Abstract
Cells respond rapidly to endoplasmic reticulum (ER) stress by blocking protein translation, increasing protein folding capacity, and accelerating degradation of unfolded proteins via ubiquitination and ER-associated degradation pathways. The ER resident type 2 deiodinase (D2) is normally ubiquitinated and degraded in the proteasome, a pathway that is accelerated by enzyme catalysis of T4 to T3. To test whether D2 is normally processed through ER-associated degradation, ER stress was induced in cells that endogenously express D2 by exposure to thapsigargin or tunicamycin. In all cell models, D2 activity was rapidly lost, to as low as of 30% of control activity, without affecting D2 mRNA levels; loss of about 40% of D2 activity and protein was also seen in human embryonic kidney 293 cells transiently expressing D2. In primary human airway cells with ER stress resulting from cystic fibrosis, D2 activity was absent. The rapid ER stress-induced loss of D2 resulted in decreased intracellular D2-mediated T3 production. ER stress-induced loss of D2 was prevented in the absence of T4, by blocking the proteasome with MG-132 or by treatment with chemical chaperones. Notably, ER stress did not alter D2 activity half-life but rather decreased D2 synthesis as assessed by induction of D2 mRNA and by [35S]methionine labeling. Remarkably, ER-stress-induced loss in D2 activity is prevented in cells transiently expressing an inactive eukaryotic initiation factor 2, indicating that this pathway mediates the loss of D2 activity. In conclusion, D2 is selectively lost during ER stress due to an eukaryotic initiation factor 2-mediated decrease in D2 synthesis and sustained proteasomal degradation. This explains the lack of D2 activity in primary human airway cells with ER stress resulting from cystic fibrosis.
The endoplasmic reticulum (ER) is an intricate and highly adaptable cellular organelle involved in critical cellular processes such as protein synthesis and folding, lipid biosynthesis, and Ca2+ homeostasis (1). The ER is said to be under “stress” whenever there is an overload of its functions such as excessive protein synthesis, protein misfolding, and/or derangement in Ca2+ homeostasis. ER stress is detected and signaled to the rest of the cell via pathways that are initiated by three main ER sensors: activating transcription factor 6-, inositol-requiring protein 1 α, and PRKR-like ER kinase (PERK). This integrated cellular response is termed the “unfolded protein response,” and once this response is initiated the cell sets in motion a series of events focused on neutralizing the stress in the ER by 1) enhancing expression of molecular chaperones, i.e. binding Ig protein (BiP); 2) accelerating protein degradation via the ER-associated degradation (ERAD) pathway; and 3) by halting protein translation (1). Failure to effectively resolve ER stress constitutes a significant disease mechanism with several studies linking ER stress to types 1 and 2 diabetes (2), as well as neurodegenerative diseases such as Alzheimer's and Parkinson's disease (3) and more recently, cystic fibrosis (CF) (4, 5).
An ER-resident protein involved in thyroid hormone activation is the thioredoxin-containing selenoprotein type 2 deiodinase (D2) that catalyzes the intracellular conversion of the prohormone T4 to its active form, T3 (6). D2 is expressed in the brain, skeletal muscle, and brown adipose tissue, where it plays a role in metabolic control and energy homeostasis (6). Notably, a number of metabolic conditions closely associated with thyroid hormone signaling have been implicated in the pathogenesis of or aggravated by ER stress such as obesity and insulin resistance (2), prompting our interest in further understanding D2 function and local thyroid hormone activation during ER stress.
D2 is a dimeric type 1 membrane protein anchored to the ER membrane that is inactivated by ubiquitination (7–9). Upon interaction of its natural substrate T4 with the enzyme's active center and subsequent catalysis (T3 production), there is ubiquitination of two critical Lys residues (K237/K244) located in the vicinity of its active center, thus inactivating the enzyme (7). This is mediated by two ubiquitin-conjugating enzymes (UBC6 and UBC7) (10, 11) and two ubiquitin ligases, the hedgehog-inducible WD repeat and SOCS box-containing 1 (12) and the highly conserved E3 ligase TEB4 (13), which has been linked to ERAD in yeast and mammals (14). Ubiquitinated D2 is not immediately taken up by the proteasomes but rather remains in the ER where it can be reactivated by ubiquitin-specific protease 20- and ubiquitin-specific protease 33-mediated deubiquitination, thus establishing an on/off switch that controls intracellular thyroid hormone activation (15).
Here we show that D2 activity and protein levels are rapidly down-regulated during ER stress, thus effectively reducing intracellular T3 production and thyroid hormone signaling in D2-expressing cells. Loss of D2 activity and local T3 production during ER stress are caused by an effective suppression in D2 mRNA translation via activation of the eukaryotic initiation factor 2 (eIF2a) pathway and not by accelerated ubiquitination/degradation of D2. This blockade of D2 synthesis can be reversed by attenuation of ER stress by treatment of cells with two different chemical chaperones, 4-phenylbutyric acid (4-PBA) and trimethylamine n-oxide (TMAO). This is physiologically relevant given that in airway cells obtained from a patient with CF, a genetic disease associated with ER stress (4, 5), there is complete lack of D2 activity despite increased Dio2 expression.
Results
ER stress triggers rapid loss of D2 activity and protein via a posttranscriptional mechanism
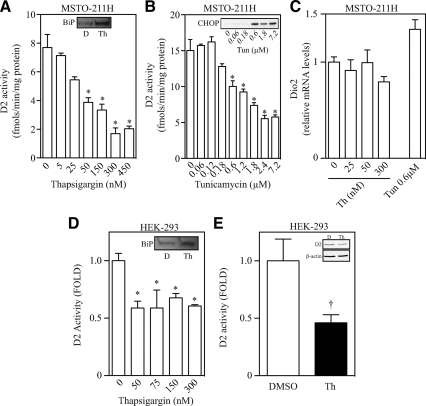
To test the hypothesis that D2 is sensitive to ER stress, we exposed MSTO-211H cells that endogenously express D2 (16), to two chemicals known to activate the ER stress response: thapsigargin, a sarco/ER Ca2+-ATPase blocker (17) or tunicamycin, an inhibitor of N′-linked glycosylation (18). Addition of thapsigargin (5–450 nm) for as little as 1 h resulted in a rapid accumulation of ER stress markers, including BiP protein and mRNA as well as C/EBP homologous protein (CHOP) mRNA levels (inset of Fig. 1A, Supplemental Fig. 1A published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). This coincided with a marked dose-dependent loss of D2 activity that reached about 25% of the levels seen in vehicle-treated cells (Fig. 1A). Notably, loss of D2 activity was detected with as little as 50 nm thapsigargin (IC50 = 30 nm), much less than the standard 300 nm commonly used by others (2).
Fig. 1.
