Abstract
During the past several years, one of the most interesting and challenging issues in endocrine genetics is determining how to integrate the findings and approaches traditionally used to understand the powerful, single-gene mutations causing endocrine syndromes with those newer techniques used to dissect the complex genetic architecture of polygenic conditions. With this overriding consideration in mind, it makes sense to begin these considerations with recent novel findings derived from the study of a particularly prismatic monogenic disorder, isolated GnRH deficiency, in defining an area of neuroendocrinology and development. Careful study of this human disease model has been employed successfully by several groups to provide unique windows through which to gain an improved understanding of the challenging issues of the developmental biology of the GnRH neurons where previous nonhuman approaches have had significant technical limitations. For example, study of this disorder has provided the field of neuroendocrinology with several unique insights into the surprising origins and early development of the GnRH neuronal network. Its associated clinical phenotypes have helped to unearth a growing list of genes responsible for GnRH neuronal specification, migration, and neuroendocrine function. Finally, this human genetic model is beginning to provide increasing evidence of interactions between these single genes, clearly demonstrating that an oligogenic genetic architecture underlies this condition.
With this introduction of monogenic/oligogenic models as a background, a focus on some quite real successes of polygenic approaches is also appropriate. These approaches fit into the broader concept of a spectrum of investigative approaches varying from monogenic to oligogenic to polygenic in their genetic architecture. Finally, a look at what is coming next will indicate the accelerating pace of all of these contributions with great promises of things to come.
An Overview of Mono- vs. Polygenic Diseases: Approaches and Rewards
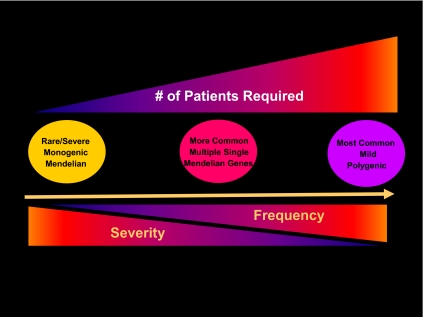
Figure 1 outlines a schema that envisions these considerations as a spectrum that emerges if one graphs the odds ratio that mutations in a given gene have upon the severity of a disease and contrast this impact with the frequency of their occurrence. One end of this spectrum is constituted by the highly deterministic contributions of the monogenic disorders. Due to their rarity, these severe but rare disorders most frequently present to clinicians in small numbers, typically as families. Despite their scarcity, they typically provide remarkable, novel insights into human biology. When the underlying mutated gene and its protein are discovered, indicting specific system biological pathways that were not previously identified are surfaced. Hence they are quite valuable prisms that provide unique insights into biology through the study of small numbers of severe cases. Taken together, however, these monogenic disorders are rare; collectively they account for only a very small percentage of the total genetic burden of disease states. Thus, they can be viewed as the tip of the genetic iceberg.
Fig. 1.
The genetic architecture of the endocrine disorders. Whereas monogenic disorders are rare, they typically have a high impact as seen on the left end of the spectrum. Oligogenic disorders represent interactions of a small number of Mendelian genes that interact to produce a more complex phenotypic spectrum of gene expression. On the right is the polygenic nature of most common genetic disorders in which mutations in several genes, each mild in its impact and fairly frequent in their incidence, act collectively to produce a disease that is common.
At the other end of this genetic spectrum lie the more common genetic disorders. These conditions typically occur as a phenotype only as the end result of synergistic mutations in multiple, mild genetic defects in a given pathway. Whereas the frequency of each of these genetic variations is relatively high in the population at large (at least when compared with their monogenic counterparts), each one individually confers only a mild risk ratio. Owing to this mildness of their impact and the commoness of their frequency, these multiple-gene defects typically require quite large populations to find. The approaches used to discover them are typically genome-wide association studies (GWAS) that demand substantial population sizes. Most common diseases (e.g. schizophrenia, inflammatory bowel disease, heart disease, hypertension, etc.) thus require a concatenation of several mutated genes to yield a phenotype. Even then, other genes and pathways can modulate their expression significantly, in essence trumping their biological effects, all of which can make them quite difficult to find.
The Spectrum of Genetic Impacts/Architectures
One consequence of viewing the genetic architecture of endocrine diseases as such a spectrum of frequency and severity in Fig. 1 is the corollary fact that the number of patients required to discover each new gene is inversely proportional to the severity of the phenotype it causes and the frequency of the genetic mutation within a population. Hence, the burden of numbers required to identify the causal genetic variants in complex diseases rapidly rises, often to tens to hundreds of thousands patients and controls. These numbers result from the individual impact of each DNA sequence variant being low and quite common in the population, typically being more than 5% of the DNA samples in a population for the currently available panel of single nucleotide polymorphisms (SNP). These logistical requirements have important consequences when the approaches required to discover them are examined. They severely restrict the applicability of these approaches to clinical research settings in which preassembled populations are already in place. This goal preassembly is typically accomplished either via previously established clinical trial networks or ongoing epidemiological studies. These requirements stand in sharp distinction from the monogenic disorders that require much smaller numbers, often the unit of study being a single family.
Isolated GnRH Deficiency
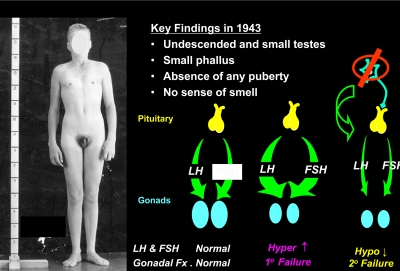
Figure 2 demonstrates a patient presenting to Massachusetts General Hospital's Endocrine Clinic in 1943 because of lack of sexual development and anosmia. He had undescended, small testes; a small phallus; absence of any pubertal development; and a complete lack of smell. His linear growth, thyroid and adrenal function, and skull film were all normal. His only defect was his low urinary gonadotropin levels, i.e. hypogonadotropic hypogonadism without a clear cause. Because his defect seemed restricted to the production of pituitary gonadotropins causing his low testicular function, he was diagnosed with “isolated gonadotropin deficiency.” This term implied a pituitary etiology for his disorder. Franz Kallmann (8) did not report this clinical association of hypogonadotropism with anosmia until 1 yr later, a condition thereafter associated with his name, i.e. Kallmann syndrome. By the time this same patient was seen in follow-up by me at the Massachusetts General Hospital in the late 1970s, the hypothalamic peptide that controls gonadotropin secretion, GnRH, had been discovered in 1971 (9, 10) and the Nobel Prize was awarded for this feat 6 yr later. Thus, the availability of this peptide for human testing raised the possibility of distinguishing a pituitary from a hypothalamic etiology.
Fig. 2.
The human disease model of isolated GnRH deficiency. A patient who presented at age 20 with absence of secondary sexual development, cryptoorchidism, microphallus, and anosmia. His primary gonadotropin levels were low and distinguished him from other cases of primary gonadal failure.
