Abstract
We recently reported that IGF-II binding to the IGF-II/mannose-6-phosphate (M6P) receptor activates the ERK1/2 cascade by triggering sphingosine kinase 1 (SK1)-dependent transactivation of G protein-coupled sphingosine 1-phosphate (S1P) receptors. Here, we investigated the mechanism of IGF-II/M6P receptor-dependent sphingosine kinase 1 (SK1) activation in human embryonic kidney 293 cells. Pretreating cells with protein kinase C (PKC) inhibitor, bisindolylmaleimide-I, abolished IGF-II-stimulated translocation of green fluorescent protein (GFP)-tagged SK1 to the plasma membrane and activation of endogenous SK1, implicating PKC as an upstream regulator of SK1. Using confocal microscopy to examine membrane translocation of GFP-tagged PKCα, β1, β2, δ, and ζ, we found that IGF-II induced rapid, transient, and isoform-specific translocation of GFP-PKCβ2 to the plasma membrane. Immunoblotting of endogenous PKC phosphorylation confirmed PKCβ2 activation in response to IGF-II. Similarly, IGF-II stimulation caused persistent membrane translocation of the kinase-deficient GFP-PKCβ2 (K371R) mutant, which does not dissociate from the membrane after translocation. IGF-II stimulation increased diacylglycerol (DAG) levels, the established activator of classical PKC. Interestingly, the polyunsaturated fraction of DAG was increased, indicating involvement of phosphatidyl inositol/phospholipase C (PLC). Pretreating cells with the PLC inhibitor, U73122, attenuated IGF-II-dependent DAG production and PKCβ2 phosphorylation, blocked membrane translocation of the kinase-deficient GFP-PKCβ2 (K371R) mutant, and reduced sphingosine 1-phosphate production, suggesting that PLC/PKCβ2 are upstream regulators of SK1 in the pathway. Taken together, these data provide evidence that activation of PLC and PKCβ2 by the IGF-II/M6P receptor are required for the activation of SK1.
The IGF-II/mannose-6-phosphate (M6P) receptor is a multifunctional single-transmembrane glycoprotein that is known to regulate diverse biological functions. It is composed of a large extracellular domain, a single transmembrane region, and a short cytoplasmic tail that lacks intrinsic catalytic activity. The receptor binds IGF-II with higher affinity than IGF-I and does not bind insulin. In addition to its role in lysosomal enzyme trafficking, clearance and/or activation of a variety of growth factors, and endocytosis-mediated degradation of IGF-II, several studies have supported its role in transmembrane signaling (1). Binding of IGF-II to the IGF-II/M6P receptor reportedly stimulates calcium influx in mouse embryo fibroblasts (2), increased glycogen synthesis in hepatoma cells (3), insulin exocytosis in pancreatic β-cells (4), cell mobility in rhabdomyosarcoma cells (5), migration of human extravillous trophoblasts (6), endothelial progenitor cells homing (7), hypertrophy in cardiomyoblasts and mitochondrial-dependent apoptosis in myocardial cells (5–7), and cholinergic neuron function in the central nervous system (8). Involvement of the IGF-II/M6P receptors in these responses has been shown by various means, including use of IGF-II analog, Leu27IGF-II, which recognizes the IGF-II/M6P receptor but not IGF-I receptor (5, 7, 9), receptor antibodies to block or mimic responses to IGF-II (4, 8, 10), and down-regulation of endogenous receptor expression by RNA interference (11). Whereas these studies provide strong evidence that IGF-II/M6P receptors engage in intracellular signaling, the mechanisms whereby the receptor mediates these effects remain poorly understood.
The protein kinase C (PKC) family of serine/threonine kinases, mediate a wide variety of cellular responses. Based on structural and cofactor requirements, PKC are divided into three subfamilies; classical or conventional PKC (α, β1, β2, γ), novel PKC (δ, ε, η, θ, μ), and atypical PKC (ζ, λ, ι). Activation of PKC is mediated primarily through increased release of diacylglycerol (DAG) and/or intracellular Ca2+ (12, 13). There is a growing body of evidence supporting the involvement of PKC in IGF-II/M6P signaling. For instance, promotion of insulin secretion by pancreatic β cells (4), vascular endothelial growth factor expression in human keratinocytes (14), and regulation of central cholinergic function (8) by IGF-II stimulation are all sensitive to PKC inhibition. Binding of Leu27IGF-II to the IGF-II/M6P receptor leads to increased phosphorylation of PKCα in cardiomyeloblast cells (5). Additionally, earlier reports suggested that the cytoplasmic tail of IGF-II/M6P receptor contains four sites that are potential substrates for protein kinases including PKC, casein kinases 1 and 2, and cAMP-dependent protein kinase (15, 16).
Sphingosine kinase 1 (SK1), a key enzyme catalyzing the formation of sphingosine 1-phosphate (S1P), has been implicated in signaling by myriad effectors including growth factors, cytokines, and agonists of various G protein-coupled receptors. We have previously reported that IGF-II binding to the IGF-II/M6P receptor stimulates MAPK through SK1-dependent transactivation of G protein-coupled S1P receptors (11). However, little is known about the underlying mechanisms by which IGF-II induces SK1 activation. Previous studies have suggested that PKC regulates SK1 activity. For example, PKC inhibition blocks phorbol myristate acetate (PMA)-induced translocation of SK1 to the plasma membrane (17). Functionally, PKC-dependent SK1 activation mediates PMA-induced angiogenesis (18), epidermal growth factor-induced breast cancer cell migration and proliferation (19), and histamine-triggered endothelial cell migration (20). Hyperglycemia stimulates SK1 activation via a PKC-dependent pathway, leading to decreased apoptosis in human aortic smooth muscle cells (21). Although SK1 possesses several putative PKC phosphorylation sites (22), purified PKC fails to stimulate SK1 activity in vitro, suggesting its effects are indirect (23). Nonetheless, PKC is thought to regulate SK1 by two mechanisms: acutely by promoting its translocation to the plasma membrane, where it has high affinity for phosphatidylserine-rich lipid bilayers (17, 24–26), and chronically by stimulating SK1 transcription (19, 23).
In the present study, we have examined the potential role of phospholipase C (PLC)/DAG/PKC in IGF-II-dependent SK1 activation. We find that PKC inhibition inhibits endogenous SK1 activation and blocks its translocation to the plasma membrane. We also find that IGF-II stimulates a rapid elevation of DAG, rapid and transient membrane translocation and phosphorylation of PKCβ2, and S1P production, responses that are all sensitive to PLC inhibition. These data suggest that activation of PLC and PKC are proximal signaling events leading to SK1 activation by the IGF-II/M6P receptor.
Results
Down-regulation of the IGF-II/M6P receptor blocks SK1 activation by IGF-II
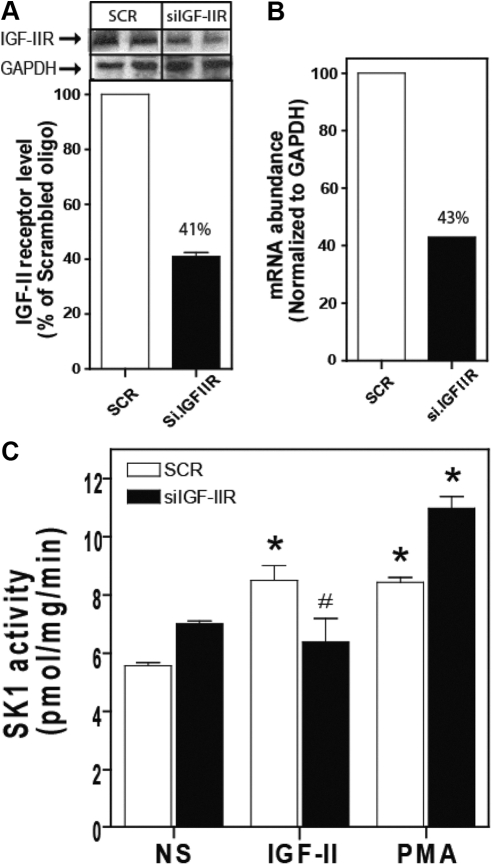
We recently reported that IGF-II binding to the IGF-II/M6P receptor stimulates ERK1/2 via a mechanism involving SK1-dependent transactivation of G protein-coupled S1P receptors (11, 27). We demonstrated that simultaneous transfection of human embryonic kidney (HEK)293 cells with two IGF-II/M6P receptor-specific small interfering RNA (siRNA) efficiently reduced receptor mRNA and protein levels and reduced IGF-II-dependent ERK1/2 activation (11). To determine the role of the IGF-II/M6P receptor in IGF-II-stimulated SK1 activation, we employed RNA interference to down-regulate IGF-II/M6P receptor and SK1 expression. Simultaneous transfection of HEK293 cells with two IGF-II/M6P receptor-specific siRNA reduced receptor protein and mRNA levels by 57% and 59%, respectively (Figs. 1, A and B). To determine whether the IGF-II/M6P receptor mediated IGF-II-stimulated SK1 activation, we directly measured SK1 activity under these conditions. As shown in Fig. 1C, 10 min exposure to either IGF-II or PMA significantly increased SK1 activity (8.51 ± 0.89 and 8.44 ± 0.30 pmol/min · mg protein, respectively, compared with basal activity of 5.57 ± 0.2 pmol/min · mg protein) in cells transfected with control scrambled siRNA. Down-regulation of the IGF-II/M6P receptor inhibited activation of SK1 by IGF-II (6.6 ± 1.4 pmol/min · mg protein compared with basal activity 7.1 ± 0.2) with no effect on PMA-dependent SK1 activation (11.0 ± 0.7 pmol/min · mg protein) consistent with our prior observation that IGF-II signals mainly by binding its cognate IGF-II/M6P receptor (11).
