transepithelial electrical resistance (TER) was measured with an epithelial voltohmmeter (EVOM) device (World Precision Instruments, Sarasota, FL, USA).
RNA extraction
Human fetal eyes at 16 or 20 WG (3 pairs each) were dissected to obtain retina, RPE, and choroid RNA and miRNA using a mirVana miRNA isolation kit (Ambion, Austin, TX, USA). RNA from cultured hfRPE, Calu-3 cells, and melanocytes were extracted using the same protocol as above. FirstChoice Human Total RNA Survey Panels containing 20 normal adult human tissues were purchased from Ambion and pooled from 3 donors. This panel includes adipose, bladder, brain, cervix, colon, esophagus, heart, kidney, liver, lung, ovary, placenta, prostate, skeletal muscle, small intestine, spleen, testes, thymus, thyroid, and trachea. Total RNA for the two matched pairs of tumor tissues and adjacent normal tissue (kidney and lung) and two tumor cell lines (MCF-7 and G-401) were purchased from Ambion.
miRNA profiling
A TaqMan MicroRNA Assays Human Panel Early Access Kit containing 157 miRNAs was used. RT was performed using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) (24).
Real-time PCR was performed on a 7900HT Sequence Detection System (Applied Biosystems). The threshold cycle (Ct) is defined as the fractional cycle number at which the fluorescence passes the fixed threshold. In cultured hfRPE, 136 miRNAs were detected, and 95% of detected miRNAs had a less than 2-fold difference between technical repeats (ΔΔCt<1) (Supplemental Fig. 1). For miRNA expression in human RPE, neuroretina, and choroid, the expression level of each miRNA relative to the mean Ct of let-7a and miR-16 was determined using the 2−ΔΔCt method (24). Expression for miRNA in normal tissue or tumor cell lines were normalized using miR-16 alone because let-7a has greater variance among different tissues. The miRNA expression profile for the NCI60 panel of 59 tumor cell lines and some normal tissues was obtained as described previously (26).
Quantitative RT (qRT)-PCR for mRNA/gene expression
Total RNA was extracted from the cultured hfRPE cells using a mirVana kit followed by an RNeasy Mini Cleanup Kit (Qiagen, Valencia, CA, USA). For RT reactions 1 μg of total RNA, oligo(dT)12–18 primer (Invitrogen, Carlsbad, CA, USA), and Omniscript Reverse Transcriptase (Qiagen) were used according to the Omniscript protocol. TaqMan PCR for 55 genes was done with an 7900HT Sequence Detection System in a 10-μl PCR reaction for every gene of each sample. These genes were selected based on miRNA target prediction of known physiological pathways. The relative mRNA quantity of each gene was normalized against total RNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the using the 2−ΔΔCt method (30).
Data analysis
For each sample, we normalized Ct for miRNAs to those of the references (mean Ct of let-7a and miR-16) using ΔCt = Ctsample − Ctreference. The sd for ΔCt is calculated as follows: sdΔCt = [(sdCtreference)2+(sdCtsample)2]1/2. In comparing RPE to neuroretina or to choroid, the latter tissue, neuroretina or choroid, is designated as a calibrator, where ΔΔCt = ΔCtsample − ΔCtcalibrator(30) and the sds for ΔΔCt and ΔCt are the same because the calibrator is set as an arbitrary constant. The range of fold difference (2−ΔΔCt) was calculated using the mean ΔΔCt from three biological repeats by incorporating the sd of ΔΔCt.
An miRNA was defined as “enriched” in RPE if its expression level was significantly higher (>10-fold) than that in retina and choroid at both 16 and 20 WG (four pairs of comparison). Statistical comparisons were made using the Student’s t test (two-tailed; unpaired samples with unequal variances). P < 0.05 was regarded as significant.
Northern blots
Total RNA was obtained from human fetal retina, RPE, and choroid as well as from primary cultures of hfRPE and analyzed by Northern blotting as described previously (31). Total RNA was similarly obtained from a panel of normal and malignant tissues (LifeSpan Biosciences, Inc., Seattle, WA, USA, and Cybrdi, Inc., Rockville, MD, USA). In brief, 10 μg of RNA pooled from three individual donors was fractionated on a precast 15% denaturing polyacrylamide gel containing 6 M urea (Invitrogen) in tris-borate-EDTA according to the manufacturer’s recommendations and transferred to the positively charged nylon membrane GeneScreen (PerkinElmer Life and Analytical Sciences, Waltham, MA, USA) in 20× SSC overnight. Blotted RNA was fixed by UV cross-linking to the membrane. LNA spiked oligonucleotide probes specific to miR-204, miR-211, and miR-96 as well as the U6 small nuclear RNA (synthesized by Exiqon, Vedbaek, Denmark) were end-labeled with [γ-32P]ATP (PerkinElmer Life and Analytical Sciences) by T4 kinase (Invitrogen) reactions. The same membrane was hybridized to each of the probes sequentially in PerfectHyb Plus hybridization solution (Sigma-Aldrich) overnight at 68°C. Hybridization signals to each of the probes were collected with a phosphor imaging screen on a Typhoon 9410 scanner (GE Healthcare, Little Chalfont, UK) with ImageQuant software. Before hybridizing to the next probe, the membrane was stripped off the previous probe in 0.1% SDS and 5 mM EDTA at 100°C for 5 min.
In situ hybridization (ISH)
A human fetal eye (16 WG) was fixed by overnight immersion in 4% paraformaldehyde and was then embedded in paraffin (HistoServ, Inc., Gaithersburg, MD, USA). From the whole eye, 6-μm-thick sections were collected, observing RNA handling techniques. Tissue depigmentation was performed following a protocol, with a minor modification, kindly provided by Dr. Enrique Rodriguez-Boulan (Dyson Vision Research Institute, Cornell University, New York, NY, USA). In brief, the slides were incubated in a buffer containing 25% deionized formamide, 25% H2O2, and 1× SSC. Pigment removal was performed by a 10-min incubation under a fluorescent tube light, which was 100 cm away. Detection of microRNAs by ISH was performed following a modified protocol of microRNA ISH with digoxigenin (Dig)-labeled LNA probes developed by Exiqon (Dr. Boye Schnack Nielsen; http://www.exiqon.com). The LNA contains a modified sugar-phosphate backbone of the oligonucleotide, which increases thermal stability and improves detection sensitivity. In brief, deparaffinized eye sections were treated with proteinase K (2 μg/ml) at 37°C. Hybridization was performed with double Dig-labeled LNA oligonucleotide (60 nM) in a hybridization buffer (Exiqon) for 2 h at 55°C. The slides were washed with 0.2× SSC at room temperature. Dig was detected using sheep anti-Dig-alkaline phosphates (APs) (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer’s protocol. The slides were incubated with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate in AP buffer containing 0.2 mM levamisole for 2 h, counterstained with Nuclear Fast Red (Vector Laboratories, Burlingame, CA, USA) and mounted with Eukitt (VWR, West Chester, PA, USA). Adjacent tissue sections were hybridized with miR-204 or miR-126 probes or small nuclear RNA (U6) probes to verify RNA preservation in the tissue or a scrambled probe (negative control).
ELISA and Western blots
hfRPE differentiated on inserts was transfected in triplicate with anti-miR-204 or anti-miR-211 for 4 d. Conditioned media were collected at 4 d after transfection and a transthyretin (prealbumin) ELISA was performed according to the manufacturer’s instructions (AssayPro, St. Charles, MO, USA). For the Western blots, 2-mo-old cultures were harvested with a cell scraper and pelleted at 4°C. Twenty micrograms of total protein was loaded into each lane of a 4–12% polyacrylamide gradient gel (NuPAGE gel; Invitrogen) under reducing conditions and transferred to PVDF membranes. The blotted membranes were incubated with antibodies against human claudin 10 (sc-25710; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), claudin 16 (ab42484; Abcam, Cambridge, MA, USA), claudin 19 (kindly provided by Dr. Furuse, University Graduate School of Medicine, Kobe, Japan), SNAIL, SNAIL2, TGF-β receptor 2 (TGF-βR2), KCNA3, and Smad3 (ab70983, ab75629, ab53168, ab54526, and ab28379; Abcam), TGF-βR1 (AF3025; R&D Systems, Minneapolis, MN, USA), Kir7.1 (generous gift from Dr. Bret Hughes, University of Michigan/Kellogg Eye Center, Ann Harbor, MI, USA), and c-Jun (sc-1694, Santa Cruz Biotechnology. Inc.). Immunoblot signals were detected using a West Dura Chemiluminescence system (Pierce Biotechnology, Rockford, IL, USA) and imaged using an Autochemie system (UVP, Upland, CA, USA). Protein bands were quantified using Labworks software (UVP).
Anti-miRNA transfection
Anti-miRNA oligonucleotides (miRIDIAN human microRNA Inhibitors; Dharmacon RNA Technologies, Lafayette, CO, USA) were transfected into cultured hfRPE cells using DharmaFECT 4 transfection reagent and miRIDIAN microRNA Inhibitor Negative Control. Confluent hfRPE cells cultured on 12-well Transwell plate (5–6×105 cells/well) were transfected with 200 nM anti-miRNA in the RPE medium containing no antibiotics. TER was measured before transfection and at various times after transfection using EVOM. Transfection was repeated every 4 d for experiments lasting >4 d.
Cell culture and reporter constructs
Cells were grown in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% heat-inactivated FBS and GlutaMAX-1 in an atmosphere of 5% CO2 at 37°C. 3′-UTR regions of miR-204 putative target genes including SNAIL 1 and 2 and TGF-βR1 and R2 were cloned into a pEZX-MT01 vector separately (GeneCopoeia Inc., Rockville, MD, USA) (see Fig. 10). The full-length sequence of the 3′-UTR from TGF-βR1 (4889 bp) was split into two parts and cloned separately into the same reporter vector. The fragment TGF-βR1a contains the miR-204 seed binding region (1027–1033 bp). The pEZX-MT01 vector, free of the relevant 3′-UTR sequence, as well as the positive control vector containing the 3′-UTR of PDCD4 gene, were purchased from GeneCopoeia Inc.
Figure 1.