Pulsatile GnRH Restores A Normal Pituitary-Gonadal Axis in Isolated Gonadotropin Deficiency (i.e. Idiopathic Hypogonadotropic Hypogonadism)
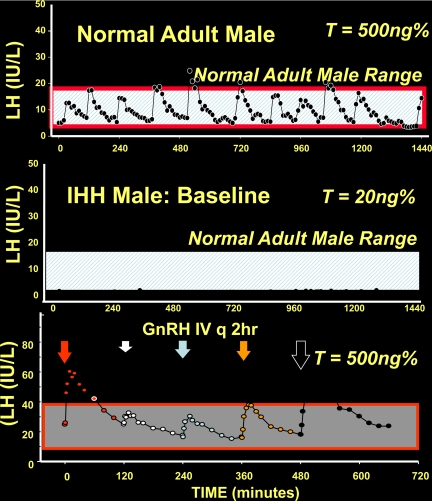
When GnRH was administered to this patient in a physiological pattern of replacement, his serum gonadotropins changed from a low, flat baseline pattern of secretion with a complete lack of any LH pulses and a hypogonadal testosterone to a pattern indistinguishable from normal men (i.e. an average of 12 GnRH-induced LH pulses/d that were GnRH induced in this patient) and a testosterone of 500 ng% as shown in Fig. 3 (11, 12). Not only did this physiological regimen of GnRH replacement establish a normal pattern of LH pulses, these GnRH-induced LH pulses completely normalized his serum testosterone and Inhibin B levels. They also induced testicular growth and eventually produced spermatogenesis and fertility in these men (12). Similarly, women with Isolated GnRH deficiency respond to pulsatile GnRH with ovulation and conception (13). Collectively, these human experiments permitted a conceptual leap in the understanding of this disease. It took our understanding from that of a pituitary gonadotropin deficiency according to Albright to a hypothalamic deficiency apparently isolated to the GnRH neurons, i.e. isolated GnRH deficiency. Subsequently, a small subset of hypogonadotropic patients exhibited a defect in their GnRH receptor gene (14) and thus represent the only exception to this thinking. However, a predominantly hypothalamic defect in most other hypogonadotropic patients has been found to represent cases of GnRH deficiency functionally and/or genetically.
Fig. 3.
Baseline characteristics and GnRH responsiveness of a GnRH-deficient male compared with a normal male. The upper panel demonstrates the typical 12 LH pulses that occur in an otherwise normal male. The middle panel represents the LH-secretory pattern of a man with isolated GnRH deficiency with a complete absence of any GnRH-induced pulses and hypogonadal serum testosterone level. The lowerpanel represents the activity of this same patient when 12 pulses of GnRH are administered each day with a corresponding appearance of a normal pattern of the pituitary-gonadal axis in response to this regimen, thus documenting the normal responsiveness of the pituitary and gonads when the physiological GnRH replacement is administered to the GnRH-deficient subject. IHH, idiopathic hypogonadotropic hypogonadism; T, testosterone; E2, estradiol.
Upon closer investigation, two distinct clinical phenotypes of these patients with isolated GnRH deficiency are immediately apparent: 1) those with anosmia that harbor a coexisting olfactory abnormality that often can be visualized by appropriate magnetic resonance imaging techniques; and 2) those with a normal sense of smell whose olfactory tracts are typically normal. Although Kallmann (8) originally described both phenotypes in his article, the anosmic group has forever borne his name as its diagnosis. The genetics of these anosmic cases have led to the discovery of several novel mutations in genes associated with GnRH neuronal migration, i.e. neurodevelopmental genes (1–4). In contrast, the normosmic group has unearthed a whole family of neuroendocrine genes responsible for controlling the hypothalamic secretion of GnRH, i.e. neuroendocrine genes. The most important point here is that although the reproductive phenotypes of these patients are quite similar, i.e. hypogonadotropism, the variability of their nonreproductive phenotypes with associated smell, renal, skeletal, and central nervous system (CNS) anomalies are often the truly important clinical clues as to the identity and biology of the new genes that underlie their condition.
This historical perspective on the pathophysiology of this human disease model was clearly enabled by the availability of the decapeptide GnRH in humans. However, several other genetic observations also prompted our group to make a large research investment in this model. First, several genetic modes of inheritance were documented in families with this disorder in online Mendelian inheritance in man with documented X-linked, autosomal recessive, and dominant families. Genetic heterogeneity of this type always translates to a network of genes that, when mutated, produce phenocopies, i.e. patients with analogous clinical phenotypes caused by different genes. Second, when the full sequence of the human genome finally became available, a remarkable observation was made. There was only one gene coding for GnRH in the entire human genome! This remarkable bioinformatic finding struck our group as bizarre. How could a gene so critical for fertility regulation, evolutionary adaptation of breeding, and consequently speciation not exhibit greater genetic redundancy? This finding stood in stark contrast to other genes important for evolution and their proteins that are typically invested with considerable genetic back-up function should any one become mutated. Consequently, it seemed clear that the mechanism(s) by which GnRH neurons and their secretion responded to various complex genetic inputs such as changes in seasonal breeding, circadian rhythms, light/dark cycles, exercise/energy/nutrition cycles, must lie upstream of the GnRH gene and potentially consist of a rich genetic network. With this working hypothesis in mind, our group then spent considerable time acquiring DNA from and phenotyping several patients/families with isolated GnRH deficiency as well as building the appropriate genetic and bioinformatic infrastructure to explore this hypothesis.
GnRH Gene Discovery Viewed in A Developmental Perspective
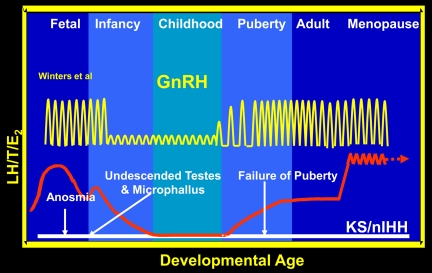
When considering the various clinical phenotypes of isolated GnRH deficiency, yet another prism through which to envision the biology of GnRH and its neuronal network's, control of the reproductive axis is with in a developmental context. Figure 4 demonstrates such a schematic of this activity of the human reproductive axis across the human life cycle. One advantage of such a vision is that it provides a useful context for mapping the clinical findings of patients with this family of disorders developmentally. Given that the pulsatile secretion of hypothalamic GnRH secretion is the key on-off switch for all this reproductive activity, its secretion must first become active to support reproductive axis activity during late fetal life as maternal human choriogonadotropin levels are waning in the third trimester of gestation. This initial burst of secretion of the GnRH neuronal network initiated in utero then spills over after delivery into the “mini-puberty” of the neonatal period lasting for months. Thereafter, a quiescence of this axis ensues during childhood as endogenous GnRH secretion and LH levels are dampened with corresponding reductions in gonadal steroidogenesis (15). Subsequently, GnRH secretion, the pilot light of reproduction, reactivates the reproductive axis during adolescence by mysterious mechanisms that remain active throughout adult life (16). Given this developmental perspective, the history of the patient in Fig. 2 can be mapped as a striking contrast to normal development.
Fig. 4.
A developmental perspective on human reproduction. This figure outlines the activity of the reproductive axis across the human life cycle in normal subjects vs. men with isolated GnRH deficiency. Certain symptoms and signs such as anosomia, undescended testes, and microphallus all indicate the absence of reproductive axis activity at the normal periods of development in which activity of the hypothalamic-pituitary axis is required.