Fig. 1.
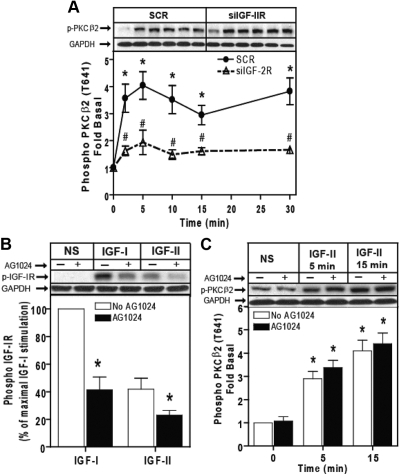
Down-regulation of IGF-II/M6P receptor expression by RNA interference inhibits IGF-II-dependent activation of SK1. A, HEK293 cells were transfected with control scrambled siRNA (SCR) or siRNA targeting the IGF-II/M6P receptor (siIGF-2R) for 48 h. The levels of IGF-II/M6P receptor and GAPDH were determined by immunoblotting whole-cell lysates. A representative IGF-II/M6P receptor and basal GAPDH immunoblots are shown above a bar graph depicting mean ± sd for three independent experiments. B, RNA was isolated and mRNA levels of IGF-II/M6P receptor and GAPDH were determined by quantitative real-time PCR. C, Serum-starved cells transfected with scrambled siRNA or siRNA targeting the IGF-II/M6P receptor were stimulated with 10 nm IGF-II or 100 nm PMA for 10 min after which SK1 activity in whole-cell lysates was assayed as described. Data shown represent the mean ± sd of three independent experiments. *, P < 0.05 vs. nonstimulated (NS); #, P < 0.05 vs. scrambled (SCR) treated.
PKC is required for IGF-II-dependent SK1 activation and green fluorescent protein (GFP)-SK1 translocation
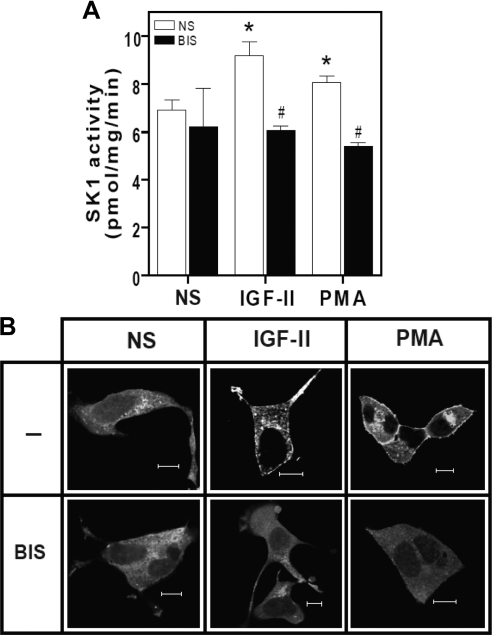
Previous studies have implicated PKC in ligand-induced IGF-II/M6P receptor signaling (5, 8, 28). In addition, several lines of evidence suggest that PKC regulates the activation of SK1 (22). To investigate whether SK1 activation by IGF-II is PKC dependent, we measured the activity of endogenous SK1 and observed the translocation of GFP-hSK1 in the presence or absence of the broad-spectrum PKC inhibitor, bisindolylmaleimide I. As shown in Fig. 2A, stimulation with either IGF-II or PMA for 10 min produced a significant increase in endogenous SK1 activity (9.2 ± 1.0 and 8.06 ± 0.49 pmol/min · mg protein, respectively, compared with basal activity of 6.93 ± 0.71 pmol/min · mg protein). This activation was abolished in the presence of the PKC inhibitor (6.08 ± 0.323 and 5.42 ± 0.23 pmol/min · mg protein, respectively), suggesting that PKC activity is required for SK1 activation in response to IGF-II, but not for the maintenance of basal SK1 activity. Next we examined the cellular distribution of GFP-hSK1 upon agonist stimulation in the presence or absence of bisindolylmaleimide I. As depicted in Fig. 2B, stimulation of HEK293 cells transiently expressing GFP-hSK1 with IGF-II or PMA for 10 min resulted in translocation of GFP-hSK1 to the plasma membrane. The IGF-II effect was blocked by PKC inhibition, similar to previous reports that PKC inhibition abrogates PMA-dependent SK1 translocation (17).
Fig. 2.
IGF-II-stimulated SK1 activation and translocation is PKC dependent. A, IGF-II-dependent-SK1 activation is sensitive to PKC inhibition. Serum-deprived HEK293 cells were pretreated with 10 nm bisindolylmaleimide I (BIS) for 30 min before stimulation with 10 nm IGF-II or 100 nm PMA. Cells were scraped and homogenized, and whole-cell lysates were assayed for SK1 activity as described. Data shown represent mean ± sd of three independent experiments. *, P < 0.05 vs. nonstimulated (NS); #, P < 0.05 vs. stimulated. B, IGF-II-induced GFP-SK1 translocation is PKC dependent. Serum-deprived HEK293 cells transiently transfected with GFP-hSK1 were preincubated with 10 nm bisindolylmaleimide I (BIS) for 30 min before stimulation with 10 nm IGF-II or 100 nm PMA for 30 min. Monolayers were fixed, and the cellular distribution of GFP-SK1 was examined by confocal fluorescence microscopy. Shown are representative confocal images from one of three independent experiments that gave similar results. Scale bar, 10 μm.
IGF-II induces rapid and transient translocation and activation of GFP-PKCβ2
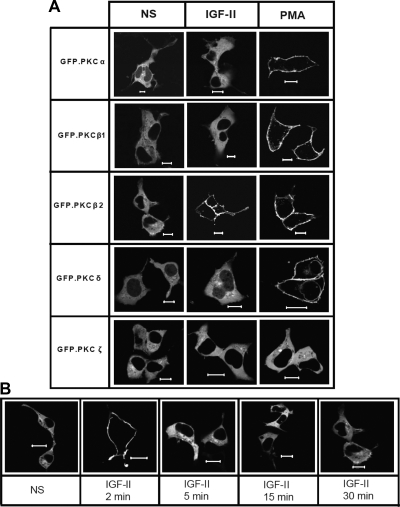
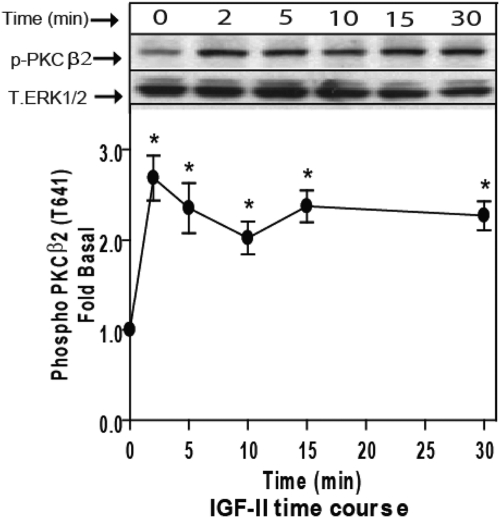
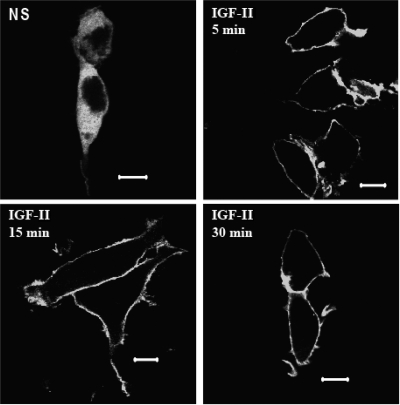
Because PKC activation is accompanied by its translocation to the plasma membrane, we employed confocal fluorescence microscopy to examine the effect of IGF-II stimulation on the cellular distribution of a panel of GFP-tagged PKC isoforms. HEK293 cells were transfected with GFP-tagged PKC constructs encoding representative classical (α, β1, β2), novel (δ), and atypical (ζ) PKC isoforms and stimulated for 2, 5, 15, or 30 min. As shown in Fig. 3A, IGF-II stimulation resulted in translocation of GFP-PKCβ2 to the plasma membrane. The effect was transient, because it was detectable only at 2 min stimulation (Fig. 3B). None of the other PKC isoforms tested responded to IGF-II at any time point. In contrast, PMA treatment produced sustained membrane translocation of all GFP-tagged classical and novel PKC isoforms. Only the atypical PKC, GFP-PKCζ, which lacks the Ca2+-binding C2 domain and cannot be activated by DAG or Ca2+ (29), failed to respond to PMA. To substantiate the selectivity of PKC activation by IGF-II, we employed isoform-specific phospho-PKC antisera to detect the changes in the phosphorylation of endogenous PKC isoforms. As shown in Fig. 4, stimulation of HEK293 cells with IGF-II increased Thr641 phosphorylation of PKCβ2 within 2 min, an effect that persisted for up to 30 min. In contrast, we failed to detect IGF-II-stimulated phosphorylation of PKCα Ser657 or Tyr687; PKCβ1 Thr642, PKCδ/θ Ser643/676; or PKCζ/λ Thr410/403 (data not shown). Together these data suggest that IGF-II stimulates isoform-specific translocation and activation of PKCβ2.