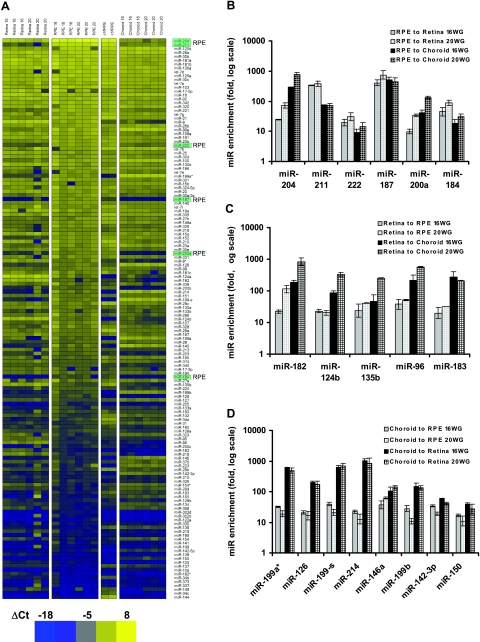
miRNA expression profile in native hfRPE, neuroretina, choroid, and primary cultures of hfRPE. A) Heat map was plotted in Heatmap Builder1.0 with ΔCt from each miRNA using the mean Ct of miR-16 and let-7a as the reference. Yellow denotes high expression; blue denotes relatively lower expression. Entire data set was assigned to 50 color bins and sorted based on ΔCt in an RPE sample, with high expression (low ΔCt) at top and low expression (high ΔCt) at bottom. At right, the 6 most highly enriched RPE miRNAs are highlighted in light green. Intensity scale: bright blue indicates lowest expression (ΔCt=−18); bright yellow indicates highest expression (ΔCt=8). Highest and lowest expression levels differ by ≈7 × 107-fold (226), which is within the detection range of TaqMan Q-PCR for miRNAs (24). B) Six miRNAs enriched in human RPE. Fold difference was calculated using average ΔΔCt from 3 biological repeats. A miRNA is considered as enriched in RPE if its expression level is significantly higher (≥10-fold) than that in neuroretina and choroid at both 16 and 20 WG. Student’s t test was run for each of the 4 pairs of comparison (P<0.05, 2-tailed, unpaired, unequal variance). C, D) Five miRNAs enriched in human neuroretina (C) and 8 miRNAs enriched in human choroid (D). Fold difference was calculated using the same criteria as for RPE in A.
Figure 2.
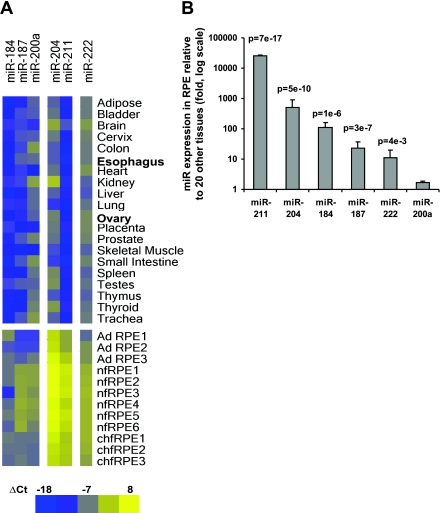
Comparison of the 6 enriched miRNAs in native hfRPE, adult RPE, hfRPE primary culture, and a panel of 20 normal human tissues (Materials and Methods) using qRT-PCR. A) Heat map of ΔCt for 6 miRNAs in 20 normal tissues, 3 native adult human RPE (AD RPE), 5 native fetal RPE (nfRPE), and 3 primary cultures of native hfRPE (chfRPE). Because the expression for miR-16 is more consistent than that for let-7a across this sample of different tissues, it was used in the normalization for ΔCt in each tissue. B) miRNA expression in cultured hfRPE (n=3) compared with the average of miR expression levels in 20 tissues using the ΔΔCt method. P value is based on Student’s t test. Intensity scale: bright blue indicates lowest miRNA expression (ΔCt=−18); bright yellow indicates highest miRNA expression (ΔCt=8). These experiments were performed before the pooled 16 and 20 WG expression data and therefore do not include miR-200b and miR-221.
Figure 3.
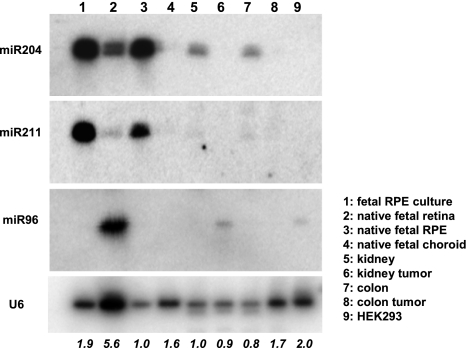
Northern blot expression of miR-204, miR-211, and miR-96 was analyzed by Northern blots with LNA-spiked and 32P-labeled oligonucleotides as probes. RNA of human fetal retina (lane 2), RPE (lane 3), and choroid (lane 4) were pooled from 3 individual donors. RNA of cultured RPE (lane 1) was obtained from passage 1 of hfRPE primary cultures. Total RNAs of normal and malignant adult human kidney (lanes 5 and 6) and colon (lanes 7 and 8) were purchased from Ambion. Total RNA (10 μg) from each preparation was loaded onto a 15% acrylamide gel. Native (lane 3) as well as cultured (lane 1) RPE is shown here highly enriched in the mature form of miR-204 and miR-211 compared with its adjacent tissues of neural retina, and choroid (lanes 2 and 4 respectively). Also, miR204 is readily detected in normal kidney (lane 5) and colon (lane 7) but is virtually undetected in the malignant counterparts of kidney (lane 6) and colon (lane 8). miR-96 is shown highly enriched in retina (lane 2) but not detected in either cultured or native RPE (lanes 1 and 3. respectively) or choroid (lane 4). RNA from HEK293 cells (lane 9) was included as a control. Hybridization to the U6 small nuclear RNA was used as the control for loading variations. Hybridization signals were visualized with a phosphor imaging screen on a Typhoon 9410 scanner. Loading variations were quantified by ImageQuant, normalized to the native RPE (lane 3), and expressed as fold changes at the bottom.
Figure 4.
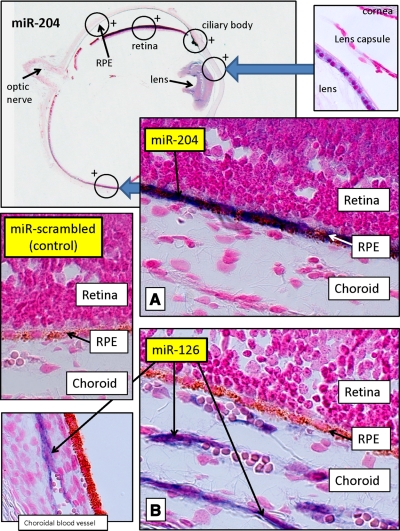
MiR-204 expression in the human eye. Cross sections of human fetal eye processed for miRNA ISH with Dig-labeled LNA probes to label miR-204. Sections were counterstained with Fast Red (red) to contrast eye structures. Top left: low-gain (×2) ISH image of entire eye section labeled for miR-204. Large amounts of MiR-204 were detected (blue) in RPE and in several other regions of eye, as indicated by circles with +. Top right inset: high-gain (×100) view of the area where positive miR-204 staining was detected in lens epithelia. A) High-gain (×100) ISH photograph of the eye section marked by the circle in the top left panel, showing intense blue staining, corresponding to large amounts of miR-204 in RPE. Weak staining for miR-204 was also detected in retina. B) Corresponding area to miR-204 magnified view in an adjacent section shows a high-gain (×100) ISH image for miR-126 labeling. miR-126, choroid enriched miR, was used as positive control and was only detected in choroid. Bottom left inset: area of the miR-126-labeled section where choroidal endothelial cells are positive. Middle left inset: scrambled miR probe from Exiqon was used as a negative control and showed no detectable labeling for RPE, choroid. or other parts of the eye.
Figure 5.
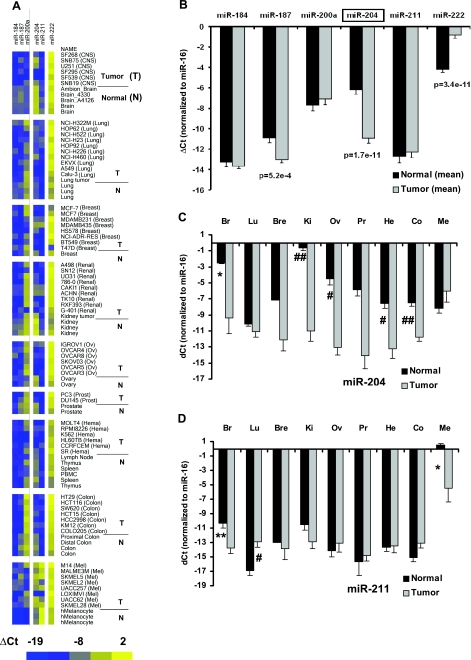
Expression profile for miRNAs in tumor cell lines and normal tissues using qRT-PCR. ΔCt is the Ct value normalizedto Ct for miR-16 in each tissue. Data presented in panel A are quantitatively analyzed in panels B–D and are presented as means ± se. A) Heat map for ΔCt of 6 miRNAs in 62 tumor cell lines, 2 tumor tissues, and 25 normal tissues. Intensity scale: bright blue indicates lowest miRNA expression (ΔCt=−19); bright yellow indicates highest miRNA expression (ΔCt=2). Highest and lowest expression levels differed by ≈2 × 106. B) Comparison of miRNA expression in tumor vs. normal tissues. ΔCt is averaged from all tumor cell lines and tissues and compared with the average from all normal tissues. C) Expression of miR-204 in 8 normal tissues and a primary culture of melanocytes vs. their matching tumor cell lines. Br, brain; Lu, lung; Bre, breast; Ki, kidney; Ov, ovary; Pr, Prostate; He, hematological cells; Co, colon; Me, melanocyte. Mean differences for breast and prostate tissue are not significant, probably because of the small sample size. D) Expression of miR-211 in 8 normal tissues and a primary culture of melanocytes vs. their matching tumor cell lines. *P < 0.05; **P < 0.01; #P < 0.005; ##P < 0.001.
Figure 6.
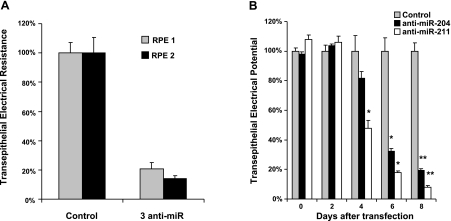
TER significantly deceased in anti-miR-treated RPE. A) TER decreased in cells transfected with a mixture of 3 anti-miRs. Control: Dharmacon anti-miRNA-negative control 2, 600 nM. Treatment: 3 anti-miRs combined (anti-miR-204, anti-miR-211, and anti-miR-222); 200 nM for each. Cells derived from different tissues were transfected in triplicate for each group in 2 separate experiments. TER was normalized as a percentage of mean TER for control transfection. B) TER deceased over time in anti-miRNA-treated RPE. Cells were repeatedly transfected with control anti-miR, anti-miR-204, or anti-miR-211 (n=3) every 3 d. Resistances were measured every 2 d and normalized to control (100%). By 6 d, cells treated with anti-miR-204 or 211 have significantly lower RT. *P < 0.05; **P < 0.005.
Figure 7.
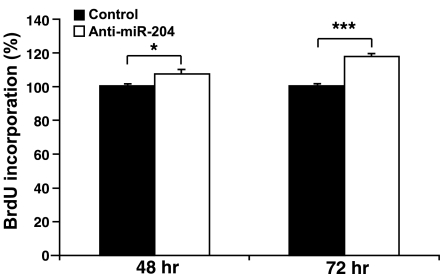
Confluent monolayers of hfRPE cell cultures were transfected with anti-miR-204 or anti-miRNA negative control oligonucleotide on d 1 and 4. Cells were collected on d 5, and BrdU incorporation experiments were performed using a cell proliferation ELISA BrdU kit. Transfection with anti-miR-204 significantly increased cell proliferation (≈7.4%, 48 h; ≈17.5%, 72 h) compared with control (n=8). *P < 0.05; ***P < 0.001).
Figure 8.