Critical Observation 1: The Origins of GnRH Cells
In 1989, Pfaff and colleagues (3) at the Rockefeller Institute made a seminal discovery. While examining the origins of the 1200 or so GnRH neurons in the mouse brain using the simple tool of immunocytochemistry, they discovered that no GnRH-positive neurons appeared in the brain until day embryonic d 9.5 (e9.5). By d 11, robust GnRH staining was visible but in a completely unexpected location, the extra CNS location of the developing olfactory placode in the nose that was completely extramural to the CNS. These precursor neurons or GnRH stem cells arose from somewhere within the olfactory system. By e9.5, they had dedicated their genetic expression and secretory activity to the GnRH pathway. After this developmental step of fate specification, the GnRH neurons commence upon a long migration from their nasal placode origins along the olfactory tract into the CNS and toward their ultimate home within the hypothalamus. Fortunately, some GnRH-positive cells are left in the wake of this migratory herd as footprints of their trek along the olfactory bulb and tract and into the hypothalamus.
Upon arrival at their ultimate resting place within the hypothalamus, this migrating cell mass of GnRH-positive neurons exits from the guidance of the olfactory tract to reside in their final anatomical home, the medial basal hypothalamus and arcuate nucleus. There, these bipolar neurons send their axons out as sentinels into the nether regions of the hypothalamus, there to sense and monitor input from various other parts of the brain relating to reading of the external environment. These afferent axons then funnel this information to the central core of GnRH-secreting cell bodies to be integrated into the functional secretion of the GnRH neuronal network that is transmitted by their dendrites into the hypophyseal portal blood supply. Such monitoring information from the rest of the brain and extramural environment then serves to coordinate the secretion of the entire GnRH neuronal network in response to changes in body weight, light-dark cycles, stress, nutrition, and exercise. The GnRH neuronal projections in the hypophyseal portal system then shower the pituitary gonadotropins with their coordinated pulsatile secretion stimulation of GnRH that result in physiological gonadotrope stimulation according to variations in the amplitude and frequency of GnRH pulses.
These landmark articles then went on to document that in the absence of the X-located KAL1 gene (3, 4), the normal migration of GnRH neurons into the CNS from the olfactory placode was completely aborted with their arrest of progression occurring at the level of the cribiform plate and olfactory bulb. In the absence of the KAL1 secreted protein, anosmin (4), no olfactory bulb and tract developed and hence the GnRH neurons could not employ these structures to support their migration into the CNS. This remarkably clever bit of translational research also signaled that the genetic era of using this human model of isolated GnRH deficiency in combination with the evolving information on the human DNA sequence had begun. Since then this human disease model has been a guiding light that has elucidated several key elements of the developmental biology and genetic architecture that control the fundamental developmental biology of GnRH.
Returning to the patient in Fig. 2 for a moment and mapping his clinical circumstances on Fig. 4, his anosmia implies that his olfactory system was defective dating back to infancy and childhood. Similarly, his cryptorchidism and microphallus attest to yet another presumed absence of normal GnRH-induced reproductive activity during the second and third trimesters of his fetal development in which the initiation of reproductive activity usually gives rise to normal penile growth and testicular descent. The initial patient found to have Kallmann's syndrome due to a deletion of the X-linked KAL1 gene were demonstrated to have this failure of GnRH neuronal migration that normally occurs along the olfactory epithelium, bulb, and track. Hence, a critical link between GnRH migration and activation of the reproductive axis was established and our patient became a presumed example of this particular form of Isolated GnRH deficiency, i.e. Kallmann syndrome, where anosmia is the key clinical finding to document involvement of the olfactory system.
New Genes Controlling GnRH Ontogeny Using Human Disease Model of Isolated GnRH Deficiency
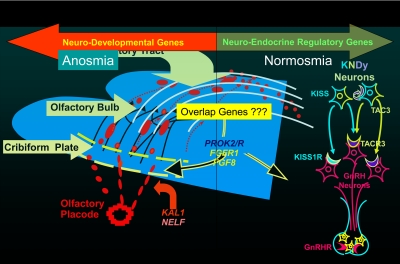
Figure 5 then portrays several of the various genes that have subsequently been described by several groups around the world as causes of this condition in relation to either their neurodevelopmental or neuroendocrine perspective, largely based upon the presence/absence of olfactory abnormalities. For example, KAL1 gene mutations (4), and the nasal embryonic LHRH-like factor (NELF), mutations (17) appear to represent defects that cause this syndrome due to defects in neurodevelopment. Subsequently, these neurons migrate over the olfactory bulb and tract into the hypothalamus. Mutations in PROK2 (18) and its receptor, PROKR2 (19–23), fibroblast growth factor (FGF)R1 (24–26) and its ligand FGF8 (27), and CHD7 (28, 29) and perhaps other new genes appear to also have roles here given their associated findings of anosmia with GnRH deficiency.
Fig. 5.
Genes causing GnRH deficiency in relation to their site of action on the ontogeny of GnRH neurons. Genes on the left half of this developmental picture typically are associated with anosomia and impair GnRH neuronal migration and/or olfactory bulb/track development and thus are neurodevelopmental in their action. Examples are KAL1, FGF8, FGFR1, and NELF. In contrast, genes on the right half of this developmental map affect the function of GnRH neurons after their migration into the hypothalamus and hence are neuroendocrine in their sites of action. These include the genes encoding the kisspeptin-, neurokinin B-, and GnRH-signaling system. Typically, patients with mutations in these genes exhibit a normal sense of smell. In the middle are genes referred to as “overlap” genes because they seem to be able to act in either mode and include the prokineticin- and FGF-signaling systems that produce individuals and families with both anosomia and normal sense of smell often within the same pedigree.
In addition, through the study of patients with Isolated GnRH deficiency with a normal sense of smell, it soon became clear through the discovery of the Kisspeptin signaling system (30–32), that there are signaling systems in neurons (with ligands and receptors) that preside over the secretion of the GnRH neuronal network. Kisspeptin neurons bearing mutations in either both the receptor (30, 31) and/or its ligand (32) are associated with normosmic GnRH deficiency. Similarly, neurokinin B deficiency in a Turkish population (33) population and in other less endogamous populations (34) exhibits an interesting phenotype associated with mutations in both ligand and receptors, TAC3 and TAC3Rs a result, this conceptual view has now divided the patients with isolated GnRH deficiency into a neurodevelopmental group, a neuroendocrine group, and a third group of genes the phenotypes of which overlap both the anosmic and nonanosmic genes often within the same family which are puzzling.
Oligogenicity: Increasing Complexity of Genetic Architecture
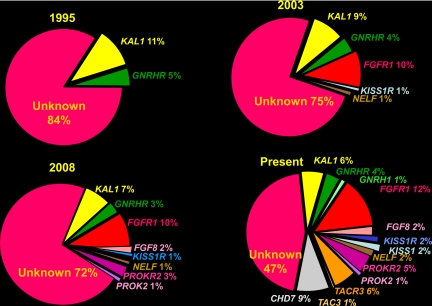
Partly because of the existence of this curious overlap group of genes, i.e. genes in which affected patients exhibited both anosmia and normosmia often with the same families as well other clinical features that incompletely segregated within and across families with the same mutation, the collective understanding of the growing complexity of the genetic architecture of this disease is improving by leaps and bounds (6, 7). Figure 6 and Fig. 7 show the growing complexity of this genetic architecture determining the GnRH neuronal network over the past 20 yr. In the most recent representation, about half the genes remain unknown.