Thapsigargin and tunicamycin treatment causes ER stress and regulates D2 activity and protein levels. Chemical ER stress causes a rapid loss in D2 levels in different cell models. A, D2 activity and BiP protein levels (inset, D = vehicle and Th = 300 nm thapsigargin) in MSTO-211H cells treated with increasing doses of thapsigargin for 1 h. B, D2 activity and CHOP protein levels (inset) in MSTO-211H cells treated with increasing doses of tunicamycin for 3 h. C, D2 mRNA levels in MSTO-211H cells treated with either increasing doses of thapsigargin (Th) for 1 h or 0.6 μm tunicamycin (Tun) for 3 h. D, D2 activity and BiP protein levels (inset, D = vehicle and Th = 300 nm thapsigargin) in HEK-293 treated with increasing doses of thapsigargin for 1 h. D2 activity was normalized to ß-galactosidase activity and is expressed as relative to control group. E, FLAG-CysD2 activity and protein (inset) levels in HEK-293 cells treated with vehicle (D) or 300 nm thapsigargin (Th) for 6 h. Both vehicle and thapsigargin-treated cells were exposed to 50 nm free T4. Gel loading was corrected to protein concentration. All values are displayed as ± sem and are representative of three experiments, where * represents P < 0.01 vs. nontreated group by one-way ANOVA followed by Dunnett's multiple-comparison test. In panel E, † represents P = 0.056 vs. vehicle-treated group by two-tailed Student's t test.
Similar findings were observed when MSTO-211H cells were exposed to tunicamycin (0.06–7.2 μm) for 3 h. Under these conditions, there was clear accumulation of CHOP protein (inset of Fig. 1B) that coincided with a dose-dependent loss of D2 activity that reached about 30% of the vehicle-treated cells (Fig. 1B). Of note, a loss of about 50% in D2 activity was also observed after glutamine starvation for 24 h (Supplemental Fig. 1B), which is a metabolic condition also known to promote ER stress (Supplemental Fig. 1C). Thus, three independent forms of ER stress resulted in consistent and marked loss of D2 activity in MSTO-211H cells. In addition, other cell types that endogenously express D2, including differentiated C2C12 myotubes (Supplemental Fig. 1, D–F) and the rhabdomyosarcoma cell line RMS-13 (Supplemental Fig. 1G) also exhibited a similar pattern of ER stress-induced loss in D2 activity. These findings are specific for D2 because the type 1 deiodinase (D1), the other T4-activating deiodinase, was not affected when HepG2 cells were exposed to similar conditions of ER stress (Table 1).
Table 1.
D1 activity and CHOP mRNA levels in HepG2 cells exposed to thapsigargin for 1 h
| Treatment | D1 activity (pmol/min · mg protein) | CHOP mRNA (relative levels) |
|---|---|---|
| Vehicle | 16.1 ± 2.11 | 0.99 ± 0.23 |
| 300 nm thapsigargin | 15.7 ± 2.90 | 7.23 ± 0.23 |
Cells were treated for 1 h with vehicle (DMSO) or 300 nm thapsigargin. Values are the mean ± sem of three different samples. All values are not statistically different.
The loss of D2 activity that followed ER stress was a result of posttranscriptional mechanisms, because D2 mRNA levels remained stable during ER stress in either MSTO-211H (Fig. 1C) or C2C12 cells (Supplemental Fig. 1H). In addition, ER stress (thapsigargin, 50–300 nm) prompted a loss in D2 activity even in human embryonic kidney (HEK)-293 cells transiently expressing human D2 under a constitutive promoter (Fig. 1D). In these cells, the increase in the BiP protein levels caused by 300 nm thapsigargin (inset of Fig. 1D) was accompanied by an approximately 40% drop in D2 activity. In a separate experiment, the drop in D2 activity correlated with a similar decrease in D2 protein levels as assessed by Western blotting in lysates of HEK-293 cells treated with thapsigargin for 6 h and transiently expressing a FLAG-tagged D2 (Fig. 1E).
Loss of D2 activity during ER stress decreases intracellular T3 production
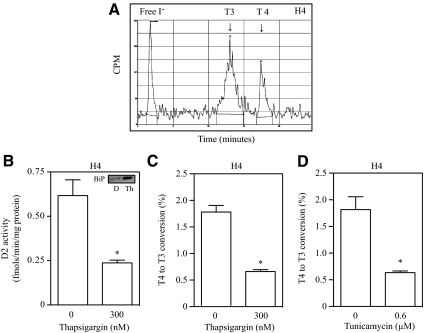
The decrease in D2 velocity observed in cell lysates suggests that T3 production in intact cells is reduced by ER stress. Thus, to assess the integrated D2-mediated T4 to T3 conversion in cells under ER stress, T4 deiodination was studied in intact glioblastoma cell line H4 during the time of the ER stress. In these cells, deiodination of 125I-labeled T4 produces the expected equimolar amounts of 125I and 125I-labeled T3 as assessed by ultra performance liquid chromatography (Fig. 2A), validating that iodide production can be used as a surrogate of T3 production in short-term experiments such as this (2 h).
Fig. 2.
ER stress decreases intracellular T3 production. A, Equimolar production of free I− and T3 production in H4 cells, as determined by ultra performance liquid chromatography. B, D2 activity and BiP protein levels (inset) in H4 cells treated with 300 nm thapsigargin (Th) for 1 h. C, Fractional conversion of T4 to T3 in intact H4 cells treated with 300 nm thapsigargin for 2 h, or in panel D, cells were treated with 0.6 μm tunicamycin for 5 h. In panels B–D, all values are displayed as ±sem and are representative of three experiments, where * represents P < 0.01 vs. nontreated group by two-tailed Student's t test.
Like the other cell lines tested, D2 activity in H4 was also sensitive to ER stress, with an approximately 50% loss in D2 activity followed by a robust increase in protein levels of the ER stress marker BiP (Fig. 2B and inset of 2B). Next, H4 cells were exposed to thapsigargin for 2 h (Fig. 2C) or tunicamycin for 5 h (Fig. 2D) and 125I-labeled T4 was added during the last 2 h of the experiment. Treatment with either chemical markedly decreased 125I release (and thus T3 production) when compared with vehicle-treated cells (Fig. 2, C and D). Similar results were observed in MSTO-211H cells treated with thapsigargin for 2 h (data not shown), confirming that ER stress reduces intracellular thyroid hormone activation in D2-expressing cells.
Rapid loss in D2 activity caused by ER stress requires intact D2 turnover mechanisms, and it does not affect enzymatic turnover rate
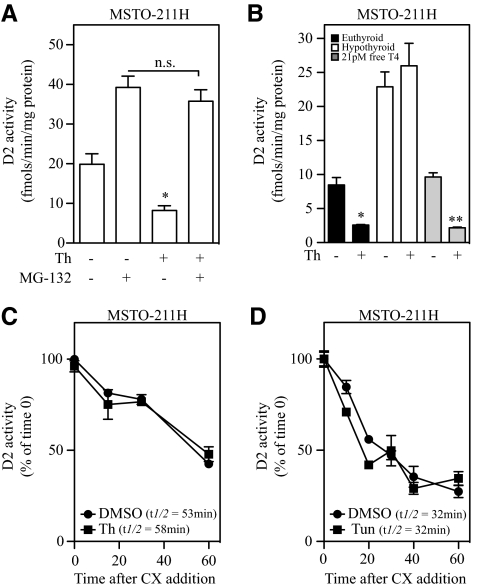
D2 is normally taken up by the proteasomes, and its turnover rate can be prolonged by proteasome inhibitors such as MG-132 (19). Thus, to test whether this pathway is involved in the loss of D2 caused by ER stress, MSTO-211H cells were treated with MG-132 for 24 h, followed by a 1-h exposure to thapsigargin. Exposure to MG-132 alone increased D2 activity by about 2-fold (Fig. 3A) while preventing thapsigargin from decreasing D2 activity (Fig. 3A). MG-132 also prevented the ER stress-induced loss in D2 activity in glutamine-starved MSTO-211H cells (Supplemental Fig. 1I).