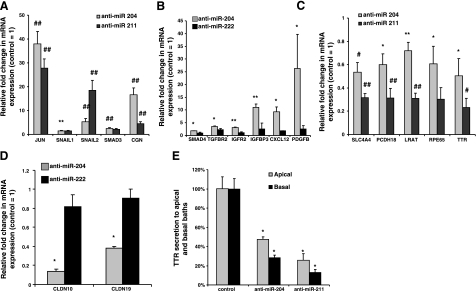
Gene expressions were up- or down-regulated in anti-miRNA-treated RPE. qRT-PCR for different genes was performed using cells treated with anti-miR-204 or anti-miR-211. Anti-miRNA derived from C. elegans and anti-miR-222 are controls that have no significant effect on TER A) Transcription factors Jun, SNAIL1, SNAIL2, Smad3, and cingulin (CGN) mRNA were assayed in anti-miR-204- or anti-miR-211-treated cells (n=6). B) Smad4, TGF-βR2 (TGFBR2), IGFR2, IGFBP3, CXCL12, and platelet-derived growth factor B (PDG) expression in anti-miR-204- or anti-miR-211-treated cells (n=4). C) SLC4A4, PCDH18, LRAT, TTR, and RPE65 are down-regulated in anti-miR-211- or anti-miR-204-treated cells (n=6). D) Claudin 10 and 19 expression in anti-miR-204-treated cells. Anti-miR-222 is a control (n=4). E) TTR secretion assayed by ELISA in cell culture medium. Cells were transfected with anti-miR-204/211 for 6 d. Fresh medium was added at d 6, and samples were collected 3 d later for ELISA. For all panels, data are means ± se from triplicates vs. anti-miR control. *P < 0.05; **P < 0.01; #P < 0.005; ##P < 0.001.
Figure 9.
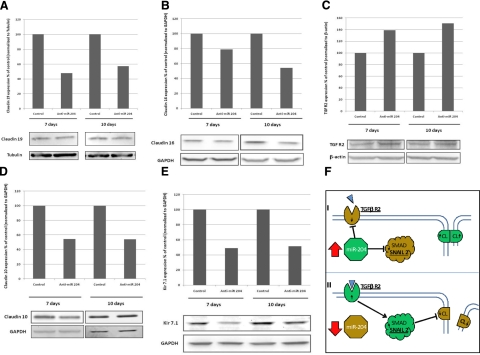
Immunoblot analysis of protein regulation by miR-204. A–E) Confluent monolayers of hfRPE cell culture were transfected with anti-miR-204 or anti-miRNA negative control oligonucleotide. Cells were collected on d 7 and 10, and 20 μg of total proteins was loaded and electrophoresed. Antibody-specific bands for claudin 19 (A), claudin 16 (B), TGF-βR2 (C), claudin 10 (D), and Kir7.1 (E) are ∼29, 26, 73, 22, and 40 kDa, respectively; GAPDH or β-actin was used as a loading control. Transfection with anti-miR-204 decreased protein expression of claudin 19, claudin 16, claudin 10, and Kir7.1. In contrast, the protein level of TGF-βR2 was increased compared with the negative control. Data are representative from ≥3 separate experiments. F) Schematic model of the miR204 regulatory pathway affecting claudin (CL) expression.
Figure 10.
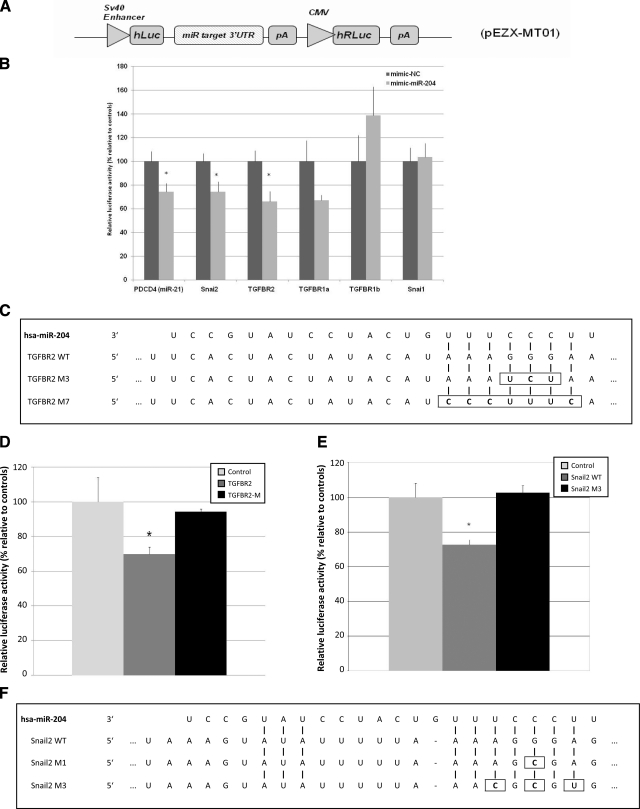
A) Schematic diagram of the luciferase reporter vector pEZX-MT101. SV40, simian virus 40. B) Results of the luciferase assay demonstrating direct binding between miR-204 and its potential target mRNAs. Relative level of luciferase activity is measured by the ratio of firefly vs. Renilla luciferase activities normalized to mimic-NC (control mimic, a C. elegans miRNA that shares minimal sequence similarity to mammalian miRNAs). Data points are means ± se of 3 separate experiments. Candidate is considered a direct target if the magnitude of decrease in luciferase activity is equal to or greater than what is measured for miR-21 and its known target PDCD4 3′-UTR (positive control) (73). C) Alignments between hsa-miR-204 and its seed binding sequence in the 3′-UTR of TGF-βR2. In the M3 and M7 mutants of the pEZX-MT01 TGF-βR2-3′-UTR vector either 3-bp (M3) or 7-bp (M7) mutations were introduced within the seed binding sequence of the wild-type 3′-UTR by site-directed mutagenesis. D) Validation of TGF-βR2 as a direct target of miR-204 by site-directed mutagenesis. HEK293 cells were either transfected with a TGF-βR2 or a TGF-βR2 M7 or cotransfected with either the wild-type (TGF-βR2) or M7 version of the pEZX-MT01 TGF-βR2-3′-UTR vector with a miR-204 mimic, and the luciferase activity was normalized to vector only control (control, as 100%). E) Luciferase activities mediated by the 3′-UTR of the human SNAIL2 in the reporter construct pEZX-MT01 SNAIL2–3′-UTR. HEK293 cells in triplicate were treated as in D. F) Sequence alignments of the seed region of hsa-miR-204 with its target site in the 3′-UTR of human SNAIL2. WT, wild-type sequence of the target site; M1 and SNAIL2 M3, mutants of the wild-type target with 1- or 3-bp substitutions (enclosed by rectangles). Values are means ± se of 3 separate experiments. Values of P < 0.05 were considered significant; Student’s t test.
Site-directed mutagenesis/transfection/luciferase reporter assay
Two mutant clones of the wild-type pEZX-MT01 TGF-βR2-3′-UTR and SNAIL2 were constructed by site-directed mutagenesis using the QuikChange Lightning site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). For TGF-βR2, two mutant clones were constructed. The seed binding region (5′-AAAGGGA-3′) was changed to 5′-CCCTTTC-3′ (7-bp replacement) in clone M7 and to AAATCTA (3-bp replacement) in clone M3. The mutagenesis primers for the two mutant clones are as follows: for M7, the forward primer is CATTCTGTAGTTCCTAAAAATACTGACTTTTTTCACTACTATACATCCCTTTCAAGTTTTATTCTTTTATGGAACACTTCAGCTGTACTCATGT, and the reverse primer is TACATGAGTACAGCTGAAGTGTTCCATAAAAGAATAAAACTTGAAAGGGATGTATAGTAGTGAAAAAAGTCAGTATTTTTAGGAACTACAGAATG; for M3, the forward primer is GTTCCTAAAAATACTGACTTTTTTCACTACTATACATAAATCTAAAGTTTTATTCTTTTATGGAACACTTCAGCTG, and the reverse primer is CAGCTGAAGTGTTCCATAAAAGAATAAAACTTTAGATTTATGTATAGTAGTGAAAAAAGTCAGTATTTTTAGGAAC. The mutagenesis reactions were performed following the manufacturer’s suggested protocol except for the annealing temperature, which was changed to 68°C for M7 and 55°C for M3.
For the SNAIL2 construct, two mutant clones were constructed. The seed binding region (5′-AAAGGGA-3′) of the first clone was changed to AAAGCGA (1-bp replacement, M1) using the QuikChange Lightning site-directed mutagenesis kit. The forward and reverse mutagenesis primers are GTAGTGCTTTAAAGTATATTTTTAAAAGCGAGGAAAAAAATAACAAGAACAAAACACAGG and CCTGTGTTTTGTTCTTGTTATTTTTTTCCTCGCTTTTAAAAATATACTTTAAAGCACTAC, respectively. The seed binding region in the second clone was changed to 5′-AACGCGT-3′ (3-bp replacement, SNAIL2 M3). To construct the second clone, the last 6 bp of the seed binding region were changed to include one copy of the MluI sequence, resulting in 5′-AACGCGT-3′ with 3 bp altered (SNAIL2 M3). To construct the SNAIL2 M3 clone, the human SNAIL2 3′-UTR sequence subcloned in pBluescript (Stratagene) was PCR-amplified with a pair of mutagenesis primers that anneal to the seed region and carry a copy of the MluI recognition sequence at the 5′ end (forward GCGCGACGCGTGGAAAAAAATAACAAGAACAAAACACAGGAGAATGTAT and reverse GGAGGACGCGTTTAAAAATATACTTTAAAGCACTACAGGTAATCAAAAA). The PCR product is thus flanked by MluI sites, and the PCR product was then digested with MluI at 37°C for 1 h, gel-purified, and self-ligated with T4 ligase. After transformation of the bacteria host DH-α5 with the self-ligation product, clones were recovered and verified by sequence analysis. All of the recovered clones were shown to be correctly mutated. The mutant 3′-UTR of the human SNAIL2 was then subcloned back into the Luciferase reporter vector pEZX-MT01, creating the final SNAIL2 M3 clone.
For all luciferase assays, cells were plated 24 h before transfection at a density of 0.5 × 105 cells/well of 24-well plates. In brief, control mimic (mimic-NC, a Caenorhabditis elegans miRNA that shares minimal sequence similarity to miRNAs in mammalian tissue) or miR-204 mimic (50 nM; Dharmacon) was transfected into HEK293 cells using Lipofectamine 2000 (Invitrogen) and was followed by a second transfection of either a miR-204 reporter construct or its mutant (50 ng). After the second transfection (24 h), the firefly and Renilla luciferase activities were determined by luminometer readings using Dual-Luciferase Reporter Assay systems (Promega, Madison, WI, USA).