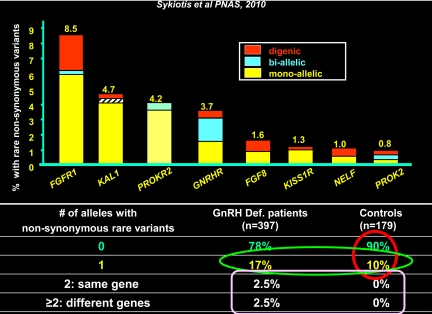
Fig. 6.
Oligogenicity in isolated GnRH deficiency. This figure demonstrates the incidence of mutations in each of eight genes known to cause human GnRH deficiency. Exomic sequencing was performed for all eight genes in 376 cases and the incidence of single mutations, two mutations in the same gene, and two mutations in different genes graphed and compared with 179 normal subjects (50).
Fig. 7.
Increasing complexity of the genetic architecture of isolated GnRH deficiency over 15 yr. Each of the four panels represents the incidence of the genes known to cause this condition from 2005 to 2011.
GnRH: Timeline of Landmark Discoveries
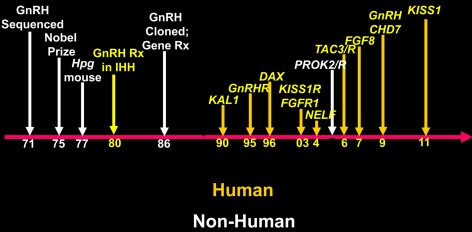
Yet another way to put the importance of genetics and the disease model of isolated GnRH deficiency into some perspective is to view the timeline of landmark discoveries in the area of GnRH including both human and nonhuman contributions (Fig. 8). The isolation and characterization of GnRH, the Nobel piece of work, was done in the 1970s. Pfaff (3, 4) first mapped the GnRH neurons and documented their extra-CNS origins in 1981 GnRH was cloned, and the first gene therapy inserting the GnRH gene into the hpg mouse was done by Mason et al. (35) at Genentech in 1986. All of these were important efforts in the basic research domain. However, since then, this entire field, with one exception (PROK2/R), was elucidated through human research using the model of isolated GnRH deficiency in the human. Figures 6 and 7 thus demonstrate quite vividly how much of our contemporary understanding of the biology of GnRH has been brought to light by human genetics. Thus, this single human model has generated many new insights and wonderful basic opportunities. For example, the kisspeptin field now has emerged with several laboratories dedicated to the study of the kisspeptin-signaling system that has now been documented to be present in all mammals, and several laboratories around the world are now working on this system. Also, many of these new genes are truly novel, and none would have been logical candidates in typical animal gene-ablation programs. In fact, the neurokinin B systems, clearly responsible for some cases of human GnRH deficiency, already failed to demonstrate any reproductive phenotype when selectively ablated in mice. Finally, the large and growing number of genes and phenotypes associated with isolated GnRH deficiency is now just beginning to hint at a series of biological networks that collectively act to define the ontogeny of GnRH neurons. And all this occurred with only about half of all patients with isolated GnRH deficiency currently having their genes identified!
Fig. 8.
A timeline of sources of information and landmark discoveries in human GnRH deficiency. Each of the major achievements is mapped on a timeline with those advances that are derived from humans in yellow and nonhuman in white.
Interactions between Mendelian Genes Contributing to Isolated GnRH Deficiency: Evidence of Oligogenicity
The most recent novel genetic insight provided by this human model demonstrated some interesting genetic features that appear to arise from the interaction between more than one of these causative genes, i.e. oligenicity. Two observations prompted this discovery. The first was the fact that subjects within the same family exhibiting identical single-gene mutation had very different phenotypes, i.e. incomplete segregation within families. In addition, the same features varied across different families with identical mutations, i.e. incomplete expressivity. Both of these observations suggested to us the existence of other genes that were interacting with the known genes to modify their expression. The fact that some patients/families with known mutations in other genes like GnRHR also exhibited additional phenotypic features such as skeletal abnormalities seen in mutations in the FGF signaling pathway or renal abnormalities such as KAL 1 mutations signaling pathways also raised this issue for us.
Widespread (11%) Oligogenicity in 376 GnRH-Deficient Probands
Initially, we documented the occurrence of such oligogenicity, i.e. where mutations in two different genes known to cause GnRH deficiency occurred simultaneously, in two different families with GnRH deficiency (6). However, to define the true incidence of this oligogenicity more precisely, we next sequenced 376 GnRH-deficient probands and nearly 200 controls for the coding sequence of all the eight genes known to cause GnRH deficiency at that time (7). Figure 6 demonstrates the rank order of the incidence of mutations in each of these genes; the incidence of compound heterozygosity in which two mutations were found in the same gene; and the incidence of digenicity in which more than one gene was discovered in these cases. Whereas several families had previously demonstrated failures of segregation when viewed in terms of a single gene mutation, when the second mutated gene was added, much of the apparent incomplete segregation was resolved. An interesting byproduct of these analyses of almost normal 200 controls for each of these genes was that 10% of the reproductively normal controls vs. 17% of probands exhibited mutations in at least one copy of one of these eight genes. This finding documented the incidence of heterozygosity in a large population of GnRH-deficient subjects for all of these genes simultaneously. Perhaps more interesting is that almost 30% of patients with demonstrated sequence mutations in one of the eight genes harbored a previously unknown mutation or second hit in yet another gene associated with isolated GnRH deficiency. Ultimately, this oligogenicity occurred in 11% of the population with demonstrable coding sequence mutations. However, it must be recalled that at the time this study was performed, only one third of the population with isolated GnRH deficiency had recognizable defects in these eight genes. Hence, it is reasonable to assume that the other two thirds of patients with positive family history will have at least this incidence of oligogenicity, if not a higher one.
Conclusion: Oligogenicity in single-gene disorders
Collectively, these studies made it clear that oligogenicity is a property of this disease model of isolated GnRH deficiency and in addition that this oligogenicity, at least in part, accounts for anomalies of segregation and expressivity. This oligogenicity has already been documented to occur in other monogenic disorders such as the ciliopathies that include Lawrence Moon Biedl Syndrome (36). This phenomenon of oligogenicity may also account for those GnRH deficienct patients with only heterozygous mutations that are known autosomal recessive in their mode of inheritance (e.g. PROKR2) when other disease-associated genes are found (i.e. the missing oligogenicity). At least six papers presented at this 2011 Endocrine Society meeting could have digenicity as the explanation for several of their genetic puzzles. This search for the ultimate incidence of oligogenicity is clearly going to be a problem that will be resolved only by the widespread availability of exomic and whole-genome sequence. However, because segregation analyses within a kinship are key criteria used to reduce the number of false positives unearthed by all of these sequencing techniques, before eliminating any putative candidate mutations, this property of oligogenicity must be borne in mind.