Fig. 3.
Under ER stress, substrate catalysis and the ubiquitin/proteasome system regulate D2 activity without affecting enzyme half-life. Proteasomal blockage and cellular hypothyroidism prevent ER stress-mediated loss of D2 activity. A, MSTO-211H cells were pretreated for 24 h with vehicle or 1 μm MG-132. After the 24-h incubation period, cells were treated with either vehicle or 25 nm thapsigargin (Th) for 1 h, where ns = not significant. B, MSTO-211H cells were incubated for 24 h in 10% FBS (euthyroid, dark bars), 10% charcoal-stripped serum (hypothyroid, clear bars), or 10% charcoal-stripped serum supplemented with 21 pm free T4 (euthyroid, light gray bars). After incubation period, cells were treated with 300 nm thapsigargin (Th) for 1 h. In panel C, MSTO-211H cells were treated with 300 nm thapsigargin (Th) in combination with 100 μm cyclohexamide (CX) for 1 h, and cells were harvested every 10 min. In panel D, MSTO-211H cells were treated with 0.6 μm tunicamycin (Tun) for 3 h, where 100 μm cyclohexamide was added in the last hour of treatment. In panels C and D, D2 activity is plotted as % of time 0 in function of time. All values are displayed as ± sem and are representative of three experiments, where *, P < 0.01 vs. vehicle-treated or **, P < 0.01 vs. T4-treated group by one-way ANOVA followed by Dunnett's posttest when two or more groups are compared with vehicle-treated group. n.s., P > 0.05.
Alternatively, another way of preventing substrate-induced ubiquitination followed by the intake of D2 in the proteasome system is to grow MSTO-211H cells in media prepared with charcoal-stripped serum, to remove thyroid hormones (20). Under hypothyroid conditions, D2 activity was not lost during thapsigargin-induced ER stress (Fig. 3B), an effect that was reversed by addition of physiological levels of T4 to the media (Fig. 3B).
ERAD is the proteasome-dependent mechanism that is activated during ER stress to minimize protein built up in the ER (14). One could expect a faster D2 turnover rate if the ERAD pathway was targeting D2 for degradation under ER stress. Thus, to test whether D2 is a target of ERAD during ER stress, MSTO-211H cells were exposed to either vehicle, thapsigargin for 1 h, or tunicamycin for 3 h in combination with 100 μm cyclohexamide, used to stop protein translation (21). Cells were harvested at 10-min intervals, with D2 activity plotted as a function of time and relative to starting activity levels. Remarkably, exposure to either thapsigargin or tunicamycin failed to modify D2 activity half-life (t1/2 = 30–50 min for both treatments, Fig. 3, C and D), indicating that D2 is not a target of ERAD under conditions of ER stress.
ER stress blocks cAMP-induced increase in D2 activity despite a robust induction in D2 mRNA
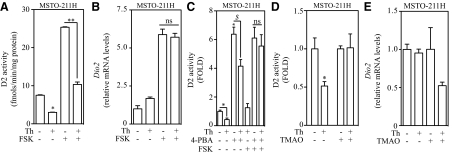
Given that D2 is not a target of ERAD, we hypothesized that D2 synthesis is slowed down as part of the cell response to ER stress, and the rapid loss in D2 activity is due to its normally rapid turnover rate. To test this hypothesis, we first exposed MSTO-211H cells to 25 nm thapsigargin for 1 h. This resulted in a 2-fold drop in D2 activity (Fig. 4A) and a 10-fold increase in CHOP mRNA levels (Supplemental Fig. 1J). Subsequently, cells were incubated for 6 h with forskolin (FSK) to activate Dio2 transcription and elevate D2 mRNA levels, driving de novo D2 synthesis.
Fig. 4.
De novo synthesis of D2 protein is impaired during ER stress, and it is sensitive to chemical chaperone treatment. Decrease in D2 levels is sensitive to 4-PBA and TMAO treatment. A, D2 activity in MSTO-211H cells treated with 300 nm thapsigargin (Th), 100 μm FSK, or a combination of both treatments. B, D2 mRNA levels of MSTO-211H cells from panel A. C, D2 activity in MSTO-211H cells pretreated with 1 mm 4-PBA or vehicle for 72 h. Cells were then exposed to 300 nm thapsigargin, 100 μm FSK, or a combination of both treatments. Values were normalized to untreated control group. D, D2 activity and D2 mRNA levels (panel E) in MSTO-211H cells pretreated with 100 mm TMAO or vehicle for 72 h. Cells were then exposed to 25 nm thapsigargin for 1 h, and activity values were normalized to respective control levels. F, CHOP mRNA levels of cells from panel D. In panels A–C total treatment time with thapsigargin and/or FSK was 6 h. All values are displayed as ± sem and are representative of three experiments, where *, P < 0.01 vs. vehicle-, **, P < 0.01 vs. FSK-, and $,P < 0.01 vs. 4-PBA-treated group by one-way ANOVA. When two or more groups are compared with vehicle-treated group, Dunnett's posttest was used. In panels B and C, ns = not significant, P > 0.05.
In vehicle-treated cells, FSK treatment increased D2 activity up to 25 fmol/mg protein/min (Fig. 4A) whereas D2 mRNA levels were up-regulated by 6-fold (Fig. 4B). On the other hand, pretreatment with thapsigargin followed by FSK treatment increased CHOP mRNA levels by more than 100-fold (Supplemental Fig. 1J) and limited the induction of D2 activity to only about 10 fmol/mg protein/min (Fig. 4A), despite almost identical D2 mRNA levels (Fig. 4B). Thus, a potent induction of Dio2 gene expression during ER stress does not lead to increased D2 activity.
Chemical chaperones have been shown to attenuate ER stress (22) and increase D2 activity via a transcriptional mechanism (23). Thus, we hypothesized that pretreatment of cells with chemical chaperones could prevent the loss of D2 activity observed during ER stress by derepressing the translational blockade of D2 mRNA. As expected, 1 h thapsigargin treatment decreased D2 activity by 60%, whereas treatment of MSTO-211H cells with 4-PBA alone for 72 h increased D2 activity by about 6-fold (Fig. 4C). When cells were pretreated with 4-PBA and then exposed to thapsigargin for 1 h there was an attenuation in the loss of D2, with an approximately 30% reduction in D2 activity, but in the presence of FSK no reduction in D2 activity was observed (Fig. 4C). The increase in CHOP mRNA levels was limited in the 4-PBA-treated cells (data not shown), indicating that by reducing ER stress the blockade in D2 synthesis can be prevented.