Fig. 3.
IGF-II stimulation causes rapid and transient translocation of GFP-PKCβ2 to the plasma membrane. Serum-starved HEK293 cells transiently transfected with GFP-PKCα, β1, β2, δ, and ζ were stimulated with 10 nm IGF-II or 100 nm PMA for (A) 2 min (panel A) or at time indicated (panel B) before fixation and examination of the cellular distribution of GFP-PKC by confocal fluorescence microscopy. Shown are representative confocal fields from one of three independent experiments that gave similar results. Scale bar, 10 μm. NS, Nonstimulated.
Fig. 4.
IGF-II induces activation of endogenous PKCβ2. Serum-starved HEK293 cells were stimulated with 10 nm IGF-II for the indicated times, and activation of PKCβ2 in whole-cell lysate samples was determined by immunoblotting with phosphorylation state-specific IgG. PKCβ2 phosphorylation is expressed as fold increase above the basal level in NS cells. A representative phospho-PKCβ2 and total ERK1/2 immunoblots are shown above a bar graph presenting mean ± sd of three independent experiments; *, P < 0.05 vs. nonstimulated (NS).
IGF-II induces persistent membrane translocation of a catalytically inactive PKCβ2 mutant
Previous work on PKCβ2 has shown that its catalytic activity is essential for its dissociation from the plasma membrane and that a kinase-deficient PKCβ2(K371R) mutant exhibits persistent membrane localization in response to activating stimuli (30). To confirm our results with GFP-tagged wild-type and endogenous PKCβ2, we tested whether IGF-II would affect the cellular distribution of GFP-PKCβ2 (K371R). As shown in Fig. 5, GFP-PKCβ2 (K371R) was distributed evenly in the cytosol of unstimulated HEK293 cells but underwent irreversible membrane translocation in response to IGF-II. This finding supports the hypothesis that IGF-II activates PKCβ2 as an early upstream event in the initiation of IGF-II/M6P receptor signaling.
Fig. 5.
IGF-II induces persistent membrane localization of the GFP-PKCβ2(K371R) mutant. Serum-deprived HEK293 cells transiently transfected with GFP-PKCβ2(K371R) were stimulated with 10 nm IGF-II for the indicated time before fixation and examination by confocal fluorescence microscopy. Shown are representative confocal images from one of three independent experiments that gave similar results. Scale bar, 10 μm. NS, Nonstimulated.
IGF-II induces PKCβ2 phosphorylation via IGF-II/M6P receptor and independent of IGF-I receptor
We have previously shown that IGF-II signals predominantly via IGF-II/M6P receptor and in part by binding to the IGF-I receptor and that the response mediated by the IGF-II/M6P receptor is independent of IGF-I receptor (11). To determine the role of both IGF-I receptors and IGF-II/M6P receptors in IGF-II-dependent PKCβ2 activation, we first employed RNA interference to down-regulate IGF-II/M6P receptor under the same conditions used in Fig. 1. Time course stimulation of IGF-II in control scrambled treated cells induced a 3- to 4-fold increase of PKCβ2 phosphorylation, and down-regulation of IGF-II/M6P receptor significantly inhibited IGF-II-dependent phosphorylation of PKCβ2 (Fig. 6A). To further determine whether IGF-I receptor plays a role in PKCβ2 activation by IGF-II, we used the IGF-I receptor-specific inhibitor, AG1024. Pretreating cells with 1 μm AG1024 for 15 min markedly inhibited IGF-I- and IGF-II-stimulated IGF-I receptor β-subunit phosphorylation by 59% and 45%, respectively (Fig. 7B). Using the same concentration of AG1024 before cell stimulation did not affect IGF-II-stimulated phosphorylation of PKCβ2 (Fig. 7C). These findings indicate that IGF-II/M6P receptor, and not IGF-I receptor, mediates IGF-II-dependent phosphorylation of PKCβ2.
Fig. 6.
IGF-II/M6P receptor mediates IGF-II-stimulated PKCβ2 phosphorylation. A, Serum-starved HEK293 cells were transfected with control scrambled siRNA (SCR) or siRNA targeting the IGF-II/M6P receptor (siIGF-2R) for 48 h were stimulated with 10 nm IGF-II for the indicated times, and activation of PKCβ2 in whole-cell lysate samples was determined by immunoblotting with phosphorylation state-specific IgG. PKCβ2 phosphorylation is expressed as fold increase above the basal level in NS cells. A representative phospho-PKCβ2 and basal GAPDH immunoblots are shown above a bar graph depicting mean ± sd for three independent experiments. *, P < 0.05 vs. nonstimulated (NS); #, P < 0.05 vs. scrambled (SCR) treated. B, Serum-deprived cells were preincubated with 1 μm AG1024 for 15 min before stimulation with 10 nm IGF-I and 10 nm IGF-II for 10 min. Basal (NS), IGF-I-stimulated, and IGF-II-stimulated IGF-I receptor β-subunit phosphorylation was determined as described. The change in IGF-I receptor phosphorylation is expressed as the percentage of maximal stimulation. p-IGF-1R, phosphorylated IGF-1R. C, Serum-starved HEK293 cells were pretreated with 1 μm AG1024 for 15 min before stimulation with 10 nm IGF-II for the indicated times, and activation of PKCβ2 in whole-cell lysate samples was determined by immunoblotting with phosphorylation state-specific IgG. PKCβ2 phosphorylation is expressed as fold increase above the basal level in NS cells. A representative phospho-PKCβ2 and basal GAPDH immunoblots are shown above a bar graph depicting mean ± sd for three independent experiments. *, P < 0.05 vs. nonstimulated (NS).
Fig. 7.
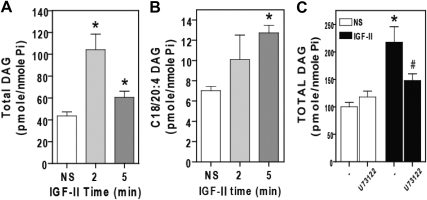
IGF-II stimulates rapid PLC inhibitor-sensitive increases in DAG. Serum-starved HEK293 cells were stimulated with 10 nm IGF-II for the indicated times, after which they were scraped and homogenized, and lipids were extracted and analyzed by quantitative mass spectrometry for total DAG (panel A) or the C18/20:4 fraction of DAG (panel B). C, Cells were pretreated with 5 μm U73122 for 30 min before stimulation with 10 nm IGF-II for 5 min. Total DAG content was assayed by mass spectroscopy. In each panel, data shown represent mean ± sd of three independent experiments. *, P < 0.05 vs. nonstimulated (NS); #, P < 0.05 vs. IGF-II stimulated.
IGF-II stimulates DAG release
The lipid second messenger, DAG, along with cytosolic Ca2+, regulates the dynamic redistribution of PKC isoforms between the cytosol and plasma membrane. DAG binds to the conserved C1 domain of classical and novel PKC isoforms, whereas Ca2+ binds the C2 domain of classical PKC. As a result, membrane translocation of classical PKC isoforms usually, but not invariably, involves binding of both DAG and Ca2+, whereas return to the cytosol results from rapid metabolism of DAG and PKC catalytic activity (12). There are multiple sources of DAG in mammalian cells: generation from phosphatidylinositols by PLC takes place mainly at the plasma membrane, de novo synthesis occurs mainly in the endoplasmic reticulum, whereas the origin of DAG generated from phospholipids by phospholipase D (PLD) and phosphatidate phosphatase is uncertain (31). Both DAG and Ca2+ are produced by phospholipase C (PLC), which catalyzes hydrolysis of phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate (IP3) and DAG. IP3, in turn, mediates release of intracellular Ca2+ by binding to receptors on the endoplasmic reticulum (32).