Bromodeoxyuridine (BrdU) incorporation and wound-healing assay
Cell proliferation was analyzed using a BrdU incorporation assay (32, 33). Confluent monolayers of hfRPE cultured in Primaria 24-well tissue culture plates (6–8 wk) were untreated or transfected with 200 nM anti-miRNA-204 oligonucleotide (miRIDIAN human microRNA inhibitors) or anti-miRNA negative control oligonucleotide (miRIDIAN microRNA Hairpin Inhibitor Negative Control 2) using DharmaFECT 4 Transfection Reagent (Thermo Fisher Scientific, Waltham, MA, USA) in the RPE medium containing no antibiotics twice (d 1 and 4). Cells were collected on d 5 using 0.25% trypsin-EDTA and seeded in tissue culture plates (96-well plate, 2.5×103/well; 24-well plate, 1.25×104/well). Cells were allowed to attach for 24 h, and culture medium was replaced with 0.4% FBS medium containing nonessential amino acids and GlutaMAX-1. After 24 or 48 h incubation, BrdU labeling solution was added and incubated for another 24 h. The proliferation rate was evaluated using a Cell Proliferation ELISA BrdU Kit (Roche Diagnostics). Ten duplicates (96-well plate) or quadruplicates (24-well plate) were used for each condition, and the experiments were repeated using cell cultures from two different donors.
A wound-healing assay was used to determine the effects of anti-miRNA-204 oligonucleotide on hfRPE cell migration, as described previously (32, 34). Confluent monolayers of hfRPE were transfected with 200 nM anti-miRNA-204 oligonucleotide or anti-miRNA negative control oligonucleotide. Cell proliferation was suppressed by incubation with 10 μg/ml mitomycin C (Sigma-Aldrich) for 2 h and a circular denuded area (7-mm diameter) was made in each well using a custom-designed cell scraper. Cell migration was quantified by counting the average number of cells that migrated into the denuded area surrounding the circumference of the denuded area after 48 and 72 h. Each condition was tested in quadruplicate using cells from two different donors.
Live/Dead assay
Cultured fully differentiated hfRPE cells were used for the Live/Dead assay (Invitrogen). The cells were incubated with 620 μM tert-butyl-hydroperoxide (TBH) (Sigma-Aldrich) in serum-free medium. TER was measured before treatment of TBH. The cells were stained with ethidium homodimer-1 and imaged with a Zeiss Axioplan 2 microscope using an ×10 objective (Carl Zeiss, Oberkochen, Germany). For whole-Transwell imaging, the scanning mode was used to take 260 mosaic images to cover the entire Transwell surface area (12,500×12,700 μm). Images for all experiments were taken using same image settings. Dead cells were counted using NIH ImageJ software with an ITCN plug-in (U.S. National Institutes of Health, Bethesda, MD, USA)
Electrophysiology
The transepithelial potential (TEP) of intact monolayers was measured using a pair of calomel electrodes and agar bridges in series with Ringer’s solution continuously perfused on each side of the tissue, which was mounted in a modified Üssing chamber as described previously (10). Concomitantly, intracellular microelectrodes were used to measure the membrane potentials, VA and VB, where TEP = VB − VA. Total TER and the ratio of apical-to-basolateral membrane resistance (RA/RB) were obtained by passing 4-μA bipolar current pulses (i) across the tissue and measuring the resultant changes in TEP, VA, and VB. The RA/RB ratio is the absolute value of the change in VA (ΔVA) divided by the change in VB (ΔVB), where RA/RB = iΔVA/iΔVB. The paracellular pathway is represented as a shunt resistor (RS), which is the parallel combination of the junctional complex resistances between neighboring cells and the resistance caused by the less-than-perfect mechanical seal around the circumference of the tissue. The control Ringer’s solution contained 120 mM NaCl, 5 mM KCl, 23 mM NaHCO3, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM glucose. Statistical comparisons were made using the Student’s t test (2-tailed; unpaired samples with unequal variances). P < 0.05 was regarded as significant. Data are reported as means ± se, unless otherwise specified.
RESULTS
miRNA expression profiles in RPE, neuroretina, and choroid
RPE enriched miRNAs were obtained by comparing the miRNA profiles from native hfRPEs and their adjacent, retinal, and choroidal tissues (3 separate samples). The heat map in Fig. 1A shows qRT-PCR data, presented as ΔCt, from 136 miRNAs in these three tissues isolated from human fetal eyes at 16 and 20 WG. For comparison, we added data from confluent monolayers of cultured hfRPE (10). As stated earlier, a miRNA was considered to be enriched in RPE if its expression level was significantly higher (by ≥10-fold; P<0.05; n=3) than that in neuroretina and choroid at both 16 and 20 WG.
At 16 WG, there are 14 miRNAs whose expression levels in RPE are up to 400-fold higher than that in neuroretina. We also identified 12 miRNAs in RPE whose expression is up to 500-fold higher than that in choroid at 16 WG (complete list in Supplemental Τable 1). At 20 WG, 16 and 18 miRNAs were up to 750-fold more highly expressed in RPE compared with neuroretina and choroid, respectively. Figure 1B summarizes the miRNAs that are enriched in RPE (miR-184, 187, 200a, 204, 211, and 222) by a factor of 10- to 754-fold compared with neuroretina or choroid at both 16 and 20 WG. In Fig. 1A, these miRNAs are shown on the right-hand side of the heat map and are highlighted in green. Five of six of these miRNAs are also highly enriched in cultured hfRPE. The top two rows of the heat map show that miR-204 and miR-211 are the most highly enriched miRNAs in RPE compared with neuroretina and choroid (25- to 754-fold higher).
Based on a similar analysis, 5 miRNAs (miR-124b, 135b, 182, 183, and 96) were identified as enriched in neuroretina (Fig. 1C), the highest (miR-182) having ≈1000 higher expression in neuroretina compared with that in RPE or choroid (Supplemental Table 2). In addition, 8 other miRNAs were identified as enriched in choroid (miR-126, 142–3p, 146a, 150, 199a*, 199b, 199-s, and 214), the latter 3 having ≈1000 higher expression in choroid than in neuroretina or RPE (Fig. 1D and Supplemental Table 3).
The expression profiles in RPE, retina, and choroid at 16 and 20 WG were very similar. Only 3 of 141 miRNAs in choroid, 1 of 141 miRNAs in retina, and none in RPE were >2-fold different. Based on these observations, we combined the data from both age groups and therefore doubled the sample size (to n=6). This allowed us to identify a larger set of enriched miRs (P<0.05; >10-fold enrichment) in RPE, neuroretina, and choroid (Table 1). Increasing the statistical power identified two additional RPE enriched miRNAs (miR-200b and 221). In addition, four additional miRNAs were identified in the choroid (miR-139, 145, 155, and 199a) and one in the neuroretina (miR-124a).
TABLE 1.
miRNAs enriched in RPE, retina, and choroid by combining 16 and 20 WG samples
| Name | RPE vs. choroid |
RPE vs. retina |
||
|---|---|---|---|---|
| Fold | P | Fold | P | |
| miR-184a | 11 | 3E−02 | 29 | 8−03 |
| miR-187a | 455 | 5E−08 | 488 | 7E−08 |
| miR-200aa | 79 | 3E−08 | 18 | 2E−05 |
| miR-204a | 691 | 2E−06 | 42 | 3E−07 |
| miR-211a | 75 | 1E−08 | 388 | 4E−06 |
| miR-222a | 11 | 1E−05 | 23 | 2E−05 |
| miR-221 | 13 | 1E−05 | 17 | 3E−05 |
| miR-200b | 72 | 3E−06 | 16 | 6E−05 |
| Retina vs. RPE | Retina vs. choroid | |||
| miR-124a | 57 | 8E−05 | 302 | 1E−04 |
| miR-124ba | 22 | 9E−06 | 147 | 6E−05 |
| miR-135ba | 32 | 7E−05 | 115 | 2E−06 |
| miR-182a | 57 | 2E−03 | 705 | 1E−03 |
| miR-183a | 27 | 2E−05 | 199 | 4E−06 |
| miR-96a | 42 | 1E−06 | 257 | 5E−04 |
| Choroid vs. RPE | Choroid vs. retina | |||
| miR-126a | 18 | 5E−06 | 187 | 1E−09 |
| miR-139 | 21 | 3E−04 | 12 | 5E−06 |
| miR-142–3pa | 25 | 2E−05 | 48 | 2E−07 |
| miR-145 | 14 | 3E−04 | 258 | 3E−05 |
| miR-146aa | 48 | 1E−07 | 119 | 1E−07 |
| miR-150a | 12 | 3E−05 | 35 | 1E−06 |
| miR-155 | 18 | 5E−04 | 53 | 3E−03 |
| miR-199a | 17 | 6E−06 | 21 | 4E−02 |
| miR-199a*a | 24 | 1E−06 | 538 | 4E−10 |
| miR-199ba | 17 | 4E−06 | 138 | 4E−09 |
| miR-199-sa | 28 | 8E−08 | 627 | 8E−08 |
| miR-214a | 17 | 8E−06 | 909 | 4E−09 |
Samples (n=6) were pooled for each tissue between two age groups (16 and 20 WG, n=3/group) before statistical analysis. Criteria for enrichment: P < 0.05 and >10-fold higher expression than the other two tissues.
Same as miRNAs identified by comparing 16 and 20 WG separately.
Validation of RPE enriched miRNAs
The heat map in Fig. 2A summarizes the relative expression levels for the RPE-enriched miRNAs, assayed in a panel of 20 normal tissues from throughout the body. Heat map colors represent expression levels that range over 7 orders of magnitude, in factors of 2, from blue (low) to yellow (high). It is apparent that miR-204/211 and 184, 187, and 222 are much more highly expressed in native human (adult and fetal) and cultured fetal RPE compared with a range of adult tissues throughout the body.
Figure 2B summarizes the relative expression levels for each of the 6 miRNAs in the 3 hfRPE cultures compared with the mean expression level in the 20 representative tissues (ΔΔCt method). Five of these miRNAs are significantly enriched in cultured hfRPE compared with tissues from the rest of body. Expression for miR-184, 187, and 222 is enriched in RPE by 112-, 22-, and 11-fold, respectively (P<0.005). Expression for miR-211 is more than 2.6 × 104-fold higher in RPE than the mean of 20 tissues (P=7×10−17), whereas miR-204 expression is 500-fold higher in RPE (P=5×10−10).
Northern blot detection and in situ localization of miRNAs
To confirm the qRT-PCR data, Northern blot analyses were performed on 1) RPE and its adjacent tissues isolated from human fetal eyes and 2) adult tissues from normal and malignant kidney and colon, respectively. The results summarized in Fig. 3 confirmed the observation that miR-204 (and miR-211) is enriched in fetal native and cultured RPE compared with neural retina and choroid. Although readily detected in normal kidney and colon, miR-204 (and mi-R211) expression is virtually undetectable under the same conditions in malignant tissues, confirming a similar observation made in our miRNA profiling experiments by qRT-PCR (see Fig. 5C). In addition, miR-96, which is enriched in retina compared with RPE and choroid (Table 1, qRT-PCR), is readily detectable in kidney tumor and HEK293 cells but not in tissues from normal kidney (Fig. 3, miR-96 panel).
