Abstract
Glucose transporter 4 (Glut4) is an important regulator of cellular glucose uptake in adipose tissue and skeletal muscle. The estrogen receptors α and β (ERα and ERβ) have been shown to regulate Glut4. However, the regulatory mechanisms are unclear, and there are conflicting results about the effects of the two ER isoforms on Glut4 activity. In this study we investigated how the lack of either ER isoform affects Glut4 expression in differentiated mouse embryonic fibroblasts. Our results demonstrate that Glut4 transcription is markedly reduced in cells lacking ERβ, both basally and upon induction by liver X receptor. These changes in Glut4 expression could not be explained by the lack of ERβ as ligand-activated transcription factor. They were rather brought about by hypermethylation of one single CpG in the Glut4 promoter in the ERβ-deficient cells. This CpG is part of an Sp1-binding site, and Sp1 binding was reduced by its methylation. Treatment with Sp1 inhibitor diminished Glut4 expression in wild-type, but not in ERβ-deficient cells, suggesting that reduced recruitment of Sp1 to the Glut4 promoter is responsible for the differences in Glut4 expression. Reintroduction of ERβ into ERβ-deficient cells partly restored Glut4 transcription and stabilized low DNA methylation after treatment with the DNA demethylating agent 5-Aza-2′-deoxycytidine. Our findings demonstrate the involvement of DNA methylation in Glut4 regulation and imply a novel function for ERβ in mediating epigenetic events and thereby regulating gene expression.
Glucose transporter 4 (Glut4) is responsible for insulin-mediated glucose uptake in adipose tissue, skeletal muscle, and heart. Upon insulin stimulation, Glut4 translocates from intracellular storages to the plasma membrane and mediates glucose intake into the cell from the circulation (1). Glut4 activity is regulated both through protein translocation to the plasma membrane and at the level of gene expression (2). Studies in mouse models showed that loss of Glut4 expression leads to decreased insulin sensitivity, whereas Glut4 overexpression in insulin-resistant mice restores insulin sensitivity (3). Transcription factors such as liver X receptor (LXR) α and Sp1 have been shown to positively regulate Glut4 expression, whereas peroxisome proliferators-activated receptor α and γ, for example, suppress Glut4 expression (2). Furthermore, Glut4 expression is also under epigenetic regulation, and studies have shown that DNA demethylation may play a role in activating Glut4 expression during adipogenesis (4).
Estrogens [17β-estradiol (E2)] regulate a number of essential physiological processes such as reproduction, brain development, and bone mass (5). They also play a role in controlling body weight, food intake, and fat distribution (6). Many of the cellular responses to E2 are mediated by the estrogen receptors (ERs), ERα and ERβ. The ER belong to the nuclear receptor family of transcription factors and share a common structural arrangement. Upon activation by estrogen, the ERs bind to DNA enhancer elements known as estrogen response elements and recruit additional coactivators, which potentiate the ability of the ERs to activate transcription of their target genes (7). Interestingly, the ERs have been suggested to regulate Glut4 levels (2, 8). However, it is currently not clear whether Glut4 is a direct ER target gene, and the effects of ER stimulation on Glut4 are contradictory and may be cell or tissue specific. In a hamster ovary cell line, it has been shown that selective activation of ERβ induces Glut4 expression (9). On the other hand, studies using ERα- and ERβ-deficient mice suggest that ERβ reduces, whereas ERα enhances, Glut4 protein levels in muscle and white adipose tissue (8).
The goal of this study was to address the question of how the different ER isoforms contribute to the regulation of Glut4 expression. We show here that in adipocytes derived from ERβ-knockout (βerko) mouse embryonic fibroblasts (MEFs), basal Glut4 expression and inducibility by LXR are significantly lower than in adipocytes derived from wt and ERα-knockout (erko) MEFs. We show further that one specific CpG in the Glut4 promoter is hypermethylated in the MEFs lacking ERβ. Reintroduction of ERβ into ERβ-deficient cells partly restores Glut4 transcription and stabilizes low DNA methylation after treatment with the DNA-demethylating agent 5-Aza-2′-deoxycytidine. Furthermore, we find that the hypermethylated CpG is part of an Sp1-binding site and that Sp1 binding is impaired by methylation of this site, which leads to decreased basal and LXR-induced Glut4 expression.
Results
Glut4 expression is reduced in the absence of ERβ
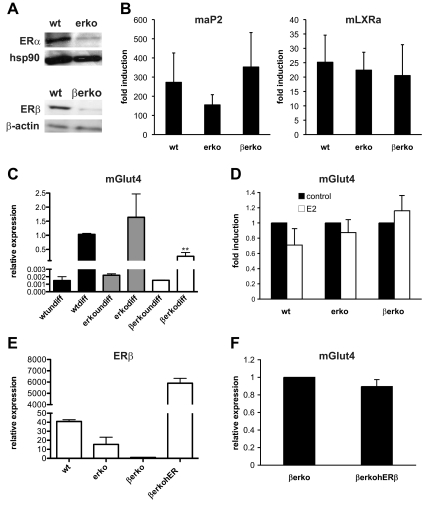
MEFs derived from wild-type (wt), erko, and βerko mice were chosen as models to study the contribution of the two ER isoforms on Glut4 transcription. ER expression and lack of expression in the knockout (KO) MEFs were confirmed by Western blot (Fig. 1A). In mice, Glut4 expression is restricted to skeletal muscle, heart, and adipose tissue (1). We therefore differentiated the MEFs into adipocytes to induce Glut4 expression and compare it between the ER-proficient and -deficient cells. MEFs derived from wt, erko, and βerko mice were differentiated according to a standard protocol, and differentiation status was monitored using the adipocyte marker gene aP2 as well as the LXRα by real-time PCR (Fig. 1B). The expression of aP2 was lower in erko-derived cells; however, the difference was not significant (P = 0.39). LXRα expression was similar between adipocytes derived from wt and ERα- and ERβ-deficient MEFs. Glut4 expression was similar in undifferentiated MEFs but hardly measurable by real-time PCR. Upon differentiation, Glut4 levels were induced and interestingly, they were about two thirds lower in βerko MEFs-derived adipocytes than in wt (P < 0.01) and erko (Fig. 1C). These findings suggest a role for ERβ in regulating Glut4 expression levels.
Fig. 1.