To examine the mechanism by which IGF-II activates PKCβ2, we employed tandem mass spectrometry to look for changes in DAG levels upon IGF-II exposure. Stimulation of HEK293 cells with IGF-II led to a significant elevation of total DAG at 2 min [104.2 ± 25.03 pmol/nmol phosphate (Pi)] and 5 min (60.5 ± 9.76 pmol/nmol Pi) compared with basal (43.6 ± 6.48 pmol/nmol Pi) (Fig. 7A). Interestingly, the polyunsaturated fraction of DAG (C18/20:4) was also significantly elevated at 2 min (11.18 ± 3.76 pmol/nmol Pi) and 5 min (12.74 ± 1.29 pmol/nmol Pi) compared with basal (7.03 ± 0.69 pmol/nmol Pi), representing about 34% of the total increase in DAG (Fig. 7B). The rise in polyunsaturated DAG level implicates phosphatidylinositol/PLC in IGF-II signaling, because phosphatidylinositol is formed primarily from the polyunsaturated fatty acids such as arachidonic acid. However we were unable to detect a significant increase in intracellular calcium in response to IGF-II in either HEK293 or primary rat vascular smooth muscle cells by calcium fluorometry using a fluorometric imaging plate reader (data not shown).
To directly test whether PLC contributed to IGF-II-stimulated DAG generation, we employed the PLC inhibitor (U73122). As shown in Fig. 7C, whereas IGF-II stimulation at 5 min increased generation of DAG (217.89 ± 28.20 pmol/nmol Pi) over basal (100.04 ± 8.27 pmol/nmol Pi), preincubation with U73122 significantly attenuated IGF-II-dependent DAG generation (147.91 ± 12.2 pmol/nmol Pi). The U73122-sensitive fraction represented 60% of the net increase in total DAG, suggesting that PLC is a significant source of the DAG produced in response to IGF-II.
PLC inhibition prevents IGF-II-dependent activation of PKCβ2
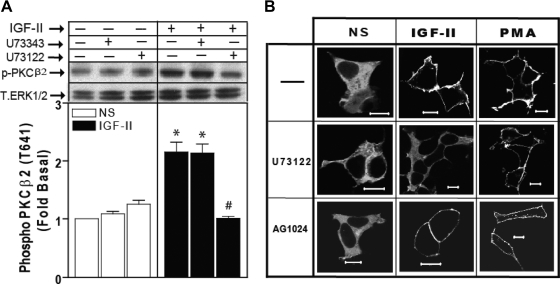
We next tested whether PLC is required for IGF-II-stimulated PKCβ2 activation. Western blot analysis of HEK293 cell lysates demonstrated a 2-fold increase in PKCβ2 phosphorylation within 2 min of IGF-II stimulation that was abrogated by pretreating cells with U73122. The inactive analog U73343 has no effect on the response (Fig. 8A). As shown in Fig. 8B, U73122 also blocked the IGF-II-dependent membrane translocation of GFP-PKCβ2 (K371R) whereas AG1024 did not, suggesting that DAG generated by PLC is required for recruitment of PKCβ2 to the plasma membrane and independent of IGF-I receptor. Because PMA bypasses the requirement for PLC by mimicking the action of DAG (33), both U73122 and AG1024 did not affect PMA-dependent membrane translocation of GFP-PKCβ2 (K371R).
Fig. 8.
IGF-II-stimulated PKCβ2 activation and translocation are PLC dependent. A, PLC inhibition prevents IGF-II-stimulated PKCβ2 phosphorylation. Serum-deprived HEK293 cells preincubated with 5 μm U73122 or its inactive analog, U73343, for 30 min before stimulation with 10 nm IGF-II. PKCβ2 activation was determined in whole-cell lysates by immunoblotting with antiphospho (T641) PKCβ2 IgG. PKCβ2 phosphorylation is expressed as fold increase above the basal level in nonstimulated (NS) transfected cells. A representative phospho-PKCβ2 immunoblot is shown above a bar graph presenting mean ± sd of three independent experiments. A total ERK1/2 performed on the same filter is shown as a loading control. *, P < 0.05 vs. NS; #, P < 0.05 vs. IGF-II stimulated. B, IGF-II-dependent membrane translocation of the GFP-PKCβ2(K371R) mutant is PLC dependent. Serum-starved HEK293 cells transiently transfected with GFP-PKCβ2(K371R) were exposed to 5 μm U73122 and 1 μm AG1024 before stimulation with 10 nm IGF-II and 100 nm PMA for 30 min. Cells were then fixed and examined by confocal fluorescence microscopy. Shown are representative confocal images from one of three independent experiments that gave similar results. Scale bar, 10 μm.
PLC is involved in IGF-II-dependent S1P production
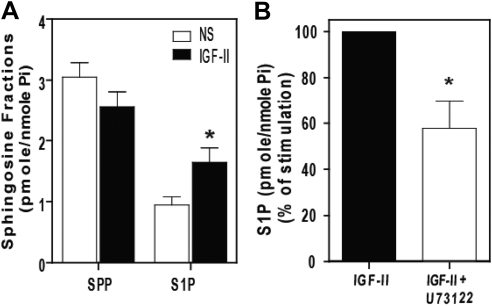
Because PLC was required for IGF-II-stimulated PKCβ2 activation, we tested whether it was also required for SK1 activation. As shown in Fig. 9A, mass spectrometric analysis of sphingolipid levels performed on HEK293 cells revealed that the IGF-II treatment produced a significant increase in cellular S1P level accompanied by a decrease in sphingosine (SPP). This is consistent with activation of SK1, which utilizes SPP to produce S1P, and agrees with our previous results obtained using in vivo radiolabeling and S1P separation by thin layer chromatography (27). As shown in Fig. 9B, pretreatment with U73122 significantly inhibited IGF-II-stimulated S1P production, indicating that PLC plays a role in IGF-II-stimulated SK1 activation.
Fig. 9.
PLC inhibition attenuates IGF-II-stimulated S1P production. A, Serum-starved HEK293 cells were stimulated with 10 nm IGF-II for 5 min, after which lipids were extracted and assayed for SPP and S1P by quantitative mass spectrometry. B, Cells were preincubated with 5 μm U73122 for 30 min before stimulation with 10 nm IGF-II for 5 min and determination of S1P. In each panel, data shown represent mean ± sd of three independent experiments; *, P < 0.05 vs. nonstimulated (NS) (panel A) and IGF-II stimulated (panel B).
Down-regulation of the PKCβ2 blocks C17-S1P production by IGF-II
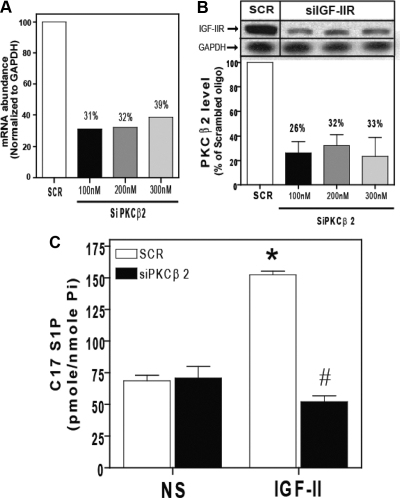
To further confirm that PKCβ2 mediated IGF-II-dependent SK1 activation, we employed RNA interference specifically targeting PKCβ2. Using three concentrations of siRNA, 100 nm, 200 nm, and 300 nm, efficiently down-regulated PKCβ2 protein levels by 74%, 68%, and 67%, respectively; and RNA levels by 69%, 68% and 61%, respectively (Fig. 10, A and B). Then we used in situ C17-sphingosine labeling. C17-sphingosine lacks one carbon in their nonpolar moiety compared with the natural C18-sphingosine and used recently for mass spectrometry measurement of S1P production as an indicator for SK1 activation (34). As shown in Fig. 10C, IGF-II stimulation for 15 min caused about a 2-fold increase in C17-S1P production that was abolished when PKCβ2 was down-regulated using the 100 nm concentration of siRNA of PKCβ2, indicating the requirement of PKCβ2 for SK1 activation by IGF-II.
Fig. 10.