Figure 4 summarizes a series of ISH studies performed using human fetal eyes (18 WG). The top left-hand panel shows a low-magnification view of a transverse eye section with several major structures identified (optic nerve, RPE, ciliary body, cornea, and lens). The circles with plus signs indicate regions where miR-204 was detected. The image inset at top right is a high-gain (×100) view of the lens epithelium showing positive staining for miR-204. The ciliary body is also highly labeled. The large middle panel (Fig. 4A) is a high-gain image of the posterior pole (midperiphery) showing that miR-204 is much more highly decorated in RPE (blue label) with relatively little stain in the retina and no stain in the choroid, corroborating the qRT-PCR data (Table 1) and Northern blots (Fig. 3). In this same series of sections, we also examined the localization of another miR, one that is highly expressed in choroid relative to RPE and retina. This localization of miR-126 serves as a specificity control for miR-204 and is of value in its own right. The large panel at the bottom right (Fig. 4B) is a high-gain image of an adjacent region (same eye, different section) showing that the histochemical stain for miR-126 is not visible in retina or RPE but is clearly present in the choroid (black arrows). This result confirms the expression data summarized in Table 1 showing that miR-126 is highly enriched in choroid compared with retina and RPE.
miR-204/211 and 222 expression in tumors
Figure 5A shows that among the 6 RPE-enriched miRNAs, miR-204 stands out as being consistently more highly expressed in normal than in tumorigenic tissues. These data, quantitatively summarized in Fig. 5B, show that the mean expression level of miR-204 is ∼30-fold lower in tumors than in normal tissues (P<2×10−11), whereas expression of miR-187 is ∼6-fold lower (P<6×10−4). In striking contrast, the expression level for miR-222 is 10-fold higher in tumor vs. normal tissues (P<4×10−11), suggesting its further investigation as a possible oncogenic marker. Figure 5C shows that miR-204 expression is significantly lower, by 20- to 1361-fold (P<0.05), in tumor than in normal tissue in 5 of the 9 tissue types (brain, kidney, ovary, hematological cells, and colon). miR-204 expression is also much lower in breast and prostate tumor tissue than in normal tissues, but this difference did not reach statistical significance possibly because of the small sample size of normal tissues (n=1 and 2, respectively). As summarized in Fig. 5D, miR-211 expression is considerably higher in melanocytes than in eight other normal tissues from throughout the body. In melanoma tissues (n=8), miR-211 expression is 66-fold lower (P<0.05) than in normal melanocytes (n=3).
Anti-miR-204/211 decreases TER
The data summarized in Fig. 5 shows that miR-204 expression levels are reduced in a wide variety of tumors, suggesting the possibility that a reduction in miR-204 expression can trigger the progressive disruption of epithelial barriers. To begin the evaluation of this hypothesis, we tested the effects of anti-miR204/211 on the TER of fully confluent, intact hfRPE monolayers that were efficiently transfected (Fig. 6 and Supplemental Fig. 2). qRT-PCR was then used to determine whether specific miRNA mimics or anti-miRNAs could increase or decrease the relative levels of miRNAs. Because both miRNA-204 and 211 are highly expressed in human RPE, transfection with mimics did not increase miRNA levels (data not shown). However, anti-miR-204 or 211, or 222, or a mixture of all three anti-miRs decreased mature miRNA levels by ≈80% (Supplemental Fig. 2D).
These preparations had an initial TER of 200-1000 Ω · cm2 and were transfected with a mixture of three anti-miRNAs (miR-204, 211, and 222) or individual miRNAs, each at a concentration of 200 nM. TER was monitored over a period of 8–12 d (Fig. 6). In two separate experiments, summarized in Fig. 6A, the anti-miRNA mixture significantly decreased TER by 79 ± 4% (n=3; P<0.005) and 86 ± 2% (n=3; P<0.05), respectively, compared with control cells. Figure 6B shows that the anti-miRNA-induced decrease in TER took several days to develop; the first noticeable change occurred at 4 d, when anti-miR-211-treated cells decreased TER by more than a factor of 2 (P<0.05). Control cells had a TER of 538 ± 56 Ω · cm2 that decreased to 257 ± 28 Ω · cm2 4 d after transfection with anti-miR-211 (P<0.05). At 6–8 d, the TER of cells treated with anti-miR-204 or 211 was decreased even further compared with control cells (n=3; P<0.05 at d 6–8).
It is possible that the miRNA-induced changes in TER were partly the result of transfection-induced toxicity, for example, necrosis or apoptosis. To quantify the magnitude of this putative effect we treated the monolayers with DharmaFECT 4 transfection reagent or anti-miR oligonucleotides and in each case measured the percentage of cell death and concomitant change in TER. These comparisons, summarized in Supplemental Fig. 3A, B, show that the TER decrease in anti-miR-204-treated cells is significantly larger than the effect of the transfection agent alone.
Effects of miR-204 on hfRPE migration/proliferation
The lower expression levels of miR-204 in surveyed tumors and the anti-miR-204-induced decrease in total tissue resistance further suggest a possible role for miR-204 in the regulation of RPE migration/proliferation. In a wound-healing assay (32), anti-miR-204 had no significant effect on hfRPE cell migration compared with the anti-miRNA negative control oligonucleotide, although miR-204 expression was reduced by 80% (Supplemental Fig. 2D). In contrast, transfection with anti-miR-204 significantly increased hfRPE cell proliferation compared with the negative control. Figure 7 summarizes the data from eight cell proliferation experiments using hfRPE cells from two different donors. Consistent with the tumor data summarized in Fig. 3, hfRPE proliferation was significantly increased by 7.4% (P=0.04) and 17.5% (P=1.2× 10−7) after 48 and 72 h, respectively.
Anti-miR-204/211-induced changes in RPE gene and protein expression
In various cell types, it has been shown that Jun/Fos, SNAIL1, SNAIL2, Smad3, Smad4, and cingulin are transcriptions factors capable of regulating epithelial TJ protein expression (35, 36). We focused on the effects of anti-miR-204 and 211 because their expression is highest among all miRNAs examined (Fig. 1A) and anti-miR-204/211 significantly decreases total transepithelial resistance. Figure 8A shows that the expression of many of these transcription factors is significantly increased after treatment with anti-miR-204 or 211: 1) Jun expression increased 28- to 38-fold (n=6; P<0.001), 2) SNAIL1 expression increased by 2-fold (n=6; P<0.05) and SNAIL2 expression increased significantly by 5- to 20-fold (n=6; P<0.001), whereas Smad3 expression was up-regulated by 2- to 3-fold (n=6; P<0.001); and 3) finally, cingulin mRNA increased by 4- to 16-fold (n=6; P<0.001) in anti-miR-204 or anti-miR-211 experiments.
Anti-miR-204 also increased expression of cytokines, growth factors and their receptors, and downstream signaling molecules. Figure 8B shows that anti-miR-204 significantly up-regulated mRNA expression levels for TGF-βR2 and Smad4, IGF receptor 2 (IGFR2), IGF-binding protein (IGFBP3), CXC chemokine ligand 12 (CXCL12), and platelet-derived growth factor (PDGF) B by 2- to 25-fold (n=4), whereas anti-miR-222, which does not affect TER (data not shown), did not significantly change the expression of these mRNAs.
Anti-miR-204 or 211 reduced the expression of several functionally important genes as summarized in Fig. 8C, D. For example, SLC4A4 is an NaHCO3 transporter that controls cell pH and transepithelial fluid transport in hfRPE (12) and PCDH18 is a member of the cadherin superfamily and therefore may be an important determinant of RPE barrier function. LRAT, RPE65, and TTR are three genes known to be associated with the visual cycle and vitamin A transport in the RPE-retina complex (6). Figure 8C shows that treatment with anti-miR-204/211 significantly reduces the mRNA expression levels of these five genes compared with the control but anti-miR-204 is less potent than anti-miR-211.
Previous work in the laboratory demonstrated that the TJ proteins, claudins 19 and 10, and other TJ proteins are highly expressed in human RPE (10). Figure 8D shows that anti-miR-204 significantly decreased claudin 10 and 19 mRNA expression by 60–80%. Transthyretin (TTR) is also an important marker for epithelial barrier integrity (37) and critical for vitamin A transport across the RPE. Figure 8E shows that anti-miR-204 or anti-miR-211 significantly decreased TTR secretion, by 50–80%, and based on ELISA measurements preferentially shifted the balance of secretion to the apical bath.
To better understand the physiological effects of miR-204, Western blots were used to determine the protein level changes for indirect and putative direct targets. Figure 9 provides illustrative examples of semiquantitative Western blots from a series of experiments in which we transfected hfRPE with anti-miR-204 for 7 or 10 d. Figure 9A, B, D shows a notable decrease in protein expression for claudins 10, 16, and 19. Each experiment was done 7 times, and a similar result was obtained in all experiments, at least on d 7 or 10. A similar result (Fig. 9E) was obtained for Kir7.1, a potassium channel highly expressed in RPE, which mediates photoreceptor-RPE interactions during transitions between light and dark. TGF-βR2 is a predicted (TargetScan) direct target of miR-204, suggesting that anti-miR-204 would increase protein expression as observed in Fig. 9C (this result was obtained in 4 separate experiments).
SNAIL1, SNAIL2, TGF-βR1, and TGF-βR2 are among in silico targets of miR-204 (TargetScan). To demonstrate direct interactions between miR-204 and these potential targets, the 3′-UTR of each was cloned downstream of the luciferase reporter construct pEZX-MT01 vector to generate pEZX-MT01–3′-UTR vectors (Fig. 10A). These vectors served as targets of the miR-204 mimic and were cotransfected separately into HEK293 cells with miR-204 mimic or its control (mimic-NC, see Materials and Methods). Renilla luciferase activity carried within the same vector was used to normalize variations in transfection efficiency. miR-21 and its known target PDCD4 3′-UTR are included as a positive control for the luciferase experiment (Fig. 10B). miR-21 significantly decreased the luciferase activity by 26% (P<0.05) 24 h after transfection, which is similar to the criteria for a direct target effect after miR-204 mimic treatment. The miR-204 mimic significantly decreased the luciferase activity from the pEZX-MT01 TGF-βR2-3′-UTR by 32.6% (n=3; P<0.02) and from the pEZX-MT01 SNAIL2–3′-UTR by 27% (n=3; P<0.03), respectively. The direct target effects of miR-204 on TGF-βR2 were further examined by site-directed mutagenesis of the seed binding region of the 3′-UTR. The native seed binding sequence was changed by either 3 (M3) or 7 (M7) nucleotides as indicated in Fig. 10C. The data summarized in Fig. 10D show that there is no significant difference (n=3; P>0.77) in luciferase activity between the control (empty vector) and the TGF-βR2 mutant construct (M7). Likewise, there is no significant difference (n=3; P>0.8) in luciferase activity between the control (empty vector) and the SNAIL2 mutant construct (SNAIL2 M3). Taken together, these data show that TGF-βR2 and SNAIL2 are direct targets of miR-204.