Glut4 expression in adipocytes derived from wt, erko, and βerko MEFs. A, Western blot analysis of ER expression in the MEFs. Nuclear extracts of wt, erko, and βerko MEFs were separated by PAGE and transferred to a nitrocellulose membrane. Shown is a representative Western blot using antibodies against ERα, ERβ, and hsp90 and β-actin as loading controls. B, AP2 (left panel) and LXRα (right panel) induction after MEFs differentiation into adipocytes. MEFs were differentiated for 8 d, and mRNA transcription was analyzed by real-time PCR. The results were normalized to 18S rRNA and to expression before differentiation. Data are represented as mean + sd. C, Glut4 expression in the differentiated MEFs. Real-time PCR results were normalized to 18S rRNA and to Glut4 expression of differentiated MEFs. Data are represented as mean + sd. **, P < 0.01 βerko compared with wt. D, Induction of Glut4 expression by estradiol in the differentiated MEFs. After differentiation, cells were treated with 10 nm E2 for 6 h. Subsequently, Glut4 mRNA was quantified by real-time PCR. Results were normalized to 18S rRNA and to Glut4 expression in the absence of E2. Data are represented as mean + sd. E, Relative expression of ERβ in βerko human (h) ERβ-cells. βerko MEFs were infected with lentivirus particle containing a hERβ construct and selected for 2 wk in blasticidin-containing medium. Then, RNA was extracted and analyzed using real-time PCR. Data are represented as mean + sd. F, Relative expression of Glut4 in βerko hERβ cells. βerko and βerko hERβ MEFs were differentiated, and Glut4 mRNA was analyzed using real-time PCR. Results were normalized to 18S rRNA and to Glut4 expression in the βerko cells. Data are represented as mean ± sd.
To investigate whether Glut4 is induced by liganded ER, MEFs-derived adipocytes were treated with E2 for 6 h, and Glut4 induction was assessed by real-time PCR (Fig. 1D). However, Glut4 expression was not significantly induced by E2, neither in the absence nor in the presence of ERβ. To further assess the role of ERβ on Glut4 expression, we decided to reintroduce ERβ into βerko MEFs. βerko MEFs were infected with lentivirus particles containing an ERβ expression vector. After selection, the stable cell line βerko human (h)ERβ was obtained, which expressed high amounts of ERβ as assessed by real-time PCR (Fig. 1E). After differentiation, Glut4 expression between βerko hERβ cells and mock-transfected cells was compared. However, reintroduction of ERβ per se did not increase Glut4 levels (Fig. 1F) despite considerably higher levels of ERβ expression. These findings suggest that in these MEFs, ERβ does not act as ligand-activated transcription factor to induce Glut4 expression transiently, but rather that its absence leads to a sustained decrease in Glut4 levels.
One CpG of the Glut4 promoter is hypermethylated in βerko MEFs
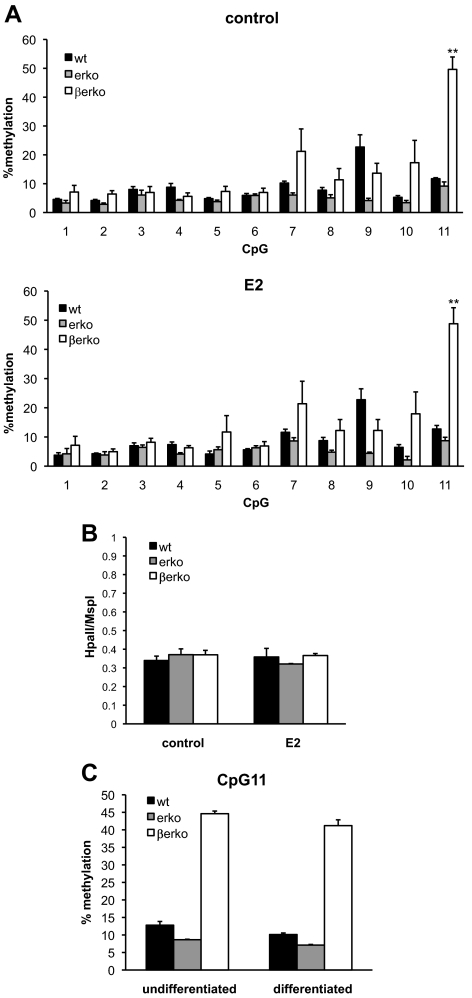
Gene expression is modulated by a number of different mechanisms, and recently, epigenetic regulation has emerged as a major regulatory event that controls transcriptional events. Generally, DNA methylation of promoter regions leads to gene silencing and plays an important role in cell differentiation (10). Interestingly, Glut4 expression has been suggested to be regulated by DNA methylation (4). We hypothesized that changes in DNA methylation of the Glut4 promoter could account for the differences in gene expression between ERβ-proficient and -deficient cells. Therefore, we assessed the methylation levels of the two CpG islands of Glut4 promoter by pyrosequencing of bisulfite-converted DNA from the MEFs. The cells were treated with E2 or vehicle control for 4 d. Subsequently, genomic DNA was isolated, bisulfite treated, and analyzed by pyrosequencing. The primer bias and pyrosequencing mismatch were negligible as assessed using different ratios of bisulfite-treated methylated and unmethylated DNA (Supplemental Fig. 1A published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). As shown in Fig. 2A, one specific CpG in island 1 (CpG11) was highly methylated in βerko compared with erko and wt MEFs (P < 0.01). In contrast, methylation status of the other CpG in island 1 (CpG 1–10 in Fig. 1A) and in island 2 (data not shown) was not significantly altered, although methylation content of the CpG adjacent to CpG11 was increased. Interestingly, E2 treatment had no visible effect on DNA methylation. Higher methylation at CpG11 in βerko MEFs was confirmed in two other clones of wt, erko, and βerko mice (Supplemental Fig.1B). Furthermore, the methylation pattern at CpG11 in wt, erko, and βerko MEFs was confirmed by methylation-sensitive restriction enzyme digest using FauI, the recognition site of which comprises CpG11 (Supplemental Fig. 1C). These observations suggest a role for ERβ in regulation of DNA methylation at a specific site in the Glut4 promoter.
Fig. 2.