Down-regulation of PKCβ2 expression by RNA interference inhibits IGF-II-dependent activation of SK1. A, HEK293 cells were transfected with control scrambled siRNA (SCR) or siRNA targeting siRNA targeting the PKCβ2 (siPKCβ2) for 48 h. RNA was isolated and mRNA levels of PKCβ2 and GAPDH were determined by quantitative real-time PCR. B, The levels of PKCβ2 and GAPDH were determined by immunoblotting whole-cell lysates. A representative PKCβ2 and basal GAPDH immunoblots are shown above a bar graph depicting mean ± sd for three independent experiments. C, Serum-deprived cells transfected with scrambled siRNA or siRNA targeting the PKCβ2 (100 nm) were stimulated with 10 nm IGF-II for 10 min, after which C17 S1P in whole-cell lysates was assayed as described. Data shown represent the mean ± sd of three independent experiments. *, P < 0.05 vs. nonstimulated (NS); #, P < 0.05 vs. scrambled (SCR) treated.
IGF-II-induced proliferation of human mesangial cells is attenuated by SK inhibitor
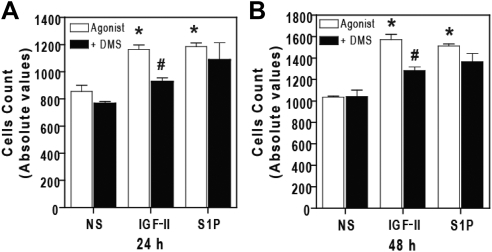
Activation of SK1 and production of S1P are known to enhance proliferative pathways. To test whether the IGF-II-dependent SK1 activation pathway plays a role in cell proliferation, we exposed human mesangial cells to IGF-II in the presence or absence of SK inhibitor, dimethylsphingosine (DMS). As shown in Fig. 10, A and B, stimulation of human mesangial cells with IGF-II and S1P significantly increased cell counts (1164 ± 75.7) and (1185 ± 62.7), respectively, compared with nonstimulated (854 ± 104.8) at 24 h, and (1573 ± 107) and (1512 ± 44.2), respectively, compared with nonstimulated (1048 ± 20.8) at 48 h. Interestingly, incubation of cells with DMS (10 μm) for 30 min before stimulation inhibited cell proliferation by IGF-II to (927 ± 62.2) and (1284 ± 76.2) at 24 h and 48 h, respectively, with no effect on S1P-dependent cell proliferation (1087 ± 284.5) and (1365 ± 171.0) at 24 h and 48 h, respectively. These findings suggest that SK1 mediates IGF-II-dependent stimulation of human mesangial cell proliferation (Fig. 11).
Fig. 11.
IGF-II-induced proliferation of human mesangial cells is attenuated by SK inhibitor. Serum-starved human mesangial cells were pretreated with 20 μm DMS for 30 min before stimulation with IGF-II (10 nm) and S1P (5 nm) for 24 h (panel A) and 48 h (panel B). Cell viability assay was performed as described. Data shown represent the mean ± sd of three independent experiments. *, P < 0.05 vs. nonstimulated (NS); #, P < 0.05 vs. stimulated cells.
Discussion
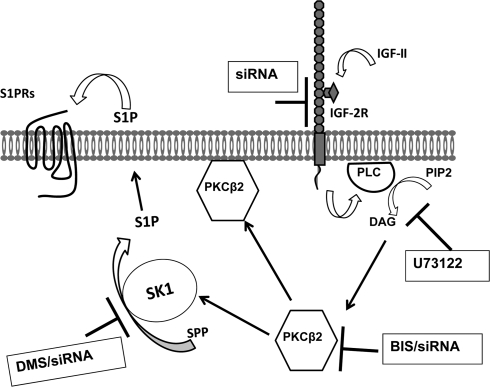
The IGF-II/M6P receptor has been suggested to function as a putative tumor suppressor because of its ability to facilitate the activation of TGF-β1, to modulate circulating LIF levels, to regulate the targeting of lysosomal enzymes to lysosomes, and to modulate local levels of IGF-II (15). On the other hand, a growing body of evidence suggests that certain cellular/biological responses of IGF-II are mediated by the IGF-II/M6P receptor via activation of a G protein-coupled pathway (noted before in the Introduction). Whether the biological processes regulated by the IGF-II/M6P receptor result primarily from its role as a clearance receptor or signal transducer, it is evident that it plays critical roles in the regulation of cell growth, both developmentally and in neoplasia. However, given that the cytoplasmic domain of the IGF-II/M6P receptor lacks intrinsic catalytic activity, the intracellular mechanism by which ligand binding induces such effects is not fully elucidated. We have previously reported that IGF-II binding to IGF-II/M6P receptors activates ERK1/2 via SK1-dependent transactivation of G protein-coupled S1P receptors (11), a signaling pathway known to mediate proliferation, survival, and angiogenic responses (22). Supporting this finding, early observations indicated that IGF-II stimulated proliferation of K562 erythroleukemia cells, cell adhesion, and proliferation of myeloma cells, and regulated growth and development of early mouse embryo through IGF-II/M6P receptor (35–37). In the present study, we demonstrate that IGF-II stimulated human mesangial cell proliferation and that it is mediated by a SK1-dependent mechanism, which may suggest a role for IGF-II and IGF-II/M6P receptor in the progression of nephropathy. However it remains unclear how IGF-II/M6P receptors control SK1 activity. The major goal of this study was to investigate the mechanism underlying SK1 activation upon IGF-II/M6P receptor stimulation. Figure 12 illustrates our proposed model for IGF-II/M6P receptor-dependent SK1 activation through a PLC/PKCβ2-dependent pathway. We first established that in HEK293 cells, down-regulation of IGF-II/M6P receptors inhibits IGF-II-dependent SK1 activation. We next demonstrated that IGF-II-mediated SK1 activation and translocation are PKC dependent. When examining representative classical, novel, and atypical PKC isoforms in a GFP-PKC translocation assay, we found that IGF-II promotes transient and isoform-selective activation of PKCβ2, a finding that is supported by the finding that endogenous PKCβ2 is rapidly phosphorylated in response to IGF-II. We also observed that IGF-II produces a rapid rise in DAG level, and that DAG release, translocation, and phosphorylation of PKCβ2, and S1P production, are all sensitive to PLC inhibition. These data suggest that activation of PI/PLC and PKCβ2 are proximal signaling events leading to SK1 activation by the IGF-II/M6P receptor.
Fig. 12.
A schematic depiction of the proposed mechanism of PLC/PKCβ2 mediating IGF-II-dependent SK1 activation. IGF-II stimulation promotes PLC activation and generation of DAG leading to activation of PKCβ2 that, in turn, induces activation and membrane translocation of endogenous SK1and production of S1P leading to transactivation of G protein-coupled receptors.
The hallmark of PKC activation is its rapid and reversible translocation to the plasma membrane. However, kinetics of membrane translocation differs markedly between PKC isoforms. The rapid and transient translocation of PKCβ2 that we observed in response to IGF-II is consistent with previous reports demonstrating different patterns of PKC translocation upon stimulation (38–40). For example, PKCβ1 and PKCβ2 exhibit different translocation kinetics in response to angiotensin AT1A receptor activation (39). Whereas PKCβ2 translocation to the membrane is transient, PKCβ1 displayed additional response patterns, including persistent membrane localization and oscillations between the membrane and cytosol after agonist removal. Our findings are also consistent with reports that PKCβ2 catalytic activity is necessary for its release from the plasma membrane (30), because the PKCβ2(K371R) mutant exhibits persistent membrane translocation in response to IGF-II.
One inconsistency in our study is the failure to detect a rise in intracellular calcium in response to IGF-II, which might be expected if PLC is contributing to the IGF-II-stimulated rise in DAG level. Certainly G protein-coupled receptors activating PLCβ1 through heterotrimeric Gq/11 proteins evoke a robust calcium response (41). On the other hand, PLCβ2/3 activation by Gβγ-subunits and PLCγ activation by receptor tyrosine kinases often produce small/unapparent changes in intracellular calcium. The involvement of PLC in the IGF-II response is supported by our findings that the IGF-II-induced DAG pool enriched in (C19/20:4) DAG is derived from PI, and that PLC inhibition blocks IGF-II-stimulated DAG production. Moreover, PLC inhibition blocks IGF-II-stimulated PKCβ2 phosphorylation and membrane translocation, indicating that the PLC-mediated increase in DAG is sufficient to activate PKCβ2 in our system even without a robust rise in intracellular calcium. Other data suggest that if the DAG-sensitive C1 domain is bound with sufficiently high affinity, then calcium-sensitive C2 domain does not need to be fully engaged for PKC activation. Thus PMA can cause membrane translocation of classical PKC isozymes in the absence of intracellular Ca2+ (12). Recent evidence that DAG derived from the metabolism of phosphatidylcholine (PC) by PLD, which does not lead to corelease of IP3, plays an important role in classical PKC regulation (13, 42) further supports the hypothesis that a robust calcium response is not essential.