Among the different classes of molecular chemical chaperones, there are molecules that act as osmolytes, such as TMAO. This molecule is thought to stabilize protein structure by indirectly interacting with functional amino acids mediated by the enhancement of the water molecule structure (24). Pretreatment of MSTO-211H cells with TMAO for 72 h prevented the loss of D2 activity normally seen after exposure to thapsigargin (Fig. 4D) without affecting D2 mRNA levels (Fig. 4E) and by attenuating ER stress (Fig. 4F). Thus, by reducing the levels of cellular ER stress, 4-PBA and TMAO were able to prevent the ER stress-induced loss of D2.
ER stress slows down D2 synthesis via an eIF2a-mediated mechanism
During ER stress there is translational blockade mediated by the activation of the eIF2a pathway, which slows down the rate of ribosomal reading of the 5′-untranslated regions (UTR) of most ribosome-bound mRNAs (1). The fact that D2 mRNA contains intrinsic regulatory elements in both the 5′- and 3′-UTR (Supplemental Fig. 2A; and Ref. 25) prompted us to investigate whether these elements played a role. Different D2-expressing vectors that contain either the wild type or a mutant 5′- (Supplemental Fig. 2B) or 3′-UTR (Supplemental Fig. 2C) were transiently expressed in HEK-293 cells and these cells were subsequently treated with thapsigargin for 5 h. Treatment with thapsigargin resulted in similar loss in D2 activity in all vectors, i.e. wild type or mutant 5′- and 3′-UTR constructs, indicating the blockage of D2 synthesis does not require a specific element present in the D2 mRNA.
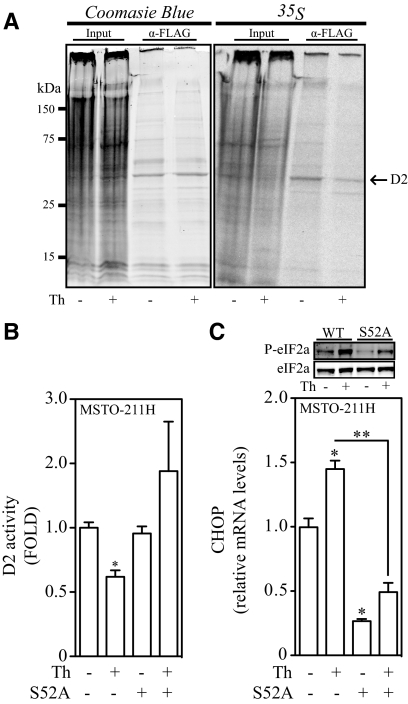
Next, using a combination of metabolic labeling with [35S]methionine and immunoprecipitation we estimated D2 synthetic rate in HEK-293 cells transiently expressing FLAG-tagged D2. Cells were pretreated for 24 h with 1 μm MG-132 to block D2 degradation, and then treated with vehicle or thapsigargin for 1 h. 35S labeling lasted 2 h and occurred during treatment with thapsigargin. ER stress decreased the amount of newly synthesized 35S-labeled D2 by about 50% when compared with the vehicle-treated cells (Fig. 5A, right panel). This took place without any changes in total pool of D2 protein (because MG-132 was used), as seen by Coomasie blue staining (Fig. 5A, left panel).
Fig. 5.
The eIF2α pathway mediates the translational arrest of D2 synthesis during ER stress. D2 protein synthesis is decreased in ER-stressed cells. A, HEK-293 cells transiently expressing a FLAG-tagged CysD2 were treated with vehicle or 300 nm thapsigargin (Th) for 1 h. After this incubation period, [35S]methionine was added to the incubation media for an additional 1 h. Total treatment time with thapsigargin was 2 h. CysD2 was immunoprecipitated and resolved on a 12% SDS-PAGE. Image shown represents a 2-d exposure to radiographic film. In panel B, D2 activity levels of MSTO-211H cells electroporated with either a control or a eIF2a-encoding vector with a inactivating S52A mutation. Cells were exposed to either vehicle or 25 nm thapsigargin for 1 h. C, Top panel, phospho and total eIF2a protein levels; in lower graph, CHOP mRNA levels of MSTO-211H cells from panel B. All values are displayed as ± sem and data were normalized to untreated group and are representative of two independent experiments. B, *, P < 0.01 vs. vehicle-treated group. C, *, P < 0.01 vs. vehicle-; **, P < 0.01 vs. S52A Th-treated group by one-way ANOVA. When two or more groups are compared with vehicle-treated group, Dunnett's posttest was used. WT, Wild type.
The activation of the eIF2a molecule during unfolded protein response is mediated by phosphorylation of a Ser residue at position 52 by PERK (26). Thus, to test the contribution of this pathway in the ER stress-induced loss of D2 activity, MSTO-211H cells transiently expressing S52A eIF2a were treated with a low dose (25 nm) of thapsigargin for 1 h. Strikingly, D2 activity in MSTO-211H cells expressing the S52A mutant was resistant to thapsigargin treatment (Fig. 5B), whereas the phosphorylation of eIF2a and the expression of the ER stress marker CHOP, a direct target of the eIF2a pathway (27), were severely impaired (Fig. 5C).
D2 activity is down-regulated in a patient with CF
CF is a genetic disease characterized by mutations in the CF transmembrane conductance regulator (CFTR) gene located on chromosome 7. The most common mutation is a deletion of a phenylanine residue at position 508 (ΔF508) of the CFTR glycoprotein (28). The CFTR-ΔF508 protein fails to properly fold in the ER, and its accumulation leads to ER stress (29), activating the ERAD pathway (5, 30, 31).
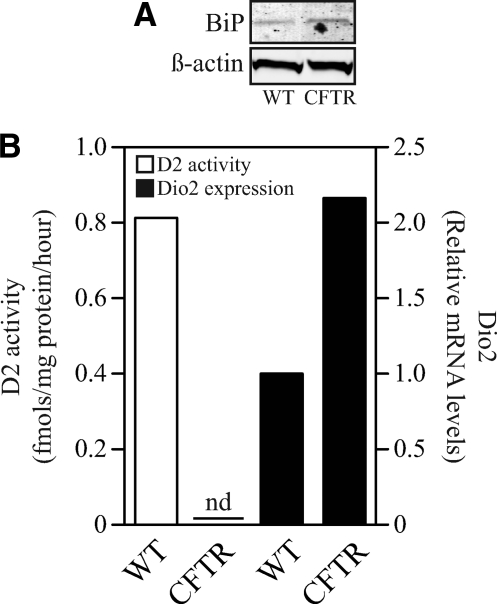
Therefore, giving the sensitivity of D2 levels to ER-stressing conditions, we hypothesized that D2 activity would be down-regulated in CF cells from a patient who is homozygous for ΔF508. Primary airway cells obtained from this patient have increased BiP protein (Fig. 6A) and CHOP mRNA levels (data not shown) after redifferentiation at the air-liquid interface in comparison with cells from a normal subject (Fig. 6A). Remarkably, CF cells had undetectable levels of D2 activity despite exhibiting higher levels of Dio2 expression (Fig. 6B), indicating that ER stress in humans results in marked down-regulation of the D2 pathway.