DNA methylation analyses in wt, erko, and βerko MEFs. A, Methylation analysis of CpG island 1 of the Glut4 promoter in the absence (upper panel) and presence (lower panel) of E2. Genomic DNA was isolated from wt, erko, and βerko MEFs, treated with 10 nm E2 for 4 d. DNA was bisulfite treated and sequenced by pyrosequencing. Data are represented as mean ± sd of three independent experiments. **, P < 0.01 βerko compared with wt and erko. B, Analysis of global DNA methylation using LUMA. MEFs derived from wt, erko, and βerko mice were treated with 10 nm E2 for 4 d, and genomic DNA was analyzed by digest with the restriction enzymes HpaII and MspI followed by pyrosequencing of the resulting fragments. Data are represented as mean + sd of three independent experiments. C, Methylation analysis after differentiation. MEFs were differentiated for 8 d, and genomic DNA was analyzed by bisulfite treatment and pyrosequencing. Shown is percent methylation at CpG11. Data are represented as mean ± sd.
To investigate whether there are global DNA methylation differences in these MEFs, LUMA (luminometric methylation assay) (11) was carried out, which is based on digest of genomic DNA with the methylation-sensitive restriction enzyme HpaII and its methylation-insensitive isoschizomere MspI. Genomic DNA of wt, erko, and βerko MEFs, treated with E2 or vehicle for 4 d, was digested with either HpaII or MspI. Subsequently, the amount of HpaII-digested DNA was quantified by pyrosequencing and normalized to the MspI digest. As shown in Fig. 2B, global methylation was similar between wt, erko, and βerko MEFs, and E2 treatment for 4 d did not change the global methylation pattern. This analysis suggests that the effect of ERβ is targeted to specific CpGs that are hypermethylated in the absence of ERβ.
DNA methylation can be altered during cell differentiation to allow for specific factors to be expressed or repressed in different developmental stages. Therefore we analyzed whether methylation at CpG11 is altered during differentiation of wt and KO MEFs to adipocytes. Genomic DNA of the MEFs-derived adipocytes was analyzed by bisulfite treatment and pyrosequencing. This analysis showed a similar methylation pattern of CpG11 in the differentiated status as in the undifferentiated MEFs (Fig. 2C). Taken together, our experiments demonstrate that methylation state of CpG11 in the Glut4 promoter is altered in MEFs lacking ERβ compared with erko or wt MEFs. This sustained change is maintained during adipocyte differentiation and correlates with decreased Glut4 expression.
Decreased methylation levels and increased Glut4 expression after 5-AZA-dC treatment in βerko hERβ but not in βerko MEFs
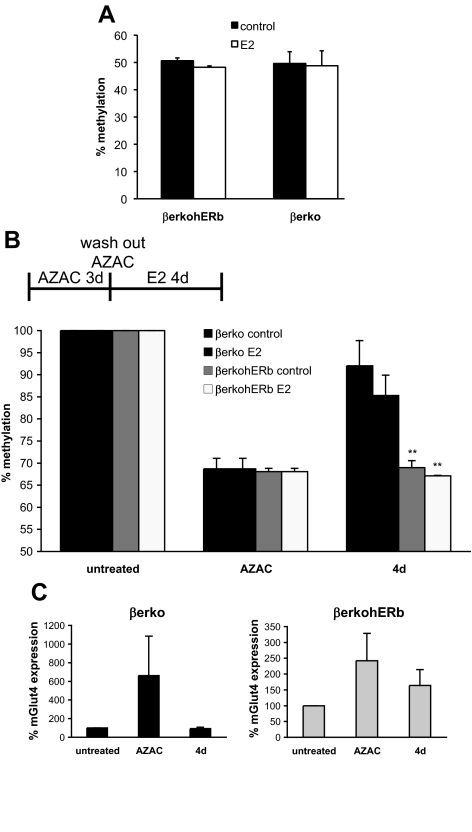
Our experiments suggest that ERβ regulates Glut4 expression by modulating DNA methylation of a specific CpG island in the Glut4 promoter. Next, we tested the effect of ERβ re-introduction into βerko MEFs on Glut4 methylation. For this purpose, Glut4 promoter methylation was analyzed and compared between βerko and βerko hERβ MEFs by bisulfite-pyrosequencing. There were no differences in methylation between βerko and βerko hERβ MEFs, neither before nor after a 4 d E2 treatment (Fig. 3A), which corresponds to the lack of Glut4 increase after ERβ reintroduction (Fig. 1E). This result likely reflects the fact that, once the DNA methylation pattern is established, ectopic expression of ERβ is not sufficient per se to alter this pattern. To simulate a situation where ERβ is already present while DNA methylation occurs, cells were treated with 5-aza-2-deoxycytidine (5-AZA-dC), a compound that inhibits DNA methyltransferase activity and thus promotes DNA demethylation (12). After 5-AZA-dC treatment, cells were left to grow for 4 d without 5-AZA-dC to reestablish the methylation pattern. As shown in Fig. 3B, 5-AZA-dC treatment induced a drop in methylation of the Glut4 promoter including CpG11. However, after 4 d of growth without 5-AZA-dC, DNA methyltransferase of CpG11 was almost as high as before the treatment in βerko MEFs. In contrast, in βerko hERβ cells, no restoration of the former DNA methyltransferase pattern could be detected. As observed previously, treatment with E2 had only a small effect. These findings suggest that ERβ can prevent DNA methyltransferase of CpG11 in the Glut 4 promoter.
Fig. 3.
DNA methylation analysis of βerko MEFs after reintroduction of ERβ. A, Methylation analysis of the CpG11 in Glut4 promoter in the absence and presence of E2. Genomic DNA was isolated from βerko and βerko hERβ MEFs that were treated with 10 nm E2 for 4 d. DNA was bisulfite treated and sequenced by pyrosequencing. Data are represented as mean ± sd. B, Methylation analysis of CpG11 after DNA methyltransferase inhibition by 5-AZA-dC treatment. βerko and βerko hERβ MEFs were treated with 5-AZA-dC for 3 d, 5-AZA-dC was washed out, and cells were allowed to grow for another 4 d in the absence or presence of E2. Genomic DNA was then isolated, bisulfited treated, and analyzed by pyrosequencing. Methylation before 5-AZA-dC treatment was set to 100%. Data are represented as mean ± sd. **, P < 0.01, βerko hERβ compared with βerko. C, Glut4 expression after DNA methyltransferase inhibition by 5-AZA-dC treatment. βerko and βerko hERβ MEFs were differentiated and subsequently treated with 5-AZA-dC for 3 d, 5-AZA-dC was washed out, and cells were allowed to grow for another 4 d. RNA was extracted and analyzed after differentiation (untreated), after 5-AZA-dC treatment (AZAC), and after 4-d wash out (4 d) using real-time PCR. Data are normalized to GAPDH expression and Glut4 expression after differentiation was set to 100%. Data are represented as mean ± sd.