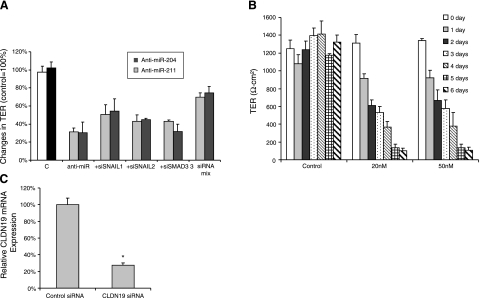
Anti-miR-204/211-induced TER decrease
The data summarized in Fig. 11A show that repression of miR-204/211 leads to significant increases in several critical regulatory transcription factors. Along with the data summarized in Fig. 8, these results suggest the possibility that SNAIL1 or SNAIL2 or Smad3 mediate the anti-miR-204/211-induced reduction in TER. However, in the presence of anti-miR-204/211, siRNA knockdown of only one of these transcription factors does not alter the anti-miRNA-induced TER decrease (Fig. 11A). In contrast, a cocktail of all three small interfering RNAs (siRNAs), for SNAIL1, SNAIL2, and Smad3, rescued TER relative to the control, suggesting that repression of all three transcription factors is needed to decrease TER. Figure 11B shows that claudin 19 siRNA, at 20 or 50 nM, significantly decreased TER by 50% at 2 d and 90% at 6 d after siRNA transfection. The data summarized in Fig. 11C show that claudin 19 mRNA decreased by 65% at 2 d after claudin 19 siRNA transfection. These data, together with the Western blots (Fig. 9), strongly suggest that anti-miR-204/211 decreased TER through the down-regulation of claudin 19 (and possibly claudins 10 and 16), which is localized (10) and highly expressed in the tight junctions of human RPE.
Figure 11.
A) Blocking transcription factors rescues anti-miRNA-induced decrease in TER. Cultured hfRPE cells were transfected with anti-miRNA or anti-miRNA plus siRNA for specific genes. Transepithelial resistance was measured on the first and last day during the experiments. Cells were treated with either anti-miR-204, anti-miR-211, anti-miR + siRNA for SNAIL1, anti-miRNA + siRNA for SNAIL2, anti-miR + siRNA for Smad3, anti-miR-211 + siRNA mixtures for SNAIL1, SNAIL2, and Smad3. In each case, anti-miRNA concentrations were 200 nM; siRNAs were 100 nM each. B) Claudin 19 siRNA induced a significant decrease in TER. Primary cultures of hfRPE were transfected twice at t = 0 and at 3 d with claudin 19 siRNA at 20 or 50 nM. TER was recorded daily by EVOM. C) Claudin 19 (CLDN19) mRNA was assayed with qRT-PCR in cells transfected with claudin 19 siRNA for 2 d. *P < 0.001.
miR-204-induced changes in membrane physiology
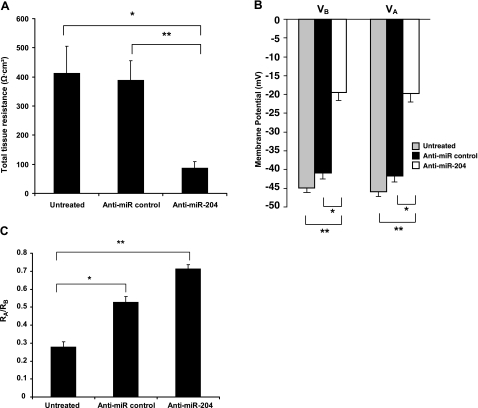
Using intact monolayers of hfRPE cultures, we measured changes in cell membrane voltage and resistance after transfection (8 or 10 d) of hfRPE with anti-miR-204. For these experiments, the initial TER of the Transwells was uniform as measured by EVOM (866±51 Ω · cm2; n=20). The data summarized in Fig. 12A show that after 10 d, TER decreased significantly to 411 ± 93 Ω · cm2 in untreated cells (n=6, P<0.05) or to 388 ± 67 Ω · cm2 in anti-miR-treated control cells (n=7; P<0.005). The cells treated with anti-miR-204 (n=7) decreased TER to 86 ± 22 Ω · cm2 in cells. In Fig. 12B, apical and basolateral membrane potentials (VA and VB) were significantly depolarized by ∼50% in cells treated with anti-miR-204 compared with those in untreated cells (P<2×10−13) or cells treated with an anti-miR control (P<2×10−10). Figure 12C shows that the ratio of the apical-to-basolateral membrane resistance (RA/RB) increased in anti-miR-204-treated cells (n=31) by 35% compared with that in the anti-miR control (n=29; P<3×10−5). The anti-miR-204-induced increase is 156% compared with that in untreated cells (n=28; P<10−13). Taken together, the results of these experiments suggest that miR-204 is a fundamental determinant of epithelial barrier integrity and of cell membrane voltage/resistance and is therefore likely to play a critical role in many of the interactions that normally occur at the blood-retinal barrier.
Figure 12.
Anti-miR-204 induced changes in membrane voltage and resistance in primary cultures of hfRPE. In these experiments, the initial TER of the Transwells were uniform as measured by EVOM (866±51 Ω · cm2; n=20). A) TER was measured after mounting confluent monolayers in a modified Üssing chamber. Data are means ±se (n=7). *P < 0.05; **P < 0.005. B) Membrane potentials and resistances were measured using intracellular microelectrode recording techniques (see Materials and Methods): VA, apical membrane potential; VB, basolateral membrane potential. *P < 2 × 10−10; **P < 2 × 10−13. C) RA/RB, ratio of apical-to-basolateral membrane resistance. *P < 3 × 10−5; **P < 10−13.
DISCUSSION
We have identified 8 miRNAs (miR-184,187, 200a, 200b, 204, 211, 221, and 222) whose expression is relatively enriched in hfRPE compared with its adjacent tissues, neuroretina, and choroidal blood supply (choroid), located at the apical and basolateral membranes, respectively. In a similar manner, we have determined separate sets of miRNAs that are enriched either in neuroretina or choroid (Table 1). The miRNAs most highly expressed in RPE are miR-204/211, and their presence, along with miR-96 (retina enriched), was confirmed by Northern blots. ISH experiments corroborated the localization of miR-204 in RPE and its paucity or absence in retina and choroid, as predicted by qRT-PCR (Table 1). The present study strongly suggests that a relatively high expression of miR-204/211 can preserve the epithelial phenotype, for example, by maintaining relatively high expression levels of key tight junction proteins (claudins) and plasma membrane channels (e.g., apical membrane Kir7.1) These proteins are critical for the function of many epithelia (38, 39) and in RPE help serve to mediate the interactions between retinal photoreceptors and RPE after transitions between light and dark (8, 12)
Expression and function of RPE enriched miRNAs
miR-200 family
miR-200 family members are enriched in epithelia (40) and are crucial inducers of the epithelial phenotype, able to suppress epithelial to mesenchymal transitions (EMTs) (41, 42). miR-200a and 200b are enriched in RPE by comparison with its surrounding tissues (Table 1). Higher expression of miR-200a/b/c has been detected in olfactory bulb epithelium compared with that in other regions of brain (43). However, as shown in Fig. 2, RPE-enriched miR-200a is not significantly different compared with the validation panel of 20 tissues from throughout the body. miR-200a and b have been shown in other systems to regulate EMT by targeting ZEB1 and ZEB2 (41). These two transcription factors, together with SNAIL1, SNAIL2, E47, and TWIST, have been identified as inducers of EMT and important in tumor invasion and metastasis (44). It will be important to determine whether these transcription factors play a similar role in RPE.
miR-221/222
The tumor association data summarized in Fig. 5B indicate that miR-222 expression is significantly higher in tumors than in normal tissue and suggest that miR-221/222 may promote tumorigenesis and RPE proliferation as observed in other systems (45). For example, miR-221/222 are overexpressed in human thyroid papillary carcinomas with a dramatic loss of tyrosine kinase receptor transcript and protein (46, 47). Increased expression of miR-221/222 also increased the proliferation potential of human prostate carcinoma, gastric cancer, and pancreatic cell lines (48) by reducing tumor suppressor p27 Kip1 (46, 47).
miR-184
miR-184 is expressed at high levels in the RPE and lens of zebrafish eyes (49). In the mouse eye, miR-184 has the highest expression levels in the basal cells of the corneal epithelium and in lens epithelial cells of the germinative zone and throughout the anterior epithelium (50). In addition, miR-184 can regulate SHIP2 activity in a variety of epithelial cells, perhaps by regulating the Akt pathway, to repress angiogenesis (51). Yu et al. (51) postulated that this regulation could help maintain the avascularity of the anterior surface of the eye by inhibiting blood vessel growth that would otherwise reduce light transmission through the dioptrics of the eye. Similarly, in the back of the eye, miR-184 may be an important deterrent of blood vessel growth across the RPE into the subretinal space, which occurs in the wet form of AMD, leading to severe visual impairment or blindness (13). In a mouse model of choroidal neovascularization, it has been shown that miR-184 is down-regulated and that intraocular injection of miR-184 may decrease choroidal neovascularization (52). It will be important to confirm these results and determine the specific RPE targets of miR-184 in human RPE.
miR-204/211
miR-204 is orders of magnitude more highly expressed in RPE than in most of the 20 adult human tissues tested (Ambion), and its expression is relatively high in brain and kidney (Fig. 2A). The estimated copy number is ∼10,000/cell (see Materials and Methods), which is in the range of those of other highly expressed miRNAs (4000–30,000 copies/cell) (24). miR-204 is highly expressed in the RPE and lens of zebrafish eyes (49). miR-204 and 211 are also enriched in the mouse eye by at least 3-fold compared with 12 regions of brain (43) and are uniformly expressed in the epithelia of the anterior region of the mouse lens, the nonpigmented epithelium of the ciliary body, and corneal anterior surface (50, 53). miR-204 has been detected in mouse RPE starting at embryonic d 10.5 and in postnatal tissue (50, 53). It was also detected in choroid plexus, which has the same embryonic origin as RPE, and in the cochlea sensory epithelium (53, 54). Figure 4 shows that in addition to RPE, miR-204 is expressed in other ocular structures of the human fetal eye, including retina, lens, and ciliary body. Taken together these data suggest that miR-204 may be a fundamentally important determinant of epithelial differentiation and function.
In most tissues, miR-211 is expressed at a very low level compared with miR-204, suggesting their functional independence. In contrast, miR-204/211 are both highly expressed in human RPE (Fig. 1A), suggesting a significant overlap in their functions. RPE and melanocytes are signature cell types in the body capable of producing melanin pigment (55). In melanocytes, miR-211 is expressed at a higher level than miR-204, suggesting a more dominant function in melanogenesis. miR-211 expression is significantly lower in melanoma than in melanocytes, which distinguishes this tissue from other tissues that develop tumors throughout the body (Fig. 5D). In the transition from melanocyte to melanoma, miR-204 is not altered (Fig. 6C), suggesting a specific role for miR-211 in the regulation of melanogenesis and melanocyte proliferation.
Although miR-204 and miR-211 share the same seed region for their targets, they seem to be involved in different physiological functions. miR-211 is an intronic miRNA that lies within the transient receptor potential melastatin 1 (TRPM1) host gene. The coding sequence of miR-204 lies within intron 6 of the human transient receptor potential melastatin 3 (TRPM3). Evidence suggests that miR-204 and TRPM3 share the same regulatory motif for transcription and are derived from a single transcription unit (56). In choroid plexus, the presence of the mature form of miR-204 and mRNA of TRMP3 is consistent with this notion (54). TRPM3 was detected by qRT-PCR in confluent monolayers of hfRPE (not shown). Given that miR-204 is enriched in hfRPE and that it has a close embryological and functional resemblance to choroid plexus, it seems likely that miR-204 and TRPM3 are also cotranscribed in RPE. In HEK293 cells, it has been shown that TRPM3 channels facilitate constitutive Ca2+ and Mn2+ entry and participate in the regulation of cell volume. These findings, taken together, suggest that TRPM3 helps regulate ion and fluid transport in epithelia. This function is particularly important in the eye where active ion-mediated fluid transport is critical for maintenance of cornea and lens transparency, intraocular pressure (ciliary body), and retinal attachment (RPE) (12, 57).