Fig. 6.
A patient with CF, homozygous for ΔF508, has null D2 activity in primary airway cells despite increased levels of D2 mRNA. Panel A shows BiP protein levels and panel B depicts D2 activity (left Y axis, clear bars) and D2 mRNA levels (right Y axis, dark bars) in human primary lung cells isolated from one subject homozygous for the ΔF508 mutation (CFTR) and one subject that did not carry this mutation (wild type). In panel B data are presented as relative levels to WT subject.
Discussion
Thyroid hormone is a key signaling molecule in development and metabolic control (32). In the present study ER stress has been linked to a rapid loss in D2 activity and intracellular T3 production (Figs. 1 and 2). Notably, this is not achieved by pushing D2 toward ERAD but rather by slowing down D2 synthesis via an eIF2a-dependent pathway (Fig. 5). Because this mechanism is observed in a number of cell models, it is conceivable that the interference caused by ER stress on D2 activity and thus local T3 production affects thyroid hormone signaling in multiple systems in which D2 is expressed.
The fact that UBC6, UBC7, and TEB4 are all involved in D2 ubiquitination and proteasome degradation would suggest that D2 could be a passive ERAD target. However, the data indicate otherwise given that D2's turnover rate was not accelerated during ER stress (Fig. 3). In fact, ER stress did not affect the rate or the properties of D2 clearance, remaining highly sensitive to substrate availability and proteasome inhibition (Fig. 3). These observations led us to hypothesize that D2 synthesis instead was the target of ER stress because it is known that treatment with thapsigargin or tunicamycin activates the PERK-eIF2a pathway (33, 34), which, in turn, transiently and selectively blocks mRNA-to-protein translation (1). Supporting this hypothesis is the clear evidence that 1) rapid induction of D2 mRNA (∼6-fold) does not result in the expected increase in D2 activity (Fig. 4, A and B); 2) there was much less 35S-D2 labeling in cells transiently expressing FLAG-tagged D2 (Fig. 5A); and 3), expression of the inactive S52A eIF2a in D2-expressing cells treated with thapsigargin crippled the eIF2a pathway (as seen by a robust deficit in CHOP expression) and prevented the loss of D2 activity during times of ER stress (Fig. 5, B and C).
Chemical chaperones are known to alleviate ER stress by facilitating protein folding and accelerating ER protein trafficking (35). Logically, we next used two different chemical chaperones, 4-PBA and TMAO, to test reversibility of the D2 loss caused by ER stress, having found that indeed it was preventable (Fig. 4, C and D). Although the chemical chaperone 4-PBA is known to increase D2 expression through transcriptional mechanisms (23), this interference did not prevent D2 from being rescued during ER stress (Fig. 4C). In addition, TMAO also rescued D2 activity without interfering with Dio2 transcriptional activity (Fig. 4E). Thus, both results support, at least in part, the beneficial metabolic effects of chemical chaperones in a mouse model of diet-induced obesity (23).
The present study was not able to identify specific elements in the D2 mRNA, either at the 5′-UTR or the 3′-UTR that would play a role in the translational regulation of D2 mRNA by ER stress (Supplemental Fig. 2, A–C). Notably, D2 is a selenoprotein and, as with other selenoproteins, incorporation of Sec requires a specific element in the 3′-UTR termed “SECIS” that mediates the insertion of a Sec residue into the growing polypeptide chain (36). However, we discarded the possibility that Sec incorporation in D2 is the target of ER stress by showing an unquestionable ER stress-induced loss of D2 synthesis, activity, and protein levels in cells transiently expressing a Sec-less D2 mutant (Cys133SecD2; Fig. 1E and Fig. 5A). In addition, studies indicate that other ER-resident selenoproteins are up-regulated during ER stress, e.g. 15-kDa selenoprotein (37), selenoprotein S (38), and selenoprotein K (39).
The physiological implications of the present findings are multifold given the studies indicating that D2 activity does mediate thyroid hormone signaling in a number of settings including pituitary thyrotrophs (TSH-producing cells) (20) and in a neuronal/glial coculture system (40). In both models, changes in D2 and D2-mediated T3 production during relatively short periods of time have been shown to affect downstream targets of T3, i.e. expression of T3-responsive genes (20, 40). This is particularly exciting in the lung where thyroid hormone signaling has been linked with proper lung function. In this tissue, T3 acts as a stimulant of alveolar fluid clearance (41, 42) as well as branching of airways (43) and differentiation of airway epithelial cells (44), whereas D2 plays a protective role in two types of acute lung injury (45), suggesting that the D2-T3 pathway is necessary for proper homeostasis of mammalian airway cells. The single observation that a patient with CF displays mild levels of ER stress (Fig. 6A) with a complete lack of D2 activity despite elevated D2 mRNA levels is striking. This suggests that in the lung the D2-regulatory mechanisms are more sensitive to alterations of ER homeostasis and rapidly shut down D2 synthesis.
That ER stress leads to a rapid decrease in D2 activity and D2-mediated T3 production supports a novel model in which during cellular stress there is down-regulation of the T3 signaling pathway (46). The effects of ER stress seem to be restricted to D2, the key player in thyroid hormone signaling. Nonetheless, it is conceivable that the apparent lack of D1 responsiveness to ER stress is due to D1's much longer half-life (47).
The present data identify a novel mechanism by which D2 is regulated, while linking thyroid hormone signaling to ER stress. The relevance of these results stems from the metabolic role played by thyroid hormone and the fact that ER stress has been linked to types 1 and 2 diabetes (2). In fact, D2 is expressed and plays a role in brown adipose tissue and skeletal muscle, two metabolically relevant tissues in diabetes that are targets of thyroid hormone (48, 49). T3 changes gene expression and accelerates energy expenditure in both tissues, and ER stress is expected to decrease these pathways via loss of D2. The central nervous system represents an additional locus where D2 regulation by ER stress could be relevant given that ER stress is detected in neurodegenerative diseases such as Alzheimer's and Parkinson's disease (3), and that a large percentage of the T3 acting in the brain is generated locally via D2 (50, 51). Finally, the novel finding that these suppressive effects on D2 levels and ER stress are present in a patient with CF and that this same effect is reversible via utilization of chemical chaperones in different cell models is exciting and opens a therapeutic opportunity to preserve D2 activity in conditions of ER stress.
Materials and Methods
Reagents and materials
Unless specified otherwise, all reagents were purchased from Sigma Chemical Co. (St. Louis, MO) or Calbiochem (La Jolla, CA). Outer ring-labeled T4 (specific activity, 4400 Ci/mmol) and Easytag 35S-labeled methionine (specific activity >1000 Ci/mmol), were from New England Nuclear/PerkinElmer (Boston, MA). Lipofectamine-2000 was from Invitrogen (Carlsbad, CA).
DNA constructs
The D2 encoding constructs and the D2 5′- and 3′-UTR constructs have been previously described (19, 52). The S52A eIF2a vector was from Addgene (Cambridge, MA; vector no. 21808).