To investigate whether these changes in DNA methyltransferase affect Glut4 expression, RNA was collected before and after the 5-AZA-dC treatment and after the 4 d washout period in the βerko and βerko hERβ MEFs. Glut4 expression was analyzed using real-time PCR. As shown in Fig. 3C, Glut4 levels increased after 5-AZA-dC treatment in both cell types. Glut4 expression was back to the initial low values in the βerko MEFs 4 d after the end of the 5-AZA-dC treatment. On the other hand, in the βerko hERβ MEFs, Glut4 expression was still elevated, although not as much as directly after the treatment (Fig. 3C). This demonstrates that the DNA methylation status of CpG11 during and after 5-AZA-dC treatment correlates with Glut4 expression, and that the presence of ERβ can prevent Glut4 silencing caused by DNA methylation.
ERβ, but not ERα, is recruited to the Glut4 promoter
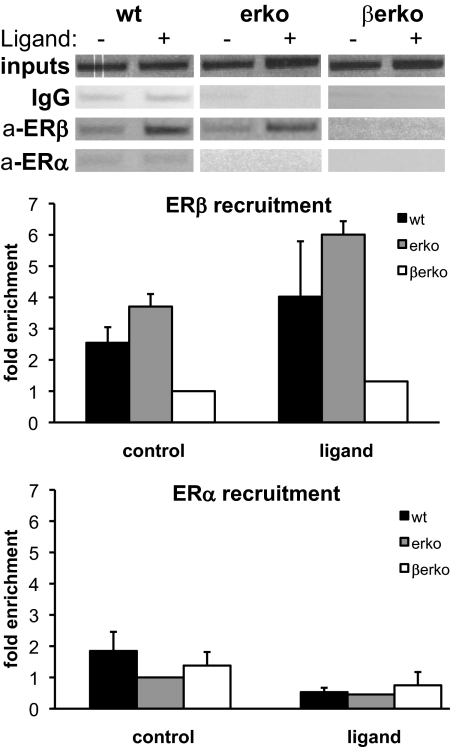
Because our results suggest that ERβ plays an active role in preventing CpG11 hypermethylation, we investigated whether ERβ is recruited to this region of the Glut4 promoter. As a control, ERα recruitment was assessed as well. We performed chromatin immunoprecipitation (ChIP) assays in wt and KO MEFs treated with ligand for 45 min. DNA-protein complexes were precipitated using antibodies against either ERβ or ERα, and a region around CpG11 was amplified by conventional or real-time PCR. As shown in Fig. 4, ERβ was efficiently recruited to CpG island 1 in the Glut4 promoter in both wt and erko cells, and its recruitment was somewhat increased upon ligand treatment. In contrast, ERα recruitment to this region could not be detected (Fig. 4), whereas in control experiments, ERα was recruited to the ER-regulated lactoferrin promoter (Supplemental Fig. 2). These ChIP results demonstrate that ERβ, but not ERα, is recruited to the region of Glut4 promoter containing CpG11 and support our observation that ERβ, but not ERα, is important for epigenetic regulation of the Glut4 promoter.
Fig. 4.
ER recruitment to CpG island 1 of Glut4 promoter in wt, erko, and βerko MEFs. ChIP assays for ERβ and ERα in the different MEFs. Cells were treated with 10 nm E2 (in wt and βerko) or DPN (in erko) for 45 min. ChIPs were analyzed by conventional PCR (upper panel) and real-time PCR (lower panel). Real-time PCR results were normalized to inputs and recruitment in βerko MEFs for ERβ and in erko MEFs for ERα. Real-time PCR data are represented as mean ± sd.
CpG11 is part of an Sp1-binding site, and its hypermethylation affects Sp1 binding
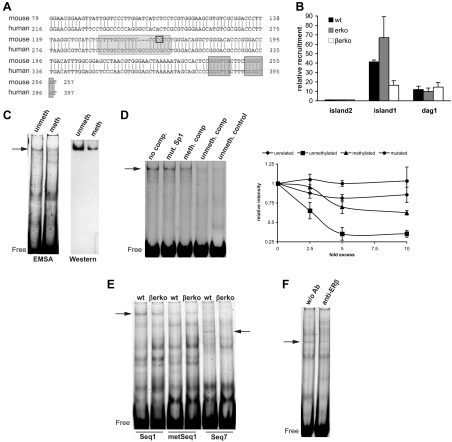
Next, we wanted to investigate how hypermethylation of a single CpG can affect Glut4 expression. In silico analysis of the Glut4 promoter region revealed that CpG11 is part of a conserved Sp1 site (Fig. 5A). Sp1 is known to regulate Glut4 expression (2); however, binding of Sp1 to this particular site has not been demonstrated. To investigate whether Sp1 binds to CpG island 1 of the Glut4 promoter, we performed ChIP assays in the wt, erko, and βerko MEFs using Sp1 antibodies followed by real-time PCR quantification. As shown in Fig. 5B, Sp1 bound to CpG island 1 in wt and erko MEFs, whereas in βerko MEFs, Sp1 recruitment was substantially impaired. No recruitment to CpG island 2 was detected, whereas in control experiments Sp1 recruitment to Dag1, a Sp1 target in MEFs (13), was comparable in all three cell lines (Fig. 5B).
Fig. 5.
Sp1 binding to the Glut4 promoter. A, Alignment of human and mouse Glut4 promoter sequence using BLAST. CpG11 (marked by a black rectangle) lies in a conserved region in the Glut4 promoter and is part of an Sp1-binding site (light gray shading). Marked with dark gray shading is the LXR-binding site. B, ChIP assays for Sp1 in wt, erko, and βerko MEFs. ChIPs were analyzed by real-time PCR (right panel). Real-time PCR results were normalized to inputs and recruitment to CpG island 2. Real-time PCR data are represented as mean ± sd. C, Mobility shift assays (left panel) and Western blot of the gel shifts (right panel) using nuclear extracts of wt MEFs and labeled unmethylated or methylated Seq1 oligonucleotides. Unmeth, Unmethylated probe; meth, methylated probe. Shown are representative gels and blots of three independent assays. D, Competition assays on labeled unmethylated Seq1 with 5-fold excess of different unlabeled oligonucleotides. Left panel, shows representative gel. Lane 1, No competitor; lane 2, 5× Seq1 with mutated Sp1 site; lane 3, 5× methylated Seq1; lane 4, 5× unmethylated Seq1; lane 5, unmethylated Sp1 control. Right panel shows quantification of at least three independent competition assays with different concentrations of an unrelated oligonucleotide (rhombes), unmethylated Seq1 (squares), methylated Seq1 (triangles), and Seq1 with mutated Sp1 site (circles). Shown are means ± sd. E, Mobility shift assays using nuclear extracts of wt and βerko MEFs with Seq1 (lanes 1 + 2), methylated Seq1 (lanes 3 + 4), and Seq7 (lanes 5 + 6). F, Mobility shift assays using wt extracts and Seq7 in the absence (lane 1) and presence (lane 2) of 1 μg anti-ERβ antibody. Ab, Antibody.