MITF is a transcription factor critical for the development of RPE (58). It plays an important role in RPE melanogenesis (59) and cell fate and induces expression of TRPM1 (60) and miR-211 as a result of cotranscription (53). Cotranscription of miR-211 is consistent with the proposed role of miR-211 in melanogenesis. The physiological function of TRPM1 in RPE has been implicated in congenital stationary night blindness in Appaloosa horses, in which decreased TRPM1 expression is associated with a reduced ON-bipolar cell depolarization in electroretinogram recordings (61).
miR-204/211: proliferation and maintenance of epithelial phenotype
TGF-β signaling is a critical determinant of the epithelial phenotype and plasticity and EMT (62,63,64). SNAIL2 and TGF-βR2 are direct targets of miR-204 and are also part of the TGF-β signaling pathway. In other epithelia, it has been shown that SNAIL proteins normally repress genes that code for claudins and other proteins critical for maintaining epithelial structure and function (65). The present data can be understood in terms of the schematic model shown in Fig. 9F and contains four key elements: 1) TGF-βR2 is a direct target of miR-204 (Fig. 10); 2) the data summarized in Figs. 8A and 10 show that SNAIL2 is also a direct target of miR-204; 3) anti-miR204 increased TGF-βR2 and decreased claudin 10, 16, and 19 expression (Western blots), all concomitant with a significant decrease in total tissue resistance (Figs. 9 and 11); and 4) addition of a siRNA cocktail for Smad3 and SNAIL1/2 was sufficient to partially rescue the anti-miR-204-induced decrease of total tissue resistance. The latter finding (Fig. 11A) and the model in Fig. 9F suggest that these transcription factors could activate downstream elements of the TGF-β signaling pathway and the dissolution of TJs (62). It has been shown in several systems that claudins 10, 16 and 19 determine TJ selectivity and resistance (4, 38).
In case I of the proposed model (Fig. 9F) the levels of miR-204 in RPE are normally high and inhibit TGF-βR2 as well as Smad3 and SNAIL1/2. The latter transcription factors are claudin repressors that allow high claudin expression, high TER, and a normal epithelial phenotype. In proliferative disease or in the early stages of EMT, the epithelial TJs disintegrate and TER can significantly decrease. Case II of Fig. 9F suggests that miR-204 may be a critical signal that initiates this process. A decrease in miR-204 would release the repression of its direct targets (TGF-βR2 and perhaps Smad3 and SNAIL1/2) and allow their activation by TGF-βR2. This pathway activates SNAIL and other transcription factors that have been shown to repress claudin expression, decrease TER, and lead to cell separation and proliferation (62). The latter prediction is supported by the data summarized in Fig. 7, showing that anti-miR-204 significantly increased RPE proliferation.
Anti-miR-204/211 also increased expression of CXCL12 and PDGF BB, which are known to be important in cell proliferation (32) and retinal diseases such as proliferative vitroretinopathy. Anti-miR-204/211 also decreased expression of genes critical for continuous operation of the visual cycle; for example, LRAT, RPE65, and TTR (Fig. 8C). These findings indicate an important role for miR-204/211 in mediating photoreceptor-driven alterations in RPE biochemistry (6).
miR-204/211 regulate epithelial cell physiology
In addition to decreasing TER (∼80%), our data show that anti-miR-204 significantly depolarized the apical and basolateral membranes. The latter effect could be caused by changes in plasma membrane electrogenic transporters or channels known to be located at the apical and basolateral membranes of human RPE (10). It has been shown that Kir7.1 is the most abundant potassium channel in human RPE, and it has been localized to the apical membrane (66,67,68) (B. Hughes, personal communication). The Western blot data in Fig. 9 show that Kir7.1 expression is decreased by anti-miR-204, possibly mediated by the activation of TGF-βR2, downstream signaling of protein kinase C, and subsequent inhibition of Kir7.1, as shown in other systems (69, 70).
According to our model, anti-miR-204/211 would allow TGF-βR2 activation and lead to a decrease in Kir7.1 at the apical membrane consistent with the observed depolarization (Fig. 12B). In addition, the anti-miR-204-induced increase in RA/RB is consistent with a decrease in apical membrane conductance caused possibly by a closure or reduction in apical membrane channels, perhaps Kir7.1 (Fig. 12C). Conversely, the present data show that in the presence of normal (high) levels of miR-204 the apical membrane potential is relatively hyperpolarized, which is critical for normal photoreceptor-RPE interactions (8, 12, 71).
Total transepithelial resistance (RT) is a direct measure of epithelial barrier integrity and consists of cellular and paracellular components. The cellular pathway also has two components, consisting of the apical (RA) and basolateral membrane resistance (RB). The paracellular path resistance is denoted by RS. By equivalent circuit analysis, the relationship between RT, RS, RA, and RB is 1/RT = 1/RS + 1/[RA+RB]. Previously we demonstrated in mammalian RPE (72) that [RA+RB] is ∼8-fold larger than RS, and a similar result has been demonstrated for cultured hfRPE (25-fold). This experimental result means that RT is determined mainly by RS and that an 80% reduction in RT reflects, to within 10%, an 80% reduction in paracellular path resistance (RS). TJ proteins determine paracellular path resistance and ionic selectivity, and these experiments, together with the claudin siRNA knockdown data, provide strong evidence that high TER is maintained by high levels of miR-204 and therefore plays a critical role in the ability of the RPE to protect the health and integrity of the distal retina.
The present experiments provide the first comparative survey of miRNAs enriched in human neuroretina, RPE, and choroid and the first functional information on miRNAs enriched in human RPE and their direct targets. In RPE, the two most highly expressed miRNAs (204 and 211) are involved in regulating several critical physiological functions. The maintenance of the outer blood-retina barrier in a quiescent state is a hallmark of normal adult epithelia.
Supplementary Material
Acknowledgments
It is the authors’ pleasure to thank George Reed and Peter Munson for statistical advice and Caifu Chen, Bret Hughes, and James Handa for their critical advice on an earlier version of the manuscript. The authors are also grateful to Boye Schnack Nielsen (Exiqon, Inc., Vedbaek, Denmark) for his help in modifying the Exiqon hybridization protocol for these studies and Roberto Pezza for help with semiquantative Western blots.
References
- Shin K., Fogg V. C., Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Bryant D. M., Mostov K. E. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie C. M., Anderson J. M. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- Hou J., Renigunta A., Gomes A. S., Hou M., Paul D. L., Waldegger S., Goodenough D. A. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A. 2009;106:10350–10355. doi: 10.1073/pnas.0907724106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. K., Steinberg R. H. Origin and organization of pigment epithelial apical projections to cones in cat retina. J Comp Neurol. 1982;206:131–145. doi: 10.1002/cne.902060204. [DOI] [PubMed] [Google Scholar]
- Travis G. H., Golczak M., Moise A. R., Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot E. F., Chang Y., Finnemann S. C. αvβ5 integrin receptors at the apical surface of the RPE: one receptor, two functions. Adv Exp Med Biol. 2008;613:369–375. doi: 10.1007/978-0-387-74904-4_43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallemore R., Hughes B., Miller S. Light-induced responses of the retinal pigment epithelium. Marmor M. F., Wolfensberger T. J., editors. Oxford University Press; New York: The Retinal Pigment Epithelium. 1998 [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Maminishkis A., Chen S., Jalickee S., Banzon T., Shi G., Wang F. E., Ehalt T., Hammer J. A., Miller S. S. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economopoulou M., Hammer J., Wang F., Fariss R., Maminishkis A., Miller S. S. Expression, localization, and function of junctional adhesion molecule-C (JAM-C) in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2009;50:1454–1463. doi: 10.1167/iovs.08-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adijanto J., Banzon T., Jalickee S., Wang N. S., Miller S. S. CO2-induced ion and fluid transport in human retinal pigment epithelium. J Gen Physiol. 2009;133:603–622. doi: 10.1085/jgp.200810169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenblatt R. B., Ferris F., 3rd Age-related macular degeneration and the immune response: implications for therapy. Am J Ophthalmol. 2007;44:618–626. doi: 10.1016/j.ajo.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A., Chew E., Rickman C., Abecasis G. Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu Rev Genomics Hum Genet. 2009;10:19–43. doi: 10.1146/annurev.genom.9.081307.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. J., Zeiss C., Chew E. Y., Tsai J. Y., Sackler R. S., Haynes C., Henning A. K., SanGiovanni J. P., Mane S. M., Mayne S. T., Bracken M. B., Ferris F. L., Ott J., Barnstable C., Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. O., Ritter R., 3rd, Abel K. J., Manning A., Panhuysen C., Farrer L. A. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Haines J. L., Hauser M. A., Schmidt S., Scott W. K., Olson L. M., Gallins P., Spencer K. L., Kwan S. Y., Noureddine M., Gilbert J. R., Schnetz-Boutaud N., Agarwal A., Postel E. A., Pericak-Vance M. A. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Toomes C., Bida L., Danciger M., Towns K. V., McKibbin M., Jacobson S. G., Logan C. V., Ali M., Bond J., Chance R., Swendeman S., Daniele L. L., Springell K., Adams M., Johnson C. A., Booth A. P., Jafri H., Rashid Y., Banin E., Strom T. M., Farber D. B., Sharon D., Blobel C. P., Pugh E. N., Jr, Pierce E. A., Inglehearn C. F. Loss of the metalloprotease ADAM9 leads to cone-rod dystrophy in humans and retinal degeneration in mice. Am J Hum Genet. 2009;84:683–691. doi: 10.1016/j.ajhg.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev E. V., Maniatis T. Multilevel regulation of gene expression by microRNAs. Science. 2008;319:1789–1790. doi: 10.1126/science.1152326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths-Jones S., Saini H. K., van Dongen S., Enright A. J. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L. P., Lau N. C., Garrett-Engele P., Grimson A., Schelter J. M., Castle J., Bartel D. P., Linsley P. S., Johnson J. M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S. N., Habermacher R., Martine U., Closs E. I., Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Lewis B. P., Burge C. B., Bartel D. P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Chen C., Ridzon D. A., Broomer A. J., Zhou Z., Lee D. H., Nguyen J. T., Barbisin M., Xu N. L., Mahuvakar V. R., Andersen M. R., Lao K. Q., Livak K. J., Guegler K. J. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., He X., Lim L. P., de Stanchina E., Xuan Z., Liang Y., Xue W., Zender L., Magnus J., Ridzon D., Jackson A. L., Linsley P. S., Chen C., Lowe S. W., Cleary M. A., Hannon G. J. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur A., Jewell D. A., Liang Y., Ridzon D., Moore J. H., Chen C., Ambros V. R., Israel M. A. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res. 2007;67:2456–2468. doi: 10.1158/0008-5472.CAN-06-2698. [DOI] [PubMed] [Google Scholar]