Surprising Phenotypes Revise Our Understanding of Basic Biology
Reversal of idiopathic hypogonadotropic hypogonadism/Kallmann Syndrome in 15 males (10% of population)
A longitudinal study of men with isolated GnRH deficiency was of interest in revealing a remarkable feature of the biology of this disease, i.e. its potential reversibility (37). All of the patients studied were demonstrated to be hypogonadotropic and mostly apulsatile before initiating therapy. Each was studied before and after receiving a variety of therapies (testosterone, pulsatile GnRH, human choriogonadotropin) which had been discontinued for two reasons: the paradoxical finding of their testes growing while on therapy like testosterone replacement and/or their apparent lack of need for prescription renewals after therapy was discontinued as they were transitioning to another form of therapy. So the question was, what happened and what did it have to do with their therapy? In each case, these patients with well-documented GnRH deficiency were found to have reversed, i.e. each now exhibited spontaneous pulses of LH, normal testosterone and Inhibin B levels, spontaneous testicular growth and often fertility. In these cases, the only common denominator was exposure to steroid exposure during their periods of treatment. In a prospective arm to this study, 10–15% of all cases of GnRH deficiency were found to undergo such reversals, including some men with Kallmann's syndrome. This latter finding merits particular attention as these were Kallmann's males with no olfactory bulbs or tract who were now having spontaneous GnRH pulses—a puzzle indeed as to how this occurred.
Reversal of GnRH deficiency: biological implications
From a biological perspective, this phenomenon of reversibility is remarkable because it raises important fundamental biological questions. First, it speaks to the neuroanatomy of these cases. Their GnRH neuronal network is clearly present and in the hypothalamus as documented by their appearance of spontaneous GnRH pulses after therapy but this GnRH network somehow did not function properly at puberty. The physiological considerations of this finding suggest that after sex steroid exposure, these GnRH neurons have somehow acquired the ability to secrete GnRH, now can fire in the coordinated fashion necessary to sustain pulsatile activity of the hypothalamic-pituitary-gonadal axes, and consequently are able to induce or sustain normal reproductive (hypothalamic-pituitary- gonadal) axis activity. A question remains as to whether these patients will demonstrate some unusual susceptibility of their GnRH pulse networks to changes in environment (e.g. exercise, starvation, or stress) similar to women with hypothalamic amenorrhea. From a clinician's perspective, this finding argues for periodic discontinuation of medication in these men to determine whether further therapy is needed. The question also arises then does this phenomenon occur in disease of other hypothalamic-releasing factors such as the isolated GHRH deficiency of childhood?
Transition from Rare to Common Diseases: Monogenic vs. Polygenic Disorders
Having discovered that oligogenicity occurred in a significant percentage of cases of isolated GnRH deficiency and that reversibility was now a characteristic of this disorder, the next logical question was, do these mono-/oligogenic mutations that clearly cause the rare disorder of isolated GnRH secretion play a role in the genetics of more common reproductive disorders? Hypothalamic amenorrhea, a reproductive disorder occurring in 2–3% of the normal female population (38) seemed like an excellent place to test this hypothesis in women for several reasons. First, it is the commonest reproductive disorder in either sex. Second, 25 yr ago our group described that all women with hypothalamic amenorrhea had in common an underlying spectrum of secretory abnormalities of their pulsatile GnRH-induced LH secretion (39). Third, many of these patients have both a family history and recurrent susceptibility to disruption of their menstrual cycles in relation to stress, diet, and exercise. Hence, this condition appeared to be an excellent common disorder in which to examine the role of all of the known loss of function genes already proven to cause the much rarer syndrome of isolated GnRH deficiency.
The Role of Mutations Causing Isolated GnRH Deficiency in Hypothalamic Amenorrhea
In examining 55 women with well-phenotyped hypothalamic amenorrhea, 14% were demonstrated to have heterozygous mutations in one of the genes that were associated with isolated GnRH deficiency and also previously demonstrated to be loss of function when studied in vitro (40). Each had segregated within families with a history of hypothalamic amenorrhea especially when mapped onto their strong family history of hypothalamic amenorrhea and delayed puberty in both probands and pedigrees of these probands with hypothalamic amenorrhea. These mutations were absent in all 422 control women, including 40 heavily excercising controls with normal menstrual periods.
Therefore this finding really represents that these mutations, previously surfaced as causal genes for isolated GnRH deficiency genes, also represent susceptibility genes that somehow render their underlying GnRH neuronal network as fragility to environmental stress. Thus, they predispose women to develop hypothalamic amenorrhea during stress, malnutrition, exercise, etc. This finding is both an excellent example of a genetics X environment interaction and of rare disease-causing mutations in the most common reproductive disorders.
Polygenic Disorders and GWAS
Much suspicion exists, especially within the basic scientific community, about the importance of the genes discovered by GWAS. These concerns involve the amount of resources required for their study, the large numbers of patients needed render them truly useful for discovery, often in the tens to hundreds of thousands, and their seemingly mild severity. The common feature of these approaches is that they hinge on complex statistical comparisons between the genomes of a patient population with a disease compared with otherwise normal controls. This determination, in turn, critically depends upon the biological importance of the gene in producing the phenotype, the precision of the phenotyping tools used to characterize the disease process, the degree of differential genetic loading of the disease population for common variants of interest, and the corresponding freedom of the controls of that disease now or in the future.
Complex statistical comparisons then systematically proceed through the full catalog of millions of variants in all of these human genomes, currently about 2.5 million times, supplied by the HapMap consortium, to define consistent differences between the patients and controls. Of course, not all of these differences will be not biologically meaningful similar to the spelling variants like “color” and “colour.” However, other of these variants truly change the biology of the underlying gene/protein and are thus deterministic in the presence of the disease, albeit in a far lesser order of magnitude than is seen with monogenic defects. Hence, GWAS are substantially a numbers game of frequency of mutations, their severity, and the resolution power of the genomic information with which the investigators are querying the disease and control genomes.
The Fundamental Challenge
The fundamental contemporary challenge of human genetics is how to test human genetic variation systematically for its role in disease risk. Given the 3.2 billion bases in the human genome, this task is akin to finding a needle in a haystack; therefore the best bet is to make the needle bigger and therefore easier to find. The initial approach to this problem was the HapMap consortium in which 1 × 106 common (i.e. occurring in >5% of normal subjects) single nucleotide polymorphisms (SNP) variants were chartered as location markers across the genome. Over time, these available ‘SNP variants’ increased to more than 2.5 × 106/genome and are now accompanied by other ‘address markers’ in the form of thousands of structural changes like copy number variants of gene sequences. However, compared with these large numbers of common DNA variants, relatively few are disease associated. Hence by comparing individuals with a given disease with the normal population, those variants most significantly marking the location of a disease-related gene will stand out in comparison and can be mapped by their more common occurrence in the disease vs. normal cohort. Ultimately, demonstrating that such associations are valid depends upon complex statistics, large numbers, and multiple comparisons that need to be corrected for by appropriate statistical methods for repeated testing. Consequently, in most GWASs a minimum significance score of equal to or greater than 10−8 must be achieved to withstand these correction factors. Hence, when seeking an association that is small and only occurs in subsets of most complex disease populations, these studies require very large numbers, varying from thousands to tens to more than 100,000. The precise number required to find these disease-associated genes depends upon several features of a given study among which the precision of the phenotyping of the populations, the frequency/rarity of the variant, and its biological impact are important. Most of the loci and their variations that have been discovered using GWAS are quite low in their odds ratio and biological impact (41). Collectively, these difficulties in discovery, when added to the relatively mild biological impact they exert, have raised considerable skepticism as to their importance by several observers.