The fact that Sp1 binding is reduced in βerko MEFs suggests that hypermethylation of CpG11 impairs Sp1 binding. To test directly whether Sp1 binds to the predicted binding site in the Glut4 promoter, and whether Sp1 binding is methylation dependent, mobility shift assays were carried out. We used a labeled oligonucleotide encompassing the predicted Sp1 site of Glut4 promoter (Seq1) with either unmethylated or methylated CpG11. These probes were incubated with nuclear extracts of wt MEFs. and the mixture was run on a native polyacrylamide gel. Subsequently, protein-DNA complexes could be detected as retarded bands (shifts) on the gel. As shown in Fig. 5C, a protein-DNA complex could be detected using labeled unmethylated Seq1 (Fig. 5C, left panel, lane 1). In contrast, methylation of CpG11 in labeled Seq1 resulted in substantial inhibition of protein binding (Fig. 5C, left panel, lane 2). To confirm the presence of Sp1 in the complex causing the shift, proteins were transferred from the gel to a membrane, and Western blotting using an anti-Sp1 antibody was carried out. Indeed, Western blotting confirmed that Sp1 protein was present in the nonmethylated DNA complex. However, we recovered substantially less protein from the methylated sequence (Fig. 5C, right panel), suggesting that Sp1 binding occurs preferentially to unmethylated Seq1.
To consolidate these findings, mobility shift competition assays were carried out. Protein-DNA complex formation was measured upon addition of 5-fold excess of unlabeled oligonucleotides encoding either Seq1, Seq1 with a mutation in the Sp1 binding site, methylated Seq1, or an Sp1 control sequence (14) (Fig. 5D). Unmethylated Seq1 as well as the Sp1 control sequence was able to compete with protein binding to labeled Seq1 (Fig. 5D, lanes 4 and 5). In contrast, addition of unlabeled Seq1 with a mutation in the Sp1-binding site had no effect on protein-DNA complex formation (Fig. 5D, lane 2), confirming that Sp1 binds to its predicted site. Further, unlabeled methylated Seq1 did not affect the ability of Sp1 to bind to labeled, unmethylated Seq1 (Fig. 5D, lane 3), clearly showing that Sp1 binds much more efficiently to its unmethylated than to its methylated recognition site in the Glut4 promoter.
ERβ binds to a sequence adjacent to the Sp1-binding site
To delineate the DNA sequence in the Glut4 promoter that ERβ binds to, we compared protein binding to Seq1 and sequences adjacent to the Sp1-binding site in mobility shift assays using nuclear extracts from wt and βerko MEFs. ERβ has been shown to form complexes with Sp1 and, in doing so, to bind tethered to Sp1-binding sites, enhancing Sp1 binding to DNA in vitro (15). If binding of ERβ to the Glut4 promoter is mediated by Sp1, a change of intensity and/or height of the shift corresponding to Sp1-DNA complexes would be expected. As shown in Fig. 5E, the band corresponding to Sp1-DNA complexes could be detected to the same extent in wt and βerko extracts in the presence of Seq1 (lanes 1+2). This indicates that Sp1 binding to the DNA is independent of ERβ and that ERβ does not bind to Seq1 tethered to Sp1. Again, the band intensity decreased if Seq1 was methylated (Fig. 5E, lanes 3+4). With an oligonucleotide termed Seq7, comprising a 28-bp long sequence 11 bp upstream of CpG11, one band could be detected in wt MEFs that was absent in βerko (Fig. 5E, lanes 5 vs. 6). Hence this shift could correspond to ERβ-DNA complexes. To confirm this notion, mobility shift assays in the absence or presence of an anti-ERβ antibody were performed. As shown in Fig. 5F, addition of the antibody decreased the intensity of the band substantially. Together, these findings indicate that ERβ binds to a sequence in the Glut4 promoter adjacent to the Sp1-binding site.
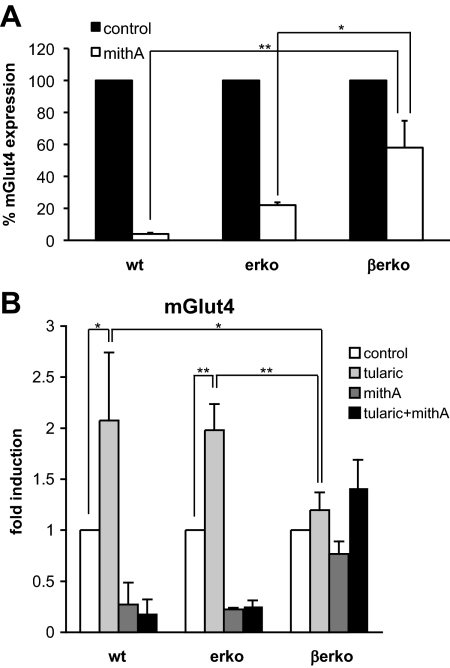
Lack of Sp1 recruitment in βerko MEFs is responsible for reduced basal transcription and decreased inducibility of Glut4 by LXR
Sp1 has been shown to regulate basal Glut4 transcription (2). Given our observation that Sp1 recruitment to the Glut4 promoter was reduced in βerko MEFs, we investigated whether decreased Glut4 expression in βerko MEFs is a result of impaired Sp1 binding to the Glut4 promoter. For this purpose, we treated the MEFs-derived adipocytes with mithramycin A (mithA), which prevents Sp1 binding to DNA (16), and subsequently measured Glut4 expression by real-time PCR (Fig. 6A). MithA treatment resulted in decreased Glut4 expression, reiterating the notion that Sp1 binding is important for basal expression of Glut4. Interestingly, however, this reduced expression of Glut4 was significantly more pronounced in wt (P < 0.01) and erko (P < 0.05) than in βerko adipocytes, suggesting that decreased Sp1 recruitment is indeed involved in the reduction of basal Glut4 expression in βerko MEFs.