Cell culture
Mesothelioma (MSTO-211H), C2C12 myocytes, rhabdomyosarcoma (RMS-13), Glioblastoma (H4), and HEK-293 were purchased from American Type Culture Collection (ATCC, Manassas, VA). Cells were grown and maintained in RPMI-1640 (MSTO-211H, RMS-13) and DMEM (HEK-293, H4, and C2C12), respectively, supplemented with 10% FBS. Sodium selenite was added to the all cell culture media at 100 nm final concentration (16, 40, 53). HEK-293 cells were transfected with 0.25 μg of a plasmid encoding a human D2, as described earlier (13) using Lipofectamine-2000. After transfection, either thapsigargin or dimethylsulfoxide (DMSO) (vehicle) was added as indicated. In all transfection experiments, cells were cotransfected with 0.25 μg of β-galactosidase, and its enzymatic activity was used to control transfection efficiency, as described previously (13). Electroporation of MSTO-211H with a control or S52A eIF2a vector was performed with a 4D-Nucleofector from Lonza (Basel, Switzerland) using 45 × 103 cells per well in a six-well dish and with program DS-130. Cells were then transferred to a six-well plate and incubated for 2 d before treatment. All experiments were performed in triplicate for each condition.
Redifferentiation of airway epithelial cells at the air-liquid interface
Normal human airways were obtained from organ donors whose lungs were rejected for transplant. Institutional review board approved consent for research with these tissues was obtained by the Life Alliance Organ Recovery Agency of the University of Miami and conformed to the declaration of Helsinki. Lungs from a CF patient were obtained with institutional review board approved consent at the time of transplant. The CF patient had an abnormal sweat test and was homozygous for ΔF508. Chamber experiments showed chloride conductance abnormalities typical for CF. Airway epithelial cells were isolated and dedifferentiated through expansion. Passage 1 cells were redifferentiated at an air-liquid interface on collagen-coated 24-mm T-clear filters (Corning, Lowell, MA) as previously described (54–57).
Western blotting, metabolic labeling, and immunopreciptation
All cell lysates analyzed by Western blot were processed as described earlier (48). Anti-CHOP (1:1000) and anti-BIP (1:1000) were from Cell Signaling Technology (Beverly, MA), and anti-β-actin was from Sigma Chemical Co. All blots were normalized to protein content and were developed using Odyssey Infrared Imager (LI-COR) (58) following manufacturer's instructions. For metabolic labeling, HEK-293 cells were plated on a 10-cm2 dish and transfected with 4 μg of a FLAG-tagged Sec133Cys mutant D2 isoform previously described (19). Cells were incubated 24 h after transfection with 1 μm of the proteasome blocker MG-132 for a period of 24 h. The next day, cells were exposed to either DMSO or 300 nm thapsigargin for 1 h, followed by an incubation of 45 min with FBS-free, methionine-free media to deplete intracellular methionine pools. Next, in addition to DMSO or 300 nm thapsigargin treatment, cells were incubated 120 μCi of 35S-labeled methionine for 1 h. After the incubation period, cells were harvested and processed for immunoprecipitation as previously described (59). Immunoprecipitation samples were resolved on a 12% SDS-PAGE gel, which was later stained with Coomasie blue for 30 min followed by destaining [40% methanol, 10% acetic acid, and 50% distilled water (vol/vol)] and finally air dried using Dryease Mini-gel drying system (Invitrogen, Carlsbad, CA) following manufacturer's instructions. Dried gels were exposed to a radiographic film for 2 d.
Deiodinase activity and T4 to T3 conversion in intact cells
D1 and D2 activities were measured as previously described (13). The production of 125I- and 125I-labeled T3 from outer ring-labeled T4 in intact cells was analyzed as described in Ref. 53. Briefly, cells were exposed to DMSO, 300 nm thapsigargin (1 h), or 0.6 μm tunicamycin (3 h) in DMEM containing 10% FBS. After the incubation period, incubating media were changed to DMEM containing 0.1% BSA and 500,000 cpm/ml [125I-labeled T4 in combination with either DMSO, 300 nm thapsigargin (1 h) or 0.6 μm tunicamycin (2 h)]. At the end of the incubation period, samples were processed and fractional T4 to T3 conversion was calculated as in Ref. 53.
Real-time quantitative PCR (qPCR)
Total RNA was extracted using TRIzol reagent (Invitrogen) and used to synthesize cDNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) following manufacturer's instructions. Extraction of RNA from human pneumocytes was performed using E.Z.N.A Total RNA kit and homogenizer mini columns from OMEGA Bio-Tek (Norcross, GA), and cDNA was synthesized as described above. Unless otherwise noted, the RT-qPCR was performed as previously described (13). RT-qPCR of Dio2 and CHOP (GADD-153) genes in human primary pneumocytes was performed using Taqman amplification assays from Applied Biosystems.
Statistical analysis
All data were analyzed using PRISM 5 software (GraphPad Software, Inc., San Diego, CA) and are expressed as mean ± sem. One-way ANOVA was used to compare more than two groups, followed by the Dunnett's posttest to detect differences between vehicle- and drug-treated groups. Student's t test (two-tailed) was used to compare differences between only two groups. P < 0.05 was used to reject the null hypothesis.
Supplementary Material
Acknowledgments
We thank David Ron (University of Cambridge) for supplying the S52A eIF2a plasmid used in these studies.
This work was supported, in part, by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK58538, NHLBI HL-60644, and HL-89399 and Hungarian Scientific Research Fund grant OTKA K81226.
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:
Ligands: Thyroid hormone.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- BiP
- Binding Ig protein
- CF
- cystic fibrosis
- CFTR
- CF transmembrane conductance regulator
- CHOP
- C/EBP homologous protein
- D2
- type 2 deiodinase
- DMSO
- dimethylsulfoxide
- eIF-2α
- eukaryotic initiation factor-2α
- ER
- endoplasmatic reticulum
- ERAD
- ER-associated degradation
- FSK
- forskolin
- HEK
- human embryonic kidney
- 4-PBA
- 4-phenylbutyric acid
- PERK
- PRKR-like ER kinase
- qPCR
- quantitative PCR
- TMAO
- trimethylamine n-oxide
- UBC
- ubiquitin-conjugating
- UTR
- untranslated region.