Our mass spectroscopic analysis of IGF-II induced-changes in DAG content also suggests the involvement of other phospholipase species in IGF-II-SK1 signaling. The polyunsaturated PI/PLC fraction of DAG represents only about 16% of the total DAG in basal state, and only 60% of the IGF-II-stimulated increase in total DAG was U73122 sensitive. This suggests that DAG derived from other pathways, such as PC/PLD or PC/PLC, may also contribute, expanding the number of possible upstream regulators that require investigation. Such complexity is consistent with published evidence that transient and localized generation of DAG by PI/PLC can lead to discrete changes in membrane properties that, in turn, activate further cellular signaling cascades (31).
Collectively, our results suggest that PKC-dependent regulation of SK1 plays an important role in IGF-II signaling, and that PLC and PKCβ2 are important upstream mediators of these effects. Previous studies have suggested that PKC activation by IGF receptors involves PLC-dependent IP3 production (39, 43), possibly derived from activation of PLC by heterotrimeric G protein Gβγ-subunits (42). Receptor phosphorylation might also play a role. Although the cytoplasmic domain of the IGF-II/M6P contains four regions that are potential substrates for Ser/Thr protein kinases, such as PKC, casein kinase 1 and 2, and cAMP-dependent protein kinase (15, 16), it is unclear whether the receptor itself is a physiologically relevant PKC substrate or how such phosphorylation might contribute to signal generation. On the other hand, there are a number of studies implicating PKC in both the activation and transcriptional regulation of SK1. For example, the PKC inhibitors staurosporine and calphostin C block PMA-dependent SK1 activation in human erythroleukemia (23) and SK1 activation and membrane translocation in HEK293 cells (17). Furthermore, sequence analysis of hSK1 reveals several putative PKC phosphorylation sites (22, 25), and PKC-dependent phosphorylation of Ser225 reportedly increases the membrane affinity of hSK1 (13). Although our data provide strong evidence for a role of PLC, possibly in coordination with other phospholipases, in the initial production of DAG that supports PKC activation, further investigation will be required to determine how the catalytically inactive IGF-II/M6P receptor regulates this complex network of lipid second messengers.
Materials and Methods
Materials
Tissue culture medium, fetal bovine serum, and penicillin/streptomycin were from Invitrogen (Carlsbad, CA). FuGENE 6 was from Roche Diagnostics (Indianapolis, IN). Double-stranded siRNAs were purchased from Xeragon (Germantown, MD). GeneSilencer transfection reagent was from Gene Therapy Systems (San Diego, CA). Primers for real-time PCR were from Integrated DNA Technologies (Coralville, IA). RNeasy kits were from QIAGEN Corporation (Valencia, CA), and iScript cDNA synthesis kits and iQ SYBR Green Supermix kits were from Bio-Rad Laboratories (Hercules, CA). IGF-II, bisindolylmaleimide, AG1024, and phorbol 12-myristate 13-acetate were from Sigma-Aldrich (St. Louis, MO). D-erythro-S1P and DMS were provided by the Medical University of South Carolina Lipidomics Core (Department of Biochemistry, Medical University of South Carolina, Charleston, SC). Mouse monoclonal antihuman CD222 mannose-6 phosphate/IGF2R was from Novus Biologicals (Littleton, CO). Goat polyclonal anti-IGF-I receptor phosphor-Tyr1158 was from Calbiochem (Gibbstown, NJ). Rabbit polyclonal anti-ERK1/2 IgG was from Cell Signaling Technology (Beverly, MA). Rabbit polyclonal anti-PKCα (phospho-S657/Y658), β1 (phospho-T642), and β2 (phospho-T641), SK1, β-actin, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were from Abcam, Inc. (Cambridge, MA). Rabbit polyclonal anti-PKCδ/θ (phospho-S642/676) and ζ/λ (phospho-T410/403) were from Cell Signaling Technology. Horseradish peroxidase-conjugated donkey antirabbit IgG was from Amersham Biosciences (Pittsburgh, PA).
cDNA constructs
All recombinant DNA procedures were carried out following standard protocols. The expression plasmid encoding GFP-tagged hSK1 was prepared as previously described (17). The wild-type pBK-CMV-GFP-PKCα, β1, β2, δ, and ζ, and the catalytically inactive pBK-CMV-GFP-K371R-PKCβ2 constructs were generously provided by Dr. Yusuf Hannun (Medical University of South Carolina).
Cell culture and transfection
HEK293 cells were obtained from the American Type Culture Collection and maintained in minimum essential medium supplemented with 10% fetal bovine serum and penicillin/streptomycin. Human mesangial cells were maintained in a 1:1 mixture of DMEM and Ham's F12 supplemented with 17% fetal bovine serum, 26 μg/ml insulin, and penicillin/streptomycin. Transient transfection of 50–60% confluent cultures was performed in 100-mm dishes using a ratio of 3 μl of FuGENE 6 per μg of plasmid DNA according to the manufacturer's protocol. Empty vector DNA was added to each transfection as needed to keep the mass of DNA constant. Transfected cells were split into multiwell plates, as appropriate, and serum deprived overnight in growth medium was supplemented with 0.5% fetal bovine serum and 10 mm HEPES, pH 7.4, before stimulation. All assays using transiently transfected cells were performed 48 h after transfection.
Down-regulation of IGF-II/M6P receptor and PKCβ2
IGF-II/M6P receptor expression was down-regulated as described previously using sequence-specific siRNAs (11, 27). Down-regulation of PKCβ2 was performed by targeting two different sites using sequence-specific siRNAs (5′-GCGACCUCAUGUAUCACAU-3′ and 5′-CGCUGGAGUACAUAGUGUA-3′) for the first site and (5′-GGGAGAAACUUGAACGCAA-3′ and 5′-CCCUCUUUGAACUUGCGUU-3′) for the second site. Scrambled siRNA sequences (5′-ACGUGACACGUUCGGAGAAAdTdT-3′ and 5′-UUCUCCGAACGUGUCACGUdTdT-3′) were used as negative control. Cells were transfected using Gene Silencer siRNA Transfection Reagent according to the manufacturer's protocol. Transfected cells were split into multiwell plates, as appropriate, and serum deprived overnight in growth medium was supplemented with 0.5% fetal bovine serum and 10 mm HEPES, pH 7.4 before stimulation. The efficiency of the knockdown was determined by quantitative real-time PCR for mRNA and immunoblotting for IGF-II receptor protein 48 h after transfection.
Quantitative real-time PCR
Total cellular RNA was isolated using the RNeasy kit according to the manufacturer's instructions. cDNA was prepared from 1 μg of total RNA with A260–A280 >1.8 using the iScript cDNA synthesis kit per the manufacturer's instructions. Quantitative real-time PCR was performed with an iCycler 1Q real-time detection system using the iQ SYBER Green Supermix kit. Reactions were performed using specific primers. For IGF-2R: forward primer, 5′-GAGGGAAGAGGCAGGAAAG-3′; reverse primer, 5′-TGTGGCAGGCATACTCAG-3′; for PKCβ2: forward primer, 5′-TACTGAGCCAGGAGGAAGG-3′; reverse primer, 5′-GGTCTCTGTTGCCATTGTTG-3′; and GAPDH: forward primer, 5′-CTGAGTACGTCGTGGAGTC-3′; reverse primer, 5′-AATGAGCCCCAGCCTTC-3′. Real-time PCR results were analyzed using Softmax Pro software (Molecular Devices Corp., Sunnyvale, CA). IGF-2R and PKCβ2 expression data were normalized to expression of GAPDH consequently as an endogenous control.
Protein immunoblotting
Appropriately transfected HEK293 cells were plated onto poly-d-lysine coated 12-well multiwell plates, serum deprived overnight, and preincubated in the presence or absence of inhibitors as described in the figure legends. Agonist stimulations were for 10 min, after which monolayers were washed once in 4 C PBS and lysed in 200 μl of Laemmli sample buffer. For ERK1/2 and PKC immunoblots, samples containing 20 μg of cell protein were resolved by SDS-PAGE, and ERK1/2 and phospho-PKC were detected by using rabbit polyclonal IgG, with horseradish peroxidase-conjugated polyclonal donkey antirabbit IgG as secondary antibody. Immune complexes were visualized by enzyme-linked chemiluminescence and quantified using a Fluor-S MultiImager.