Fig. 6.
Glut4 expression upon Sp1 inhibition and/or LXR induction. A, Effect of the Sp1 inhibitor mithA on Glut4 expression. MEFs were differentiated for 8 d and subsequently treated with mithA for 24 h. Glut4 expression was assessed by real-time PCR and normalized to 18S rRNA. Expression without mithA treatment was set to 100% for each cell type. Data are represented as mean ± sd. *, P < 0.05; **, P < 0.01. B, Glut4 induction by tularic in MEFs-derived adipocytes in the absence and presence of mithA. MEFs were differentiated for 8 d, subsequently treated with mithA or vehicle for 24 h, and then stimulated with 1 μm tularic or dimethylsulfoxide as control for 6 h. Glut4 induction was analyzed by real-time PCR. The results were normalized to 18S rRNA and to expression after control treatment. Data are represented as mean ± sd. *, P < 0.05; **, P < 0.01.
Sp1 not only regulates basal transcription but can also interact with transcription factors the binding sites of which are close to Sp1 sites. This has been shown to be the case for LXR on the ABCA1 promoter (17). Intriguingly, LXR has been reported to regulate Glut4 transcription, and its binding site is located 75 bp downstream of CpG11 (Fig. 4A). We speculated that Glut4 inducibility by LXR in the presence of LXR ligands, such as tularic, might depend on the interaction between Sp1 and LXR. To test this hypothesis, we compared Glut4 inducibility by tularic in the MEFs-derived adipocytes using real-time PCR. As shown in Fig. 6B, tularic treatment for 6 h induced Glut4 expression in adipocytes derived from wt and erko MEFs about 2-fold, whereas it had little effect in βerko MEFs-derived adipocytes. Both LXRα and LXRβ were expressed in the MEFs in comparable levels (Fig. 2A and data not shown), suggesting that differences in LXR levels are not the reason for the lack of Glut4 inducibility.
We then analyzed the involvement of Sp1 in the abolishment of tularic response in βerko MEFs. Cells were treated with mithA before tularic treatment, which abolished Glut4 induction by tularic (Fig. 6B, black bars) in wt and erko MEFs. These experiments suggest that Sp1 is involved in LXR-dependent Glut4 induction. Thus, hypermethylation of CpG11 does not only affect basal Glut4 transcription but also its inducibility by LXR via diminished Sp1 recruitment to the promoter.
Discussion
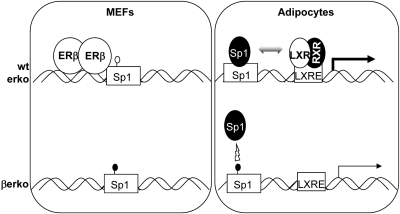
Disturbance of the estrogen system by exposure to endocrine disruptive chemicals such as bisphenol A or diethylstilbestrol during sensitive developmental stages is known to prime the exposed organism to different disease conditions such as cancer, obesity, and diabetes type 2 (18). The ER are known to regulate genes that are important for fat and glucose metabolism, which might explain the association between exposure to endocrine disruptive chemicals and metabolic dysfunctions. Glut4 is an important player in glucose uptake, and its expression can be regulated by the ER. However, the regulatory mechanisms are unclear and there are conflicting results about the effects of the two ER isoforms on Glut4 activity. In this study, we addressed the role of the different ER isoform on Glut4 regulation. Our results show that ERβ modulates Glut4 expression at the epigenetic level by a novel pathway (Fig. 7). ERβ binds to Glut4 promoter and maintains one CpG, CpG11, in an unmethylated state. CpG11 is part of a functional Sp1-binding site, and its hypermethylation diminishes Sp1 recruitment to the promoter, which in turn leads to decreased basal Glut4 transcription and abolished inducibility by LXR in the differentiated cells. In undifferentiated wt and erko MEFs, Sp1 is recruited to the Glut4 promoter; however, gene expression is extremely low, indicating that other factors specifically expressed or recruited upon differentiation contribute to Sp1-induced Glut4 transcription.
Fig. 7.
Model for ERβ action on the Glut4 promoter. In wt and erko cells, ERβ prevents methylation of CpG11 that is part of an Sp1-binding site. In adipocytes derived from these cells, Sp1 can bind to the Glut4 promoter and activate basal transcription and inducibility by LXR. In βerko cells, on the other hand, CpG11 is methylated, which prevents Sp1 from binding to its recognition site and thus both basal and inducible Glut4 transcription are reduced. RXR, Retinoid X receptor, the heterodimerization partner of LXR; LXRE, LXR response element..
It is possible that hypermethylation of CpG11 leads to changes in histone modifications, which in turn alter chromatin structure and accessibility of the Sp1-binding site. Thus in the βerko cells, DNA hypermethylation might not be the sole reason for inhibited Sp1 recruitment to the Glut4 promoter. Our EMSA experiments suggest, however, that changes in DNA methylation affects Sp1 binding by itself. These findings are in contrast to previous publications suggesting that Sp1 binding to DNA is methylation independent (14). One of the published artificial Sp1-binding sites was used as control oligonucleotide in this study. We could confirm that on the published sequence, methylation status did not affected Sp1-binding activity (data not shown). This observation suggests that methylation-dependent binding of Sp1 is sequence specific. In addition, recent publications also report that Sp1 binding is impaired by methylation of its recognition site [e.g. on the α1d-adrenergic receptor and the protein kinase C-ϵ gene ((19, 20)], supporting our results on the Glut4 promoter.
The ERs have been shown to activate genes via Sp1-binding sites by binding to Sp1 in a ligand-dependent manner (e.g Refs. 15, 21 and 22). Such an interaction possibly happens on the Glut4 promoter as well. However, our EMSA experiments show that Sp1 binding to its recognition site is ERβ independent because it occurs to the same extent with βerko as with wt extracts. Furthermore, ERβ seems to bind to a region adjacent to the Sp1 site. It is possible that there still is physical interaction between Sp1 and ERβ on the Glut4 promoter. Nevertheless, our results suggest that such an interaction is not important for direct transcriptional regulation of Glut4 because ER ligand and reintroduction of ERβ into βerko MEFs did not affect Glut4 expression per se. On the other hand, disruption of Sp1 binding to the DNA by mithA decreased Glut4 transcription drastically, demonstrating a clear role of Sp1 by itself.