Don't Confuse Genetic Contribution with Biological Importance: 3-Hydroxy-3-Methylglutaryl (HMG) Coenzyme A (CoA) Reductase
Given that the biological impact of each of these variants and their associated odds ratios are often low, a common misconception occurs in their interpretation. The notion of some observers is that the odds ratio of a genetic variant is directly proportional to its biochemical phenotype like a lipid level. In other words, its relative risk ratio is directly proportional to the biology of the new gene/protein/variant. The fact that these two properties, i.e. relative risk ratio and biological impact, can be quite unlinked is vividly portrayed by the gene HMG CoA reductase reductase gene (42). The contribution of HMG CoA to low density lipoprotein or ApoB levels is so relatively small that one might even believe that this gene is not very significant when viewed through this prism of GWAS genetics odds and relative risk ratios. On the other hand, statins, the drugs that block this enzyme, have all demonstrated a major impact upon cardiovascular disease and mortality in myocardial infarction. No clinical trials examining the impact of statins on myocardial infarctions in appropriate populations have demonstrated less than 25–30% reduction in incidence of myocardial infarction and even decreased death rates. Hence, this example indicates a circumstance that it is an important reminder not to confuse relative risk ratios with biological importance, as is commonly done.
GWAS for Lipids
Lipid disorders represent an interesting example of the use of the genetic tools for discovery used to identify genes that occur in both monogenic vs. polygenic forms. When sufficient numbers of patients and controls are systematically examined for novel DNA variants, several interesting observations emerge. In version 1.0 (43) of a GWAS using 2931 subjects, two known monogenic genes and one unknown were discovered. Version 2.0 of this study examined 8816 patients/18,554 controls (44, 45), and more known genes and new genes were discovered. Version 3.0 (44) used 19,840 patients and 19,000 controls and increased the yield even further. Finally, version 4.0 (46), the Global Lipids Genetics Consortium (n ≈ 100,000) performed across 46 countries, revealed not only more new genes, but they began to organize themselves in metabolic pathways, both old and new. Thus, by the time populations exceed 100,000, not only are the P values highly significant but the systems biology of a disease begins to emerge.
Connecting Rare and Common Variants in Lipids: ANGPTL3
These same investigators studying lipid disorders subsequently also published exomic sequencing in a family with hyperlipidemia that unearthed novel monogenic mutations in ANGPTL3 (47). This gene had already surfaced by their early GWAS in which it had been demonstrated to contribute risk of cardiovascular disease in a dose-responsive way. Therefore the indictment of this gene by both GWAS and linkage represents an impressive cross-roughing of monogenic and polygenic approaches that confirm its importance. Presumably such confirmations will now become more common as the great density of available SNP increases, the at-risk haplotypes of these particular genes and mutations become more precisely defined, and the widespread availability of whole-exome and then genome sequencing expands. There are now several examples of genes causing monogenic syndromes also harboring disease-associated common variants (44, 45, 48). Thus, with appropriately sized populations and increasing precise tools, real novelty emerges from these association studies.
The Evolving Genetic Architecture of Type 2 Disease
Finally, studies attempting to define the genetic architecture of type 2 diabetes have drawn considerable attention. These studies now comprise 150,000 patients and controls, underscoring the fact that an important lesson here is the need for international collaborations for such common diseases (49).
The Value and Number of Published GWAS
By the end of 2010, 1,200 published loci have identified a wide variety of disease-associated genes, the vast majority of which are truly novel. The question then arises as to how many patients/controls does it take to discover these novel genes and how novel and important are these discoveries? In schizophrenia and inflammatory bowel disease, the trajectory of knowledge growth is quite analogous with the slope of the rising curve of novel gene discovery vs. number of patients studied depending upon the genetic architecture of the specific disease under study. The key is that all of these studies are moving up a similar new knowledge curve. As the need for large numbers, more precise phenotyping, and new bioinformatic and statistical tools continue to open entirely new vistas of the “omics” to disease and gene discoveries, understanding of the genetics of endocrine disorders has a bright future.
Conclusion: GWAS
This approach is a valuable genetic tool with which to discover new causative genes for complex diseases that have not yielded to past investigations. Thus, the information derived from them is truly novel and valuable. Although the relative risk ratios of these genes involved in disease are typically low, their biological importance and drugability can be quite disproportionate (cf. above example of HMG CoA reductase). As more of these genes emerge, new systems biology pathways will also become apparent. The analogy that comes to mind is the building of Mount Rushmore, one chip at a time until the full façade was clear. One drawback of this kind of work is that it requires heavy infrastructure, considerable funding that is stable over long periods of time, and dedicated, collaborative teams operating internationally. Although considerable National Institutes of Health funding is going into such studies, more is coming from foundations and private funds. Eventually, at sizes of 150,000 patients and 150,000 controls, the limits of an available population can be reached even with international collaborations. Additionally, it is difficult to phenotype that number of patients in any detailed fashion. However, when a gene emerges, it is possible to Mendelianize it. That is to say take the new gene, track its phenotype within families and across populations, and contrast their phenotypic findings with that of other genes associated with a given disease. Therefore GWAS is a really demand bid for broad collaborations and longitudinal follow-up. That said, this approach represents but one end of the genetic spectrum whereas single, well-phenotyped families can find a key to any disorder if their phenotype can be found and described in detail, which is always easier to perform in small investigative units like single families.
Looking ahead: exomic sequencing
The costs of sequencing are rapidly dropping and the information is growing proportionally. However, a brief postscript is in order here. The bioinformatic and statistical filtering processes involved in these searches of 3.2 billion base pairs is considerable. First the base pair assignments (calls) must be accurate. To be certain of such accuracy, in general, a 10-fold sequencing coverage is required, given the accuracy of current sequencing techniques. Then, assignment of the sequence to the reference genome is a critical and complex next step given the vast number of redundancies present in the human genome. Next, cataloguing of these changes in relation to normal variations must be performed in which referencing the sequence variants to normal vs. disease-causing alleles is complex. The bioinformatic databases and prediction programs that can carry out this massive filtering to get hundreds of cases down to 10 or 15 variants per patient as follow-up of larger numbers is rapidly appearing. Then the final question is their functional biology and what to do about adventitious findings especially in genes that have profound clinical implications such as BRCA1, sudden death genes, etc. Despite all of these formidable challenges, it is clear that exomic and full-genome sequencing will change everything by soon providing a tsunami of data for subsequent bioinformatic analyses.
Conclusion
The bottom line here is that given these rapidly advancing genetic and genomic tools, their declining costs, and the increasing availability of software programs to filter such vast amounts of information, human genetic variations in diseases will clearly become the starting point for more and more research projects. These results, in turn, will generate more and more basic research opportunities than ever. A key advantage of these genetic approaches is that all these opportunities are grounded in a human reality by beginning with patients with a disease that will help prioritize them. In addition, this feature will also assure all investigators, basic or clinical, that they are working on genes and projects that are truly relevant to disease because that is the origin of their discovery. The public and Congress need to be convinced of this fundamental truth to ensure that the necessary resources will continue to be available to make the rather bold leaps into our understanding of the genetic architecture of human diseases that extract such a personal and economic toll on our patients.