References
- 1. Schröder M. 2008. Endoplasmic reticulum stress responses. Cell Mol Life Sci 65:862–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461 [DOI] [PubMed] [Google Scholar]
- 3. Lindholm D, Wootz H, Korhonen L. 2006. ER stress and neurodegenerative diseases. Cell Death Differ 13:385–392 [DOI] [PubMed] [Google Scholar]
- 4. Kerbiriou M, Le Drévo MA, Férec C, Trouvé P. 2007. Coupling cystic fibrosis to endoplasmic reticulum stress: differential role of Grp78 and ATF6. Biochim Biophys Acta 1772:1236–1249 [DOI] [PubMed] [Google Scholar]
- 5. Nanua S, Sajjan U, Keshavjee S, Hershenson MB. 2006. Absence of typical unfolded protein response in primary cultured cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun 343:135–143 [DOI] [PubMed] [Google Scholar]
- 6. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC. 2008. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sagar GD, Gereben B, Callebaut I, Mornon JP, Zeöld A, da Silva WS, Luongo C, Dentice M, Tente SM, Freitas BC, Harney JW, Zavacki AM, Bianco AC. 2007. Ubiquitination-induced conformational change within the deiodinase dimer is a switch regulating enzyme activity. Mol Cell Biol 27:4774–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baqui MM, Gereben B, Harney JW, Larsen PR, Bianco AC. 2000. Distinct subcellular localization of transiently expressed types 1 and 2 iodothyronine deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology 141:4309–4312 [DOI] [PubMed] [Google Scholar]
- 9. Zeöld A, Pormüller L, Dentice M, Harney JW, Curcio-Morelli C, Tente SM, Bianco AC, Gereben B. 2006. Metabolic instability of type 2 deiodinase is transferable to stable proteins independently of subcellular localization. J Biol Chem 281:31538–31543 [DOI] [PubMed] [Google Scholar]
- 10. Botero D, Gereben B, Goncalves C, De Jesus LA, Harney JW, Bianco AC. 2002. Ubc6p and ubc7p are required for normal and substrate-induced endoplasmic reticulum-associated degradation of the human selenoprotein type 2 iodothyronine monodeiodinase. Mol Endocrinol 16:1999–2007 [DOI] [PubMed] [Google Scholar]
- 11. Kim BW, Zavacki AM, Curcio-Morelli C, Dentice M, Harney JW, Larsen PR, Bianco AC. 2003. Endoplasmic reticulum-associated degradation of the human type 2 iodothyronine deiodinase (D2) is mediated via an association between mammalian UBC7 and the carboxyl region of D2. Mol Endocrinol 17:2603–2612 [DOI] [PubMed] [Google Scholar]
- 12. Dentice M, Bandyopadhyay A, Gereben B, Callebaut I, Christoffolete MA, Kim BW, Nissim S, Mornon JP, Zavacki AM, Zeöld A, Capelo LP, Curcio-Morelli C, Ribeiro R, Harney JW, Tabin CJ, Bianco AC. 2005. The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol 7:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zavacki AM, Arrojo E, Drigo R, Freitas BC, Chung M, Harney JW, Egri P, Wittmann G, Fekete C, Gereben B, Bianco AC. 2009. The E3 ubiquitin ligase TEB4 mediates degradation of type 2 iodothyronine deiodinase. Mol Cell Biol 29:5339–5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vembar SS, Brodsky JL. 2008. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol 9:944–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curcio-Morelli C, Zavacki AM, Christofollete M, Gereben B, de Freitas BC, Harney JW, Li Z, Wu G, Bianco AC. 2003. Deubiquitination of type 2 iodothyronine deiodinase by von Hippel-Lindau protein-interacting deubiquitinating enzymes regulates thyroid hormone activation. J Clin Invest 112:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curcio C, Baqui MM, Salvatore D, Rihn BH, Mohr S, Harney JW, Larsen PR, Bianco AC. 2001. The human type 2 iodothyronine deiodinase is a selenoprotein highly expressed in a mesothelioma cell line. J Biol Chem 276:30183–30187 [DOI] [PubMed] [Google Scholar]
- 17. Inesi G, Hua S, Xu C, Ma H, Seth M, Prasad AM, Sumbilla C. 2005. Studies of Ca2+ ATPase (SERCA) inhibition. J Bioenerg Biomembr 37:365–368 [DOI] [PubMed] [Google Scholar]
- 18. Prescher JA, Bertozzi CR. 2006. Chemical technologies for probing glycans. Cell 126:851–854 [DOI] [PubMed] [Google Scholar]
- 19. Gereben B, Goncalves C, Harney JW, Larsen PR, Bianco AC. 2000. Selective proteolysis of human type 2 deiodinase: a novel ubiquitin-proteasomal mediated mechanism for regulation of hormone activation. Mol Endocrinol 14:1697–1708 [DOI] [PubMed] [Google Scholar]
- 20. Christoffolete MA, Ribeiro R, Singru P, Fekete C, da Silva WS, Gordon DF, Huang SA, Crescenzi A, Harney JW, Ridgway EC, Larsen PR, Lechan RM, Bianco AC. 2006. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism. Endocrinology 147:1735–1743 [DOI] [PubMed] [Google Scholar]
- 21. Schneider-Poetsch T, Ju J, Eyler DE, Dang Y, Bhat S, Merrick WC, Green R, Shen B, Liu JO. 2010. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat Chem Biol 6:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. 2006. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313:1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. da-Silva WS, Ribich S, Arrojo e Drigo R, Castillo M, Patti ME, Bianco AC. 2011. The chemical chaperones tauroursodeoxycholic and 4-phenylbutyric acid accelerate thyroid hormone activation and energy expenditure. FEBS Lett 585:539–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zou Q, Bennion BJ, Daggett V, Murphy KP. 2002. The molecular mechanism of stabilization of proteins by TMAO and its ability to counteract the effects of urea. J Am Chem Soc 124:1192–1202 [DOI] [PubMed] [Google Scholar]
- 25. Gereben B, Salvatore D. 2005. Pretranslational regulation of type 2 deiodinase. Thyroid 15:855–864 [DOI] [PubMed] [Google Scholar]
- 26. Harding HP, Zhang Y, Ron D. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274 [DOI] [PubMed] [Google Scholar]
- 27. Ma Y, Brewer JW, Diehl JA, Hendershot LM. 2002. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 318:1351–1365 [DOI] [PubMed] [Google Scholar]
- 28. Rowe SM, Miller S, Sorscher EJ. 2005. Cystic fibrosis. N Engl J Med 352:1992–2001 [DOI] [PubMed] [Google Scholar]
- 29. Bartoszewski R, Rab A, Jurkuvenaite A, Mazur M, Wakefield J, Collawn JF, Bebok Z. 2008. Activation of the unfolded protein response by deltaF508 CFTR. Am J Respir Cell Mol Biol 39:448–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM. 2001. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol 3:100–105 [DOI] [PubMed] [Google Scholar]
- 31. Farinha CM, Amaral MD. 2005. Most F508del-CFTR is targeted to degradation at an early folding checkpoint and independently of calnexin. Mol Cell Biol 25:5242–5252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheng SY, Leonard JL, Davis PJ. 2010. Molecular aspects of thyroid hormone actions. Endocr Rev 31:139–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kojima E, Takeuchi A, Haneda M, Yagi A, Hasegawa T, Yamaki K, Takeda K, Akira S, Shimokata K, Isobe K. 2003. The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress: elucidation by GADD34-deficient mice. FASEB J 17:1573–1575 [DOI] [PubMed] [Google Scholar]