- Garzon R., Garofalo M., Martelli M. P., Briesewitz R., Wang L., Fernandez-Cymering C., Volinia S., Liu C. G., Schnittger S., Haferlach T., Liso A., Diverio D., Mancini M., Meloni G., Foa R., Martelli M. F., Mecucci C., Croce C. M., Falini B. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci U S A. 2008;105:3945–3950. doi: 10.1073/pnas.0800135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S. microRNA expression in the eyes and their significance in relation to functions. Prog Retin Eye Res. 2009;28:87–116. doi: 10.1016/j.preteyeres.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Rizzolo L. J. Development and role of tight junctions in the retinal pigment epithelium. Int Rev Cytol. 2007;258:195–234. doi: 10.1016/S0074-7696(07)58004-6. [DOI] [PubMed] [Google Scholar]
- Schmittgen T. D., Livak K. J. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Varallyay E., Burgyan J., Havelda Z. MicroRNA detection by Northern blotting using locked nucleic acid probes. Nat Protoc. 2008;3:190–196. doi: 10.1038/nprot.2007.528. [DOI] [PubMed] [Google Scholar]
- Li R., Maminishkis A., Wang F. E., Miller S. S. PDGF-C and -D induced proliferation/migration of human RPE is abolished by inflammatory cytokines. Invest Ophthalmol Vis Sci. 2007;48:5722–5732. doi: 10.1167/iovs.07-0327. [DOI] [PubMed] [Google Scholar]
- Li R., Maminishkis A., Banzon T., Wan Q., Jalickee S., Chen S., Miller S. S. IFNγ regulates retinal pigment epithelial fluid transport. Am J Physiol Cell Physiol. 2009;297:C1452–C1465. doi: 10.1152/ajpcell.00255.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Maminishkis A., Zahn G., Vossmeyer D., Miller S. S. Integrin α5β1 mediates attachment, migration, and proliferation in human retinal pigment epithelium: relevance for proliferative retinal disease. Invest Ophthalmol Vis Sci. 2009;50:5988–5996. doi: 10.1167/iovs.09-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi R. A., Leof E. B. TGF-β signaling: a tale of two responses. J Cell Biochem. 2007;102:593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- Martinez-Estrada O. M., Culleres A., Soriano F. X., Peinado H., Bolos V., Martinez F. O., Reina M., Cano A., Fabre M., Vilaro S. The transcription factors Slug and Snail act as repressors of claudin-1 expression in epithelial cells. Biochem J. 2006;394:449–457. doi: 10.1042/BJ20050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N., Fazio V., Cucullo L., Kight K., Masaryk T., Barnett G., Vogelbaum M., Kinter M., Rasmussen P., Mayberg M. R., Janigro D. Serum transthyretin monomer as a possible marker of blood-to-CSF barrier disruption. J Neurosci. 2003;23:1949–1955. doi: 10.1523/JNEUROSCI.23-05-01949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J., Renigunta A., Konrad M., Gomes A. S., Schneeberger E. E., Paul D. L., Waldegger S., Goodenough D. A. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen I., Hughes J. M., Vogels I. M., Schalkwijk C. G., Van Noorden C. J., Schlingemann R. O. Altered expression of genes related to blood-retina barrier disruption in streptozotocin-induced diabetes. Exp Eye Res. 2009;89:4–15. doi: 10.1016/j.exer.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Baskerville S., Bartel D. P. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA. 2005;11:241–247. doi: 10.1261/rna.7240905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory P. A., Bert A. G., Paterson E. L., Barry S. C., Tsykin A., Farshid G., Vadas M. A., Khew-Goodall Y., Goodall G. J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- Park S. M., Gaur A. B., Lengyel E., Peter M. E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak M., Silahtaroglu A., Moller M., Christensen M., Rath M. F., Skryabin B., Tommerup N., Kauppinen S. MicroRNA expression in the adult mouse central nervous system. RNA. 2008;14:432–444. doi: 10.1261/rna.783108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H., Olmeda D., Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Mueller D. W., Bosserhoff A. K. Role of miRNAs in the progression of malignant melanoma. Br J Cancer. 2009;101:551–556. doi: 10.1038/sj.bjc.6605204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Jazdzewski K., Li W., Liyanarachchi S., Nagy R., Volinia S., Calin G. A., Liu C. G., Franssila K., Suster S., Kloos R. T., Croce C. M., de la Chapelle A. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci U S A. 2005;102:19075–19080. doi: 10.1073/pnas.0509603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visone R., Russo L., Pallante P., De Martino I., Ferraro A., Leone V., Borbone E., Petrocca F., Alder H., Croce C. M., Fusco A. MicroRNAs (miR)-221 and miR-222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr Relat Cancer. 2007;14:791–798. doi: 10.1677/ERC-07-0129. [DOI] [PubMed] [Google Scholar]
- Sun T., Wang Q., Balk S., Brown M., Lee G. S., Kantoff P. The role of microRNA-221 and microRNA-222 in androgen-independent prostate cancer cell lines. Cancer Res. 2009;69:3356–3363. doi: 10.1158/0008-5472.CAN-08-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsimali M., Kloosterman W. P., de Bruijn E., Rosa F., Plasterk R. H., Wilson S. W. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol. 2007;8:R173. doi: 10.1186/gb-2007-8-8-r173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D. G., Oliveira-Fernandes M., Lavker R. M. MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol Vis. 2006;12:1175–1184. [PubMed] [Google Scholar]
- Yu J., Ryan D. G., Getsios S., Oliveira-Fernandes M., Fatima A., Lavker R. M. MicroRNA-184 antagonizes microRNA-205 to maintain SHIP2 levels in epithelia. Proc Natl Acad Sci U S A. 2008;105:19300–19305. doi: 10.1073/pnas.0803992105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Yang X., Xie B., Chen Y., Swaim M., Hackett S. F., Campochiaro P. A. MicroRNAs regulate ocular neovascularization. Mol Ther. 2008;16:1208–1216. doi: 10.1038/mt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karali M., Peluso I., Marigo V., Banfi S. Identification and characterization of microRNAs expressed in the mouse eye. Invest Ophthalmol Vis Sci. 2007;48:509–515. doi: 10.1167/iovs.06-0866. [DOI] [PubMed] [Google Scholar]
- Deo M., Yu J. Y., Chung K. H., Tippens M., Turner D. L. Detection of mammalian microRNA expression by in situ hybridization with RNA oligonucleotides. Dev Dyn. 2006;235:2538–2548. doi: 10.1002/dvdy.20847. [DOI] [PubMed] [Google Scholar]
- Murisier F., Beermann F. Genetics of pigment cells: lessons from the tyrosinase gene family. Histol Histopathol. 2006;21:567–578. doi: 10.14670/HH-21.567. [DOI] [PubMed] [Google Scholar]
- Harteneck C., Reiter B. TRP channels activated by extracellular hypo-osmoticity in epithelia. Biochem Soc Trans. 2007;35:91–95. doi: 10.1042/BST0350091. [DOI] [PubMed] [Google Scholar]
- Levin M. H., Verkman A. S. Aquaporins and CFTR in ocular epithelial fluid transport. J Membr Biol. 2006;210:105–115. doi: 10.1007/s00232-005-0849-1. [DOI] [PubMed] [Google Scholar]
- Bharti K., Liu W., Csermely T., Bertuzzi S., Arnheiter H. Alternative promoter use in eye development: the complex role and regulation of the transcription factor MITF. Development. 2008;135:1169–1178. doi: 10.1242/dev.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassabehji M., Newton V. E., Read A. P. Waardenburg syndrome type 2 caused by mutations in the human microphthalmia (MITF) gene. Nat Genet. 1994;8:251–255. doi: 10.1038/ng1194-251. [DOI] [PubMed] [Google Scholar]
- Zhiqi S., Soltani M. H., Bhat K. M., Sangha N., Fang D., Hunter J. J., Setaluri V. Human melastatin 1 (TRPM1) is regulated by MITF and produces multiple polypeptide isoforms in melanocytes and melanoma. Melanoma Res. 2004;14:509–516. doi: 10.1097/00008390-200412000-00011. [DOI] [PubMed] [Google Scholar]
- Bellone R. R., Brooks S. A., Sandmeyer L., Murphy B. A., Forsyth G., Archer S., Bailey E., Grahn B. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns (LP) in the Appaloosa horse (Equus caballus) Genetics. 2008;179:1861–1870. doi: 10.1534/genetics.108.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Lamouille S., Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S., Kono-Saika S., Tanaka T., Yamanaka O., Ohnishi Y., Sato M., Muragaki Y., Ooshima A., Yoo J., Flanders K. C., Roberts A. B. Smad3 is required for dedifferentiation of retinal pigment epithelium following retinal detachment in mice. Lab Invest. 2004;84:1245–1258. doi: 10.1038/labinvest.3700156. [DOI] [PubMed] [Google Scholar]
- Giampieri S., Manning C., Hooper S., Jones L., Hill C. S., Sahai E. Localized and reversible TGFβ signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrozzino F., Soulie P., Huber D., Mensi N., Orci L., Cano A., Feraille E., Montesano R. Inducible expression of Snail selectively increases paracellular ion permeability and differentially modulates tight junction proteins. Am J Physiol Cell Physiol. 2005;289:C1002–C1014. doi: 10.1152/ajpcell.00175.2005. [DOI] [PubMed] [Google Scholar]
- Yang D., Zhang X., Hughes B. A. Expression of inwardly rectifying potassium channel subunits in native human retinal pigment epithelium. Exp Eye Res. 2008;87:176–183. doi: 10.1016/j.exer.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Pan A., Swaminathan A., Kumar G., Hughes B. A. Expression and localization of the inwardly rectifying potassium channel Kir71 in native bovine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:3178–3185. doi: 10.1167/iovs.02-1189. [DOI] [PubMed] [Google Scholar]
- Hejtmancik J. F., Jiao X., Li A., Sergeev Y. V., Ding X., Sharma A. K., Chan C. C., Medina I., Edwards A. O. Mutations in KCNJ13 cause autosomal-dominant snowflake vitreoretinal degeneration. Am J Hum Genet. 2008;82:174–180. doi: 10.1016/j.ajhg.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zitron E., Bloehs R., Muller-Krebs S., Scholz E., Zeier M., Katus H., Karle C., Schwenger V. Dual regulation of renal Kir71 potassium channels by protein kinase A and protein kinase C. Biochem Biophys Res Commun. 2008;377:981–986. doi: 10.1016/j.bbrc.2008.10.110. [DOI] [PubMed] [Google Scholar]
- Yakymovych I., Ten Dijke P., Heldin C. H., Souchelnytskyi S. Regulation of Smad signaling by protein kinase C. FASEB J. 2001;15:553–555. doi: 10.1096/fj.00-0474fje. [DOI] [PubMed] [Google Scholar]
- Hughes B., Gallemore R., Miller S. Transport mechanisms in the retinal pigment epithelium. Marmor M. F., Wolfensberger T. J., editors. Oxford University Press; New York: The Retinal Pigment Epithelium. 1998 [Google Scholar]
- Joseph D. P., Miller S. S. Apical and basal membrane ion transport mechanisms in bovine retinal pigment epithelium. J Physiol. 1991;435:439–463. doi: 10.1113/jphysiol.1991.sp018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asangani I. A., Rasheed S. A., Nikolova D. A., Leupold J. H., Colburn N. H., Post S., Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.