Acknowledgments
Disclosure Summary: Dr. Crowley is a cofounder of Combinent Biomedical Systems (with Robert Langer, Ph.D.) and a consultant for Quest and Abbotts Diagnostics and Proventys Medical Care. None of these relationships influenced this work.
Footnotes
- CNS
- Central nervous system
- CoA
- coenzyme A
- FGF
- fibroblast growth factor
- GWAS
- genome-wide association studies
- HMG
- 3-hydroxy-3-methylglutaryl
- SNP
- single nucleotide polymorphisms.
References
- 1. Franco B, Guioli S, Pragliola A, Incerti B, Bardoni B, Tonlorenzi R, Carrozzo R, Maestrini E, Pieretti M, Taillon-Miller P, Crown CF, Willard HF, Lawrence C, Graziella Persico M, Camerino G, Ballabio A. 1991. A gene deleted in Kallmann's syndrome shares homology with neural cell adhesion molecules. Nature 353:529–536 [DOI] [PubMed] [Google Scholar]
- 2. Legouis R, Hardelin JP, Levilliers J, Claverie JM, Compain S, Wunderle V, Millansseau P, Le Paslier D, Dohen D, Caterina D. 1991. The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules. Cell 67:423–435 [DOI] [PubMed] [Google Scholar]
- 3. Schwanzel-Fukuda M, Bick D, Pfaff DW. 1989. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res 6:311–326 [DOI] [PubMed] [Google Scholar]
- 4. Bick D, Franco B, Sherins RJ, Heye B, Pike L, Crawford J, Maddalena A, Incerti B, Pragliola A, Meitinger T, Ballabio A. 1992. Intragenic deletion of the KALIG-1 gene in Kallmann's syndrome. N Engl J Med 326:1752–1755 [DOI] [PubMed] [Google Scholar]
- 5. Sykiotis GP, Pitteloud N, Seminara SB, Kaiser UB, Crowley WF., Jr 2010. Deciphering genetic disease in the genomic era: the model of GnRH deficiency. Sci Transl Med 2:32rv2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, Li WP, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W. 2007. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest 117:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sykiotis GP, Plummer L, Hughes VA, Au M, Durrani S, Nayak-Young S, Dwyer AA, Quinton R, Hall JE, Gusella JF, Seminara SB, Crowley WF, Jr, Pitteloud N. 2010. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci USA 107:15140–15144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kallmann F, Schoenfeld W, Barrera S. 1944. The genetic aspects of primary eunuchoidism. Am J Ment Defic 48:203–236 [Google Scholar]
- 9. Baba Y, Matsuo H, Schally AV. 1971. Structure of the porcine LH- and FSH-releasing hormone. II. Confirmation of the proposed structure of conventional sequential analyses. Biochem Biophys Res Commun 44:459–463 [DOI] [PubMed] [Google Scholar]
- 10. Amoss M, Burgus R, Blackwell R, Vale W, Fellows R, Guillemin R. 1971. Polypeptides antagonists of the hypothalamic luteinizing hormone releasing factor. Biochem Biophys Res Commun 44:205–210 [DOI] [PubMed] [Google Scholar]
- 11. Crowley WF, Jr, Filicori M, Spratt DI, Santoro NF. 1985. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res 41:473–531 [DOI] [PubMed] [Google Scholar]
- 12. Hoffman AR, Crowley WF., Jr 1982. Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med 307:1237–1241 [DOI] [PubMed] [Google Scholar]
- 13. Crowley WF, Jr, McArthur JW. 1980. Simulation of the normal menstrual cycle in Kallman's syndrome by pulsatile administration of luteinizing hormone-releasing hormone (LHRH). J Clin Endocrinol Metab 51:173–175 [DOI] [PubMed] [Google Scholar]
- 14. de Roux N, Young J, Misrahi M, Genet R, Chanson P, Schaison G, Milgrom E. 1997. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med 337:1597–1602 [DOI] [PubMed] [Google Scholar]
- 15. Dunkel L, Alfthan H, Stenman UH, Tapanainen P, Perheentupa J. 1990. Pulsatile secretion of LH and FSH in prepubertal and early boys revealed by ultrasensitive time-resolved immunofluorometric assay. Pediatr Res 27:215–219 [DOI] [PubMed] [Google Scholar]
- 16. Boyar RM, Rosenfeld RS, Kapen S, Finkelstein JW, Roffwarg HP, Weitzman ED, Hellman L. 1974. Simultaneous augmented secretion of luteinizing hormone and testosterone during sleep. J Clin Invest 54:609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miura K, Acierno JS, Seminara SB. 2004. Characterization of the human nasal embryonic LHRH factor gene, NELF, and a mutation screening among 65 patients with idiopathic hypogonadotropic hypogonadism (IHH). J Hum Genet 49:265–268 [DOI] [PubMed] [Google Scholar]
- 18. Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, Crowley WF., Jr 2007. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA 104:17447–17452 PMID:17959774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dode C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler MD, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, Morgan G, Murat A, Toublanc JE, Wolczynski S, Delpech M, Petit, Young J, Hardelin JP. 2006. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2, PLoS Genet 2:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cole LW, Sidis Y, Zhang C, Quinton R, Plummer L, Pignatelli D, Hughes VA, Dwyer AA, Raivio T, Hayes FJ, Seminara SB, Huot C, Alos N, Speiser P, Takeshita A, Van Vliet G, Pearce S, Crowley WF, Jr, Zhou QY, Pitteloud N. 2008. Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J Clin Endocrinol Metab 93:3551–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simisi AA, Asci R, Bellastella G, Maione L, Esposito D, Elefante A, De Bellis A, Bellastella A, Iolascon A. 2008. Homzygous mutation in the prokineiticn-receptor2 gene (Val274AAsp) presenting as reversible Kallmann syndrome and persistent oligozoospermia: case report. Hum Reprod 23:2380–2384 [DOI] [PubMed] [Google Scholar]
- 22. Leroy C, Fouveaut C, Leclercq S, Jacquemont S, Boullay HD, Lespinasse J, Delpech M, Dupont JM, Hardelin JP, Dodé C. 2008. Biallelic mutations in the prokineticin-2 gene in two sporadic cases of Kallmann syndrome. Eur J Hum Genet 16:865–868 [DOI] [PubMed] [Google Scholar]
- 23. Abreau AP, Trarback EB, de Castro M, Frade Costa EM, Versiani B, Maias Baptistia MT, Garmes HM, Mendonca BB, Latronico AC. 2008. Loss-of-function mutations in the genes encoding prokineticin-2 or prokineticin receptor-2 cause autosomal recessive Kallman syndrome. J Clin Endocrinol Metab 93:4113–4118 [DOI] [PubMed] [Google Scholar]
- 24. Dodé C, Levilliers J, Dupont JM, De Paepe A, Le Dû N, Soussi-Yanicostas N, Coimbra RS, Delmaghani S, Compain-Nouaille S, Baverel F, Pêcheux C, Le Tessier D, Cruaud C, Delpech M, Speleman F, Vermeulen S, Amalfitano A, Bachelot Y, Bouchard P, Cabrol S, Carel JC, Delemarre-van de Waal H, Goulet-Salmon B, Kottler ML, Richard O, Sanchez-Franco F, Saura R, Young J, Petit C, Hardelin JP. 2003. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet 33:463–465 [DOI] [PubMed] [Google Scholar]
- 25. Pitteloud N, Acierno JS, Jr, Meysing AU, Dwyer AA, Hayes FJ, Crowley WF., Jr 2005. Reversible Kallmann syndrome, delayed puberty, and isolated anosmia occurring in a single family with a mutation in the fibroblast growth factor receptor 1 gene. J Clin Endocrinol Metab 90:1317–1322 [DOI] [PubMed] [Google Scholar]