It is possible that Sp1 is also involved in the regulation of methylation at CpG11. Indeed, Sp1 has been suggested to be responsible to prevent DNA methylation of a CpG island situated in the aprt gene by acting as insulator on the periphery of CpG islands (23, 24). However, reduced binding of Sp1 in the βerko MEFs does not result in complete methylation of the CpG island (although higher methylation of the neighboring CpG could be measured). Furthermore, such a role for Sp1 has been questioned, first by the fact that Sp1 −/− mice show no increase in DNA methylation of the aprt CpG islands (25), and second, that vascular endothelial zinc finger 1 (VEZF1) seems to be sufficient for protection of DNA from being methylated (26). Thus, we suggest that. at least in the case for Glut4, the role for Sp1 is merely to regulate gene transcription.
There are basically two possibilities for how ERβ stabilizes CpG11 in its unmethylated form: either it recruits factors that are involved in active demethylation or it prevents binding of DNA methyltransferases and thus maintenance of DNA methylation throughout replication. ERα has been reported to interact both with factors possibly involved in active DNA demethylation, e.g. thymine DNA glycosylase (27, 28), as well as with the DNA methyltransferases (28). However, currently it is not known whether ERβ can interact with either DNA methyltransferases or with proteins such as DNA glycosylases. Future studies are required to characterize the precise mechanism by which ERβ protects Glut4 promoter from DNA methylation.
Introduction of ERβ into βerko MEFs was not sufficient per se to promote demethylation of CpG11. We conclude from these results that ERβ is not able to induce DNA demethylation. It rather seems that, once the methylation pattern at CpG11 has been established (probably early in development when major de- and remethylation processes occur), this pattern is rather stable. Because reintroduction of ERβ into βerko MEFs showed an effect after demethylation of the DNA, we suggest that ERβ protects CpG11 from becoming methylated. Interestingly, ERβ is highly expressed after egg fertilization (where the major de- and remethylation processes occur) but disappears later on in development (29–31). Importantly, ERα is not present at that stage (29), which suggests the ERα is not involved in establishing the methylation pattern of genes such as Glut4. On the other hand, ERα-induced gene transcription, e.g. on the pS2 promoter, has been shown to induce rapid cycles of DNA de- and remethylation without any long-term changes in DNA methylation (28, 32). Thus, ERα might be involved in promoting short-term, dynamic DNA methylation changes, whereas ERβ might contribute to the establishment of stable methylation patterns.
In the adult organism, the ERs are involved in the regulation of Glut4 protein expression by mechanisms yet to be determined: ERα enhances Glut4 protein levels in white adipose tissue whereas ERβ reduces its levels in skeletal muscle (8). Additionally, ERα, but not βerko mice, show higher fat mass, insulin resistance, and impaired glucose tolerance (33). On high-fat diet, on the other hand, βerko mice show increased body weight gain, but improved insulin sensitivity compared with wt mice (34). The latter is caused by an inhibitory action of ERβ on the peroxisome proliferator-activated receptor γ (PPARγ), a ligand-induced transcription factor that enhances adipogenesis (34) and inhibits Glut4 gene expression (2). Indeed, we have observed an inhibitory effect of ERβ on PPARγ in the course of our differentiation experiments: when omitting the PPARγ ligand troglitazone from the differentiation medium, βerko MEFs differentiated substantially faster to adipocytes than wt and erko MEFs (our unpublished observations), suggesting an increased ligand-independent action of PPARγ in the absence of ERβ. The inhibitory effect of ERβ on PPARγ could thus explain increased Glut4 expression in skeletal muscle in βerko mice. Taken together, regulation of Glut4 by ER and other factors appears complex and specific to tissue type and developmental stage. Thus the physiological implications of the change in Glut4 promoter methylation and of its consequences described here must be carefully evaluated for in vivo situations.
In conclusion, the data presented here suggest a novel epigenetic mechanism of ERβ to regulate Glut4 expression. The involvement of ERβ could provide a link between changes in glucose tolerance and epigenetic alterations observed after exposure to endocrine disruptive chemicals in early development.
Materials and Methods
Reagents, plasmids, and antibodies
17β-estradiol, 5-Aza-2′-deoxycytidine (5-AZA-dC), 3-isobutyl-1-methylxanthine, troglitazone, insulin, and dexamethasone were purchased from Sigma (St. Louis, MO), and diarylpropionitrile (DPN) was obtained from Tocris Bioscience (Bristol, UK). Reagents and buffers used for pyrosequencing were purchased from QIAGEN (Chatsworth, CA). pLenti6hERβ was a kind gift from Dr. A. Ström. Antibodies polyclonal anti-ERα H-184, monoclonal anti-Hsp90 F-8, and actin antibody sc-8432, were all purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); polyclonal anti-Sp1 was obtained from Millipore Corp. (Bedford, MA); polyclonal anti-ERβ was a kind gift from Dr. M. Warner.
Cell culture and treatments
MEFs were isolated at embryonic d 13.5 from ERα−/− (Taconic Europe, Ejby, Denmark), ERβ −/− mice (35), and wt littermates. They were kept in high-glucose DMEM without phenol red, supplemented with 10% fetal calf serum, l-glutamine, sodium pyruvate, and nonessential amino acids. For stimulation with ER agonists, cells were put for at least 2 d into DMEM with 5% dextran-coated charcoal-treated serum and treated with 10 nm E2 or DPN. For 5-AZA-dC treatment, cells were incubated with 50 μm 5-AZA-dC during 3 d, during which fresh 5- AZA-dC was added every day. For adipose differentiation, cells were treated with medium containing 500 μm 3-isobutyl-1-methylxanthine, 1 μm dexamethasone, 5 μm troglitazone, and 10 μg/ml insulin for 8 d, during which fresh medium was added every other day. For lentivirus infection, βerko MEFs were seeded 30,000 cells/well into six-well plates the day before infection. The next day, 400 μl lentivirus supernatant containing pLenti6hERβ at a concentration of 106 IU/ml together with 6 μg/ml polybrene was added to the cells and left over night. Selection with 5 μg/ml blasticidine was started 4 d later.