Assay of SK1 activity
SK1 activity was determined as previously described (31). After stimulation, cells were collected by centrifugation for 5 min at 3000 x g and resuspended in ice-cold 0.1 m phosphate buffer containing 20 mm Tris-Cl, pH 7.4, 1 mm EDTA, 0.5 mm deoxypyridoxine, 15 mm NaF, 1 mm β-mercaptoethanol, 1 mm sodium orthovanadate, 40 mm β-glycerophosphate, 0.4 mm phenylmethyl-sulfonyl fluoride, 20% glycerol, 0.1% Triton X-100, and protease inhibitor cocktail. After sonication and assay of protein concentration, equal amounts of lysate protein were incubated for 30 min at 37 C with 50 μm sphingosine (delivered in 4 mg/ml fatty acid-free BSA) and [γ-32P]ATP (10 μCi; 50 μm final concentration). The reaction was terminated by the addition of 20 μl of 1 n HCl and 800 μl of chloroform/methanol/HCl (100:200:1) and allowed to stand at room temperature for 10 min. Subsequently, 240 μl of chloroform and 240 μl of 2 m potassium chloride were added, and samples were centrifuged at 3000 × g for 5 min. The aqueous layer was aspirated, and 250 μl of the organic layer were transferred to new glass tubes. The samples were dried and resuspended in chloroform/ methanol/HCl (100:200:1). Lipids were resolved on silica thin layer chromatography plates using 1-butanol/methanol/acetic acid/water (80:20:10:20) as the solvent system. Labeled S1P was visualized by autoradiography and radioactivity corresponding to sphingosine phosphate was scraped and counted in a scintillation counter.
C17-Sphingosine labeling
Cells were labeled with C17-sphingosine (1 μm, 13 min, Avanti Polar Lipids, Inc., Alabaster, AL), washed three times with PBS, and collected. The reaction was stopped by the addition of 1 ml of extraction solvent containing ethyl acetate/2-propanol/water (60:30:10, vol/vol) supplemented with internal standard for enzyme immunoassay/liquid chromatography/mass spectrometry analysis. Lipids are extracted twice, dried under a stream of nitrogen, and resuspended into 150 μl of 1 mm NH4COOH in 0.2% HCOOH in methanol and analyzed by enzyme immunoassay/liquid chromatography/mass spectrometry.
Analysis of sphingolipids
Sphingolipid fractions were analyzed using a Thermo Finnigan TSQ7000 triple quadrupole mass spectrometer, operating in a multiple reaction monitoring positive ionization mode as previously described (44). HEK293 cells were plated in 60-mm dishes before starvation and stimulation for the indicated times in the presence or absence of inhibitors. The media were removed, and cells were scraped in 200 μl of cold lysis buffer (1% Triton X-100; 50 mm Tris, pH 7.5; 150 mm NaCl; 1 mm EDTA; 1:200 protease inhibitor cocktail, 1:200 phosphatase inhibitor cocktail), sonicated and placed in 1.5-ml plastic tubes and immediately frozen in liquid nitrogen. Samples were stored at −80 C until analyzed. Sphingolipid levels were calculated and normalized to phosphate (Pi).
Confocal fluorescence microscopy
For visualization of GFP-SK1 and GFP-PKC isoforms, appropriately transfected HEK293 cells were plated onto collagen-coated 35-mm glass-bottom dishes, serum deprived overnight, preincubated with or without inhibitors, and stimulated as described in the figure legends. After stimulation, cells were fixed with 4% paraformaldehyde for 30 min at room temperature and washed with PBS before examination. Confocal microscopy was performed using a Zeiss LSM 510 laser-scanning microscope (Carl Zeiss, Thornwood, NY) with 488-nm excitation and 516- to 560-nm emission filter sets.
Cell viability assay
Human mesangial cells were plated in 96-well plate and serum starved and then stimulated for the indicated times in the presence or absence of inhibitors. Cell proliferation was assessed using CellTiter-Glo Luminescent Cell Viability Assay kit (Promega Corp., Madison, WI) per the manufacturer's instructions.
Statistical analysis
Experiments were each performed at least in triplicate, and error bars represent standard deviation. Statistical analyses were performed by Student's t test. P values are defined in the figure legends.
Acknowledgments
We thank Dr. Yusuf Hannun for his valuable discussion and the Hollings Cancer Center Molecular Imaging Facility at Medical University of South Carolina.
This work was supported by a grant from South Carolina Center for Biomedical Research Excellence in Lipidomics and Pathology (to H.M.E.), National Institutes of Health (NIH) Grants DK55524 (to L.M.L.) and NIH/NCRR P20 RR17677 (to L.M.O.), HL087986 and HL077192 (to A.A.J.), Department of Veterans Affairs Research Enhancement Award (L.M.L.), and Veteran Affairs Merit Award (to L.M.O.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DAG
- Diacylglycerol
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- GFP
- green fluorescent protein
- HEK
- human embryonic kidney
- IP3
- inositol 1,4,5-triphosphate
- M6P
- mannose-6-phosphate
- PC
- phosphatidylcholine
- Pi
- phosphate
- PKC
- protein kinase C
- PLC
- phospholipase C
- PLD
- phospholipase D
- PMA
- phorbol myristate acetate
- S1P
- sphingosine 1-phosphate
- siRNA
- small interfering RNA
- SK1
- sphingosine kinase 1
- SPP
- sphingosine.
References
- 1. Hawkes C, Amritraj A, Macdonald RG, Jhamandas JH, Kar S. 2007. Heterotrimeric G proteins and the single-transmembrane domain IGF-II/M6P receptor: functional interaction and relevance to cell signaling. Mol Neurobiol 35:329–345 [DOI] [PubMed] [Google Scholar]
- 2. Matsunaga H, Nishimoto I, Kojima I, Yamashita N, Kurokawa K, Ogata E. 1988. Activation of a calcium-permeable cation channel by insulin-like growth factor II in BALB/c 3T3 cells. Am J Physiol 255:C442–C446 [DOI] [PubMed] [Google Scholar]
- 3. Hari J, Pierce SB, Morgan DO, Sara V, Smith MC, Roth RA. 1987. The receptor for insulin-like growth factor II mediates an insulin-like response. EMBO J 6:3367–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Q, Tally M, Larsson O, Kennedy RT, Huang L, Hall K, Berggren PO. 1997. Insulin-like growth factor II signaling through the insulin-like growth factor II/mannose-6-phosphate receptor promotes exocytosis in insulin-secreting cells. Proc Natl Acad Sci USA 94:6232–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chu CH, Tzang BS, Chen LM, Kuo CH, Cheng YC, Chen LY, Tsai FJ, Tsai CH, Kuo WW, Huang CY. 2008. IGF-II/mannose-6-phosphate receptor signaling induced cell hypertrophy and atrial natriuretic peptide/BNP expression via Gαq interaction and protein kinase C-α/CaMKII activation in H9c2 cardiomyoblast cells. J Endocrinol 197:381–390 [DOI] [PubMed] [Google Scholar]
- 6. Chu CH, Tzang BS, Chen LM, Liu CJ, Tsai FJ, Tsai CH, Lin JA, Kuo WW, Bau DT, Yao CH, Huang CY. 2009. Activation of insulin-like growth factor II receptor induces mitochondrial-dependent apoptosis through Gαq and downstream calcineurin signaling in myocardial cells. Endocrinology 150:2723–2731 [DOI] [PubMed] [Google Scholar]
- 7. Huang CY, Hao LY, Buetow DE. 2002. Hypertrophy of cultured adult rat ventricular cardiomyocytes induced by antibodies against the insulin-like growth factor (IGF)-I or the IGF-I receptor is IGF-II-dependent. Mol Cell Biochem 233:65–72 [DOI] [PubMed] [Google Scholar]
- 8. Hawkes C, Jhamandas JH, Harris KH, Fu W, MacDonald RG, Kar S. 2006. Single transmembrane domain insulin-like growth factor-II/mannose-6-phosphate receptor regulates central cholinergic function by activating a G-protein-sensitive, protein kinase C-dependent pathway. J Neurosci 26:585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Minniti CP, Kohn EE, Grubb JH, Sly WS, Oh Y, Müller HL, Rosenfeld RG, Helman LJ. 1992. The insulin-like growth factor II (IGF-II)/mannose 6-phosphate receptor mediates IGF-II-induced motility in human rhabdomyosarcoma cells. J Biol Chem 267:9000–9004 [PubMed] [Google Scholar]
- 10. Maeng YS, Choi HJ, Kwon JY, Park YW, Choi KS, Min JK, Kim YH, Suh PG, Kang KS, Won MH, Kim YM, Kwon YG. 2009. Endothelial progenitor cell homing: prominent role of the IGF2-IGF2R-PLCβ2 axis. Blood 113:233–243 [DOI] [PubMed] [Google Scholar]