- 26. Pitteloud N, Acierno JS, Jr, Meysing A, Eliseenkova AV, Ma J, Ibrahimi OA, Metzger DL, Hayes FJ, Dwyer AA, Hughes VA, Yialamas M, Hall JE, Grant E, Mohammadi M, Crowley WF., Jr 2006. Mutations in fibroblast growth factor receptor 1 cause both Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA 103:6281–6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Falardeau J, Wilson CJ, Chung, Beenken A, Raivio T, Lacey Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Simon HS, Pearce, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N. 2008. Decreased FGFβ signaling causes deficiency of gonadotropin releasing hormone in humans and mice. J Clin Invest 118:2822–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jongmans MC, van Ravenswaaij-Arts CM, Pitteloud N, Ogata T, Sato N, Claahsen-van der Grinten HL, van der Donk K, Seminara S, Bergman JE, Brunner HG, Crowley WF, Jr, Hoefsloot LH. 2009. CHD7 mutations in patients initially diagnosed with Kallmann syndrome–the clinical overlap with CHARGE syndrome. Clin Genet 75:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim HG, Kurth I, Lan F, Meliciani I, Wenzel W, Eom SH., Kang GB, Rosenberger G, Tekin M, Ozata M, Bick DP, Sherins RF, Walker SL, Shi Y, Gusella JF, Layman LC. 2008. Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am J Hum Genet 83:511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KISS1-dervied peptide receptor, GPR43. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 32. Chan YM, Broder-Fingert S, Paraschos S, Lapatto R, Cerrato F, De Guillebon AD, Wu I, Wahab F, Dwyer A, Kirsch S, Quinton R, Cheetham T, Ozata M, Ten S, Chanoine JP, Pitteloud N, Martin K, Schiffmann R, Van der Kamp H, Nader S, Hall JE, Kaiser UB, Seminara SB. GnRH-deficient phenotypes in humans and mice with heterozygous variants in KISS1/Kiss1. J Clin Endocrinol Metab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. 2009. TAC3 and TAC3R mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the centeral control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonça BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. 2010. Jun TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab 95:2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mason AJ, Pitts SD, Nikolics K, Szonyi E, Wilcox JN, Seeburg PH, Stewart TA. 1986. The hypogonadal mouse: reproductive functions restored by gene therapy. Science 234:1372–1378 [DOI] [PubMed] [Google Scholar]
- 36. Hildebrandt F, Benzing T, Katsanis N. 2011. Ciliopathies. N Engl J Med 364:1533–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, Hughes VA, Cole LW, Pearce SH, Lee H, Boepple P, Crowley WF, Jr, Pitteloud N. 2007. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med 357:863–873 [DOI] [PubMed] [Google Scholar]
- 38. Pettersson F, Fries H, Nillius SJ. 1973. Epidemiology of secondary amenorrhea. I. Incidence and prevalence rates. Am J Obstet Gynecol 117:80–86 [DOI] [PubMed] [Google Scholar]
- 39. Santoro N, Filicori M, Crowley WF., Jr 1986. Hypogonadotropic disorders in men and women: diagnosis and therapy with pulsatile gonadotropin-releasing hormone. Endocr Rev 7:11–23 [DOI] [PubMed] [Google Scholar]
- 40. Caronia LM, Martin C, Welt C, Sykiotis GP, Quinton R, Thambundit A, Avbelj M, Dhruvakumar S, Plummer L, Hughes VA, Seminara S, Boepple PA, Sidis Y, Crowley WF, Martin K, Hall JE, Pitteloud NA. 2011. A genetic basis for functional hypothalamic amenorrhea. N Engl J Med 364:2155–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khoury MJ, Wacholder S. 2009. Invited commentary: from genome-wide studies to gene-environment interaction studies—challenges and opportunities Am J Epidemiol 169:227–230; discussion 234–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hardy J, Singleton A. 2009. Genomewide association studies and human disease. N Engl J Med 360:1759–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saxena R, Vight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshulder D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Boström K, Isoma B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, et al. 2007. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336 [DOI] [PubMed] [Google Scholar]
- 44. Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, Strait J, Duren WL, Maschio A, Busonero F, Mulas A, Albai G, Swift AJ, Morken MA, Nasisu N, Bennett D, Parish S, Shen H, Galan P, Meneton P, Hercberg S, et al. 2008. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 40:161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, Anand S, Engert JC, Samani NJ, Schunkert H, Erdmann J, Reilly MP, Rader DJ, Morgan T, Spertus JA, Stoll M, Girelli D, McKeown PP, Patterson CC., Soscovick DS, O'Donnell CJ, Elosua R, Peltonen L, Salomaa V, Schwartz SM, et al. 2009. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet 41:334–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Styianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X., et al. 2010. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466:707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Musunuru K, Pirruccello JP, Do R, Peloso GM, Guiducci C, Sougnez C, Garimellta KV, Fisher S, Abreu J, Barry AJ, Fennell T, Banks E, Ambrogio L, Cibulskis K, Kernytsky A, Gonzalez E, Rudzicz N, Engert JC, DePristo MA, Daly MJ, Cohen JC, Hobbs HH, Altshuler D, Schonfeld G, Gabreil SB, et al. 2010. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med 363:2220–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kathirsan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, Cooper GM, Roos C, Voight BF, Havulinna AS, Wahlstrand B, Hednew T, Coretta D, Tai ES, Ordovas JM, Berglund G, Vartiainen E, Jousilahti P, Hedblad B, Taskinen MR, Newton-Cheh C, Salomaa V, Peltonen L, Groop L, Altshulder DM, et al. 2008. Six new loci associated with blood low-density lipoprotein cholesterol, high-density liporprotein cholesterol or triglycerides in humans. Nat Genet 40:189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, McCulloch LJ, Ferreira T, Grallert H, Amin N, Wu G, Willer CJ, Raychaudhuri S, McCarroll SA, Langenberg C, Hofmann OM, Dupuis J, Qi L, Segre AV, van Hoek M, Navarro P, et al. 2010. Twelve type 2 diabetes susceptibility loci identified through large-scale association analyses. Nat Genet [Erratum (2011) 43:388] 42:579–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sykiotis G, Plummer L, Hughes V, Au M, Durrani S, Nayak-Young S, Dwyer A, Quinton R, Hall J, Gusella J, Seminara S, Crowley W, Pitteloud N. 2010. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. P Nat Acad Sci 107:34, 15140–15144 [DOI] [PMC free article] [PubMed] [Google Scholar]