- 34. Brewer JW, Hendershot LM, Sherr CJ, Diehl JA. 1999. Mammalian unfolded protein response inhibits cyclin D1 translation and cell-cycle progression. Proc Natl Acad Sci USA 96:8505–8510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Engin F, Hotamisligil GS. 2010. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab 12(Suppl 2):108–115 [DOI] [PubMed] [Google Scholar]
- 36. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- 37. Labunskyy VM, Yoo MH, Hatfield DL, Gladyshev VN. 2009. Sep15, a thioredoxin-like selenoprotein, is involved in the unfolded protein response and differentially regulated by adaptive and acute ER stresses. Biochemistry 48:8458–8465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gao Y, Feng HC, Walder K, Bolton K, Sunderland T, Bishara N, Quick M, Kantham L, Collier GR. 2004. Regulation of the selenoprotein SelS by glucose deprivation and endoplasmic reticulum stress—SelS is a novel glucose-regulated protein. FEBS Lett 563:185–190 [DOI] [PubMed] [Google Scholar]
- 39. Du S, Zhou J, Jia Y, Huang K. 2010. SelK is a novel ER stress-regulated protein and protects HepG2 cells from ER stress agent-induced apoptosis. Arch Biochem Biophys 502:137–143 [DOI] [PubMed] [Google Scholar]
- 40. Freitas BC, Gereben B, Castillo M, Kalló I, Zeöld A, Egri P, Liposits Z, Zavacki AM, Maciel RM, Jo S, Singru P, Sanchez E, Lechan RM, Bianco AC. 2010. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J Clin Invest 120:2206–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Folkesson HG, Norlin A, Wang Y, Abedinpour P, Matthay MA. 2000. Dexamethasone and thyroid hormone pretreatment upregulate alveolar epithelial fluid clearance in adult rats. J Appl Physiol 88:416–424 [DOI] [PubMed] [Google Scholar]
- 42. Bhargava M, Runyon MR, Smirnov D, Lei J, Groppoli TJ, Mariash CN, Wangensteen OD, Ingbar DH. 2008. Triiodo-L-thyronine rapidly stimulates alveolar fluid clearance in normal and hyperoxia-injured lungs. Am J Respir Crit Care Med 178:506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Archavachotikul K, Ciccone TJ, Chinoy MR, Nielsen HC, Volpe MV. 2002. Thyroid hormone affects embryonic mouse lung branching morphogenesis and cellular differentiation. Am J Physiol Lung Cell Mol Physiol 282:L359–L369 [DOI] [PubMed] [Google Scholar]
- 44. Yoon JH, Gray T, Guzman K, Koo JS, Nettesheim P. 1997. Regulation of the secretory phenotype of human airway epithelium by retinoic acid, triiodothyronine, and extracellular matrix. Am J Respir Cell Mol Biol 16:724–731 [DOI] [PubMed] [Google Scholar]
- 45. Ma SF, Xie L, Pino-Yanes M, Sammani S, Wade MS, Letsiou E, Siegler J, Wang T, Infusino G, Kittles RA, Flores C, Zhou T, Prabhakar BS, Moreno-Vinasco L, Villar J, Jacobson JR, Dudek SM, Garcia JG. 17. June 2011 Type 2 deiodinase and host responses of sepsis and acute lung injury. Am J Respir Cell Mol Biol 10.1165/rcmb.2011-0179OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simonides WS, Mulcahey MA, Redout EM, Muller A, Zuidwijk MJ, Visser TJ, Wassen FW, Crescenzi A, da-Silva WS, Harney J, Engel FB, Obregon MJ, Larsen PR, Bianco AC, Huang SA. 2008. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest 118:975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baqui M, Botero D, Gereben B, Curcio C, Harney JW, Salvatore D, Sorimachi K, Larsen PR, Bianco AC. 2003. Human type 3 iodothyronine selenodeiodinase is located in the plasma membrane and undergoes rapid internalization to endosomes. J Biol Chem 278:1206–1211 [DOI] [PubMed] [Google Scholar]
- 48. Hall JA, Ribich S, Christoffolete MA, Simovic G, Correa-Medina M, Patti ME, Bianco AC. 2010. Absence of thyroid hormone activation during development underlies a permanent defect in adaptive thermogenesis. Endocrinology 151:4573–4582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grozovsky R, Ribich S, Rosene ML, Mulcahey MA, Huang SA, Patti ME, Bianco AC, Kim BW. 2009. Type 2 deiodinase expression is induced by peroxisomal proliferator-activated receptor-γ agonists in skeletal myocytes. Endocrinology 150:1976–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Larsen PR, Silva JE, Kaplan MM. 1981. Relationships between circulating and intracellular thyroid hormones: physiological and clinical implications. Endocr Rev 2:87–102 [DOI] [PubMed] [Google Scholar]
- 51. Galton VA, Wood ET, St Germain EA, Withrow CA, Aldrich G, St Germain GM, Clark AS, St Germain DL. 2007. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology 148:3080–3088 [DOI] [PubMed] [Google Scholar]
- 52. Gereben B, Kollár A, Harney JW, Larsen PR. 2002. The mRNA structure has potent regulatory effects on type 2 iodothyronine deiodinase expression. Mol Endocrinol 16:1667–1679 [DOI] [PubMed] [Google Scholar]
- 53. da-Silva WS, Harney JW, Kim BW, Li J, Bianco SD, Crescenzi A, Christoffolete MA, Huang SA, Bianco AC. 2007. The small polyphenolic molecule kaempferol increases cellular energy expenditure and thyroid hormone activation. Diabetes 56:767–776 [DOI] [PubMed] [Google Scholar]
- 54. Bernacki SH, Nelson AL, Abdullah L, Sheehan JK, Harris A, Davis CW, Randell SH. 1999. Mucin gene expression during differentiation of human airway epithelia in vitro. Muc4 and muc5b are strongly induced. Am J Respir Cell Mol Biol 20:595–604 [DOI] [PubMed] [Google Scholar]
- 55. Schmid A, Bai G, Schmid N, Zaccolo M, Ostrowski LE, Conner GE, Fregien N, Salathe M. 2006. Real-time analysis of cAMP-mediated regulation of ciliary motility in single primary human airway epithelial cells. J Cell Sci 119:4176–4186 [DOI] [PubMed] [Google Scholar]
- 56. Nlend MC, Bookman RJ, Conner GE, Salathe M. 2002. Regulator of G-protein signaling protein 2 modulates purinergic calcium and ciliary beat frequency responses in airway epithelia. Am J Respir Cell Mol Biol 27:436–445 [DOI] [PubMed] [Google Scholar]
- 57. Fragoso MA, Fernandez V, Forteza R, Randell SH, Salathe M, Conner GE. 2004. Transcellular thiocyanate transport by human airway epithelia. J Physiol 561:183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Markovic D, Punn A, Lehnert H, Grammatopoulos DK. 2008. Intracellular mechanisms regulating corticotropin-releasing hormone receptor-2β endocytosis and interaction with extracellularly regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling cascades. Mol Endocrinol 22:689–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Curcio-Morelli C, Gereben B, Zavacki AM, Kim BW, Huang S, Harney JW, Larsen PR, Bianco AC. 2003. In vivo dimerization of types 1, 2, and 3 iodothyronine selenodeiodinases. Endocrinology 144:937–946 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.