- 11. El-Shewy HM, Lee MH, Obeid LM, Jaffa AA, Luttrell LM. 2007. The insulin-like growth factor type 1 and insulin-like growth factor type 2/mannose-6-phosphate receptors independently regulate ERK1/2 activity in HEK293 cells. J Biol Chem 282:26150–26157 [DOI] [PubMed] [Google Scholar]
- 12. Newton AC. 2001. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev 101:2353–2364 [DOI] [PubMed] [Google Scholar]
- 13. Idkowiak-Baldys J, Baddys A, Raymond JR, Hannun YA. 2009. Sustained receptor stimulation leads to sequestration of recycling endosomes in a classical protein kinase C- and phospholipase D-dependent manner. J Biol Chem 284:22322–22331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim HJ, Kim TY. 2005. Regulation of vascular endothelial growth factor expression by insulin-like growth factor-II in human keratinocytes, differential involvement of mitogen-activated protein kinases and feedback inhibition of protein kinase C. Br J Dermatol 152:418–425 [DOI] [PubMed] [Google Scholar]
- 15. Dahms NM, Hancock MK. 2002. P-type lectins. Biochim Biophys Acta 1572:317–340 [DOI] [PubMed] [Google Scholar]
- 16. Hawkes C, Kar S. 2002. Insulin-like growth factor-II/mannose-6-phosphate receptor in the spinal cord and dorsal root ganglia of the adult rat. Eur J Neurosci 15:33–39 [DOI] [PubMed] [Google Scholar]
- 17. Johnson KR, Becker KP, Facchinetti MM, Hannun YA, Obeid LM. 2002. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA). J Biol Chem 277:35257–35262 [DOI] [PubMed] [Google Scholar]
- 18. Taylor CJ, Motamed K, Lilly B. 2006. Protein kinase C and downstream signaling pathways in a three-dimensional model of phorbol ester-induced angiogenesis. Angiogenesis 9:39–51 [DOI] [PubMed] [Google Scholar]
- 19. Döll F, Pfeilschifter J, Huwiler A. 2005. The epidermal growth factor stimulates sphingosine kinase-1 expression and activity in the human mammary carcinoma cell line MCF7. Biochim Biophys Acta 1738:72–81 [DOI] [PubMed] [Google Scholar]
- 20. Huwiler A, Döll F, Ren S, Klawitter S, Greening A, Römer I, Bubnova S, Reinsberg L, Pfeilschifter J. 2006. Histamine increases sphingosine kinase-1 expression and activity in the human arterial endothelial cell line EA.hy 926 by a PKC-α-dependent mechanism. Biochim Biophys Acta 1761:367–376 [DOI] [PubMed] [Google Scholar]
- 21. You B, Ren A, Yan G, Sun J. 2007. Activation of sphingosine kinase-1 mediates inhibition of vascular smooth muscle cell apoptosis by hyperglycemia. Diabetes 56:1445–1453 [DOI] [PubMed] [Google Scholar]
- 22. Taha TA, Hannun YA, Obeid LM. 2006. Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol 39:113–1131 [DOI] [PubMed] [Google Scholar]
- 23. Buehrer BM, Bardes ES, Bell RM. 1996. Protein kinase C-dependent regulation of human erythroleukemia (HEL) cell sphingosine kinase activity. Biochim Biophys Acta 1303:233–242 [DOI] [PubMed] [Google Scholar]
- 24. Olivera A, Rosenthal J, Spiegel S. 1996. Effect of acidic phospholipids on sphingosine kinase. J Cell Biochem 60:529–537 [DOI] [PubMed] [Google Scholar]
- 25. Pitson SM, Moretti PA, Zebol JR, Xia P, Gamble JR, Vadas MA, D'Andrea RJ, Wattenberg BW. 2000. Expression of a catalytically inactive sphingosine kinase mutant blocks agonist-induced sphingosine kinase activation. A dominant-negative sphingosine kinase. J Biol Chem 275:33945–33950 [DOI] [PubMed] [Google Scholar]
- 26. Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, Vadas MA, Wattenberg BW. 2003. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J 22:5491–5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El-Shewy HM, Johnson KR, Lee MH, Jaffa AA, Obeid LM, Luttrell LM. 2006. Insulin-like growth factors mediate heterotrimeric G protein-dependent ERK1/2 activation by transactivating sphingosine 1-phosphate receptors. J Biol Chem 281:31399–31407 [DOI] [PubMed] [Google Scholar]
- 28. Kang-Park S, Lee JH, Shin JH, Lee YI. 2001. Activation of the IGF-II gene by HBV-X protein requires PKC and p44/p42 MAP kinase signalings. Biochem Biophys Res Commun 283:303–307 [DOI] [PubMed] [Google Scholar]
- 29. Luiken JJ., Ouwens DM, Habets DD, van der Zon GC, Coumans WA, Schwenk RW, Bonen A, Glatz JF. 2009. Permissive action of protein kinase C-ζ in insulin-induced CD36- and GLUT4 translocation in cardiac myocytes. J Endocrinol 201:199–209 [DOI] [PubMed] [Google Scholar]
- 30. Feng X, Hannun YA. 1998. An essential role for autophosphorylation in the dissociation of activated protein kinase C from the plasma membrane. J Biol Chem 273:26870–26874 [DOI] [PubMed] [Google Scholar]
- 31. Goñi FM, Alonso A. 1999. Structure and functional properties of diacylglycerols in membranes. Prog Lipid Res 38:1–48 [DOI] [PubMed] [Google Scholar]
- 32. Becker KP, Hannun YA. 2004. Isoenzyme-specific translocation of protein kinase C (PKC)βII and not PKCβI to a juxtanuclear subset of recycling endosomes: involvement of phospholipase D. J Biol Chem 279:28251–28256 [DOI] [PubMed] [Google Scholar]
- 33. Feng X, Becker KP, Stribling SD, Peters KG, Hannun YA. 2000. Regulation of receptor-mediated protein kinase C membrane trafficking by autophosphorylation. J Biol Chem 275:17024–17034 [DOI] [PubMed] [Google Scholar]
- 34. Anelli V, Gault CR, Snider AJ, Obeid LM. 2010. Role of sphingosine kinase-1 in paracrine/transcellular angiogenesis and lymphangiogenesis in vitro. FASEB J 24:2727–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tally M, Li CH, Hall K. 1987. IGF-2 stimulated growth mediated by the somatomedin type 2 receptor. Biochem Biophys Res Commun 148:811–816 [DOI] [PubMed] [Google Scholar]
- 36. Pantaleon M, Jericho H, Rabnott G, Kaye PL. 2003. The role of insulin-like growth factor II and its receptor in mouse preimplantation development. Reprod Fertil Dev 15:37–45 [DOI] [PubMed] [Google Scholar]
- 37. Nishiura T, Karasuno T, Yoshida H, Nakao H, Ogawa M, Horikawa Y, Yoshimura M, Okajima Y, Kanakura Y, Kanayama Y, Matsuzawa Y. 1996. Functional role of cation-independent mannose 6-phosphate/insulin-like growth factor II receptor in cell adhesion and proliferation of a human myeloma cell line OPM-2. Blood 88:3546–3554 [PubMed] [Google Scholar]
- 38. Kitatani K, Idkowiak-aldys J, Hannun YA. 2007. Mechanism of inhibition of sequestration of protein kinase C α/βII by ceramide. Roles of ceramide-activated protein phosphatases and phosphorylation/dephosphorylation of protein kinase C α/βII on threonine 638/641. J Biol Chem 282:20647–20656 [DOI] [PubMed] [Google Scholar]
- 39. Poiraudeau S, Lieberherr M, Kergosie N, Corvol MT. 1997. Different mechanisms are involved in intracellular calcium increase by insulin-like growth factors 1 and 2 in articular chondrocytes: voltage-gated calcium channels, and/or phospholipase C coupled to a pertussis-sensitive G-protein. J Cell Biochem 64:414–422 [PubMed] [Google Scholar]
- 40. Policha A, Daneshtalab N, Chen L, Dale LB, Altier C, Khosravani H, Thomas WG, Zamponi GW, Ferguson SS. 2006. Role of angiotensin II type 1A receptor phosphorylation, phospholipase D, and extracellular calcium in isoform-specific protein kinase C membrane translocation responses. J Biol Chem 281:26340–26349 [DOI] [PubMed] [Google Scholar]
- 41. Hubbard KB, Hepler JR. 2006. Cell signalling diversity of the Gqα family of heterotrimeric G proteins. Cell Signal 18:135–150 [DOI] [PubMed] [Google Scholar]
- 42. Becker KP, Hannun YA. 2005. Protein kinase C and phospholipase D: intimate interactions in intracellular signaling. Cell Mol Life Sci 62:1448–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rogers SA, Purchio AF, Hammerman MR. 1990. Mannose 6-phosphate-containing peptides activate phospholipase C in proximal tubular basolateral membranes from canine kidney. J Biol Chem 265:9722–9727 [PubMed] [Google Scholar]
- 44. Bielawski J, Szulc ZM, Hannun YA, Bielawska A. 2006. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods 39:82–91 [DOI] [PubMed] [Google Scholar]