LUMA
LUMA was carried out as described previously (36). Briefly, cells were treated for 4 d with E2, and genomic DNA was extracted using the Invisorb Spin Cell Mini kit (Invitek Inc., Hayward, CA). DNA (500 ng) was cleaved with either HpaII+EcoRI or MspI+EcoRI for 4 h. Resulting overhangs were analyzed using a Pyrosequencer PSQ96 MA system (QIAGEN). The HpaII/EcoRI and MspI/EcoRI ratios were calculated as (dGTP + dCTP)/dATP for the respective reactions. The HpaII/ MspI ratio was defined as (HpaII/EcoRI)/(MspI/EcoRI).
Bisulfite treatment and pyrosequencing of individual promoters
Genomic DNA (200–500 ng) was bisulfite treated and purified using the EZ DNA Methylation kit (Zymo Research Corp., Orange, CA). Converted DNA (1 μl) was used for nested PCR amplification (for primer sequence see Supplemental Table 1), and the PCR product was sequenced by pyrosequencing in a Pyromark Q24 (QIAGEN). Bias of the PCR primers and pyrosequencing mismatches were tested using 5-AZA-dC-treated genomic DNA from MEFs and Universal Methylated Mouse DNA (Zymo Research) in different ratios (Supplemental Fig. 1A).
Methylation analyses using FauI
Genomic DNA (1 μg) from wt, erko, and βerko MEFs was subjected to FauI (New England Biolabs, Inc., Beverly, MA) digest for 1 h at 55 C. Subsequently, 1/20 of this reaction was used in a real-time PCR amplifying Glut4 promoter. DNA fragments of either SHIP promoter or 18S rRNA (neither exhibit a FauI site) were used for normalization. Percentage methylation was calculated by generating a standard curve using FauI-digested 5-AZA-dC-treated genomic DNA from MEFs and Universal Methylated Mouse DNA (Zymo Research) in different ratios.
RNA isolation and real-time PCR
RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. Total RNA (1 μg) was treated with deoxyribonuclease I and reverse transcribed using random hexamer primers (Invitrogen). Of the resulting cDNA, 1 μl was then used for real-time PCR using a 7500 Real time PCR system and SYBR Green-based detection (Applied Biosystems, Foster City, CA) (37, 38). Gene transcripts were normalized to the 18s rRNA content (primers listed in Supplemental Table 1). All results are based on the ΔΔCT method and represent the mean of at least three independent experiments.
ChIP
After treatment of cells with 10 nm E2 or DPN for 45 min, ChIP assays were performed as described elsewhere (39). DNA fragments were analyzed by conventional and/or by real-time PCR (primers listed in Supplemental Table 1).
Mobility shift assays
Nuclear extracts of MEFs were prepared as described previously (40). The mobility shift reaction contained 2 μg nuclear extracts, 2 μl 5× EMSA buffer (20% glycerol; 5 mm MgCl2; 2.5 mm EDTA; 2.5 mm dithiothreitol; 250 mm NaCl; 50 mm Tris-HCl, pH 7.5), 0.5 μg polydeoxyinosinic deoxycytidylic acid·polydeoxyinosinic deoxycytidylic acid, 1 pmol fluorescein-labeled oligonucleotide (sequences listed in Supplemental Table 1), in a total volume of 10 μl. The reaction was incubated for 30 min at 25 C, and DNA was separated on a 6% polyacrylamide gel in 0.5× Tris-buffered EDTA. For competition assays, 5–10 pmol unlabeled competitor oligonucleotides (sequences listed in Supplemental Table 1) were added after 30 min of incubation for an additional 10 min. Anti-ERβ antibody was preincubated with nuclear extracts for 10 min at 25 C before addition of the probe. Fluorescent signals were detected using a Typhoon 9400 variable mode imager (Amersham Biosciences, Piscataway, NJ), and signals were quantified using ImageQuant TL (GE Healthcare, Piscataway, NJ).
Statistical analyses
Statistical significance was assessed using the unpaired, two-tailed t test. The level of significance was selected as P < 0.05.
Bioinformatical analyses
For computer-based analysis of transcription factor-binding sites, MatInspector, a program of the Genomatix software suite, was used.
Supplementary Material
Acknowledgments
We thank Dr. Sebastian Lewandowski (Karolinska Institutet, Stockholm, Sweden) for the wt, erko, and βerko MEFs. We also thank Dr. Anders Ström (University of Houston, Houston, TX) for the pLentihERβ construct and valuable help with the lentivirus infections. Thanks also to Dr. Ivan Nalvarte (Friedrich Miescher Institute, Basel Switzerland) for critically reading and correcting the manuscript.
This work was supported by the Swiss National Science Foundation, and the European Union-funded Chemicals as contaminants in the food chain (CASCADE), Consortium for Research into Nuclear Receptors in Development and Aging (CRESCENDO), and Marie Curie SME-academia nuclear receptor knowledge transfer (SME-RECEPTOR) projects.
Current address for J.P.: Department of Biomedicine, Institute of Biochemistry and Genetics, University of Basel, Basel, 4058 Switzerland.
Current address for N.B.K.: School of Biological Sciences, University of Auckland, Auckland, 1142 New Zealand.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 5-AZA-dC
- 5-Aza-2-deoxycytidine
- ChIP
- chromatin immunoprecipitation
- DPN
- diarylpropionitrile
- E2
- 17β-estradiol
- ER
- estrogen receptor
- erko
- ERα-knockout
- βerko
- ERβ-knockout
- Glut4
- glucose transporter 4
- hERβ
- human ERβ
- KO
- knockout
- LUMA
- luminometric methylation assay
- LXR
- liver X receptor
- MEFs
- mouse embryonic fibroblasts
- mithA
- mithramycin A
- PPAR
- peroxisome proliferator-activated receptor
- wt
- wild-type.
References
- 1. Kahn BB. 1996. Lilly lecture 1995. Glucose transport: pivotal step in insulin action. Diabetes 45:1644–1654 [DOI] [PubMed] [Google Scholar]
- 2. Im SS, Kwon SK, Kim TH, Kim HI, Ahn YH. 2007. Regulation of glucose transporter type 4 isoform gene expression in muscle and adipocytes. IUBMB Life 59:134–145 [DOI] [PubMed] [Google Scholar]
- 3. Graham TE, Kahn BB. 2007. Tissue-specific alterations of glucose transport and molecular mechanisms of intertissue communication in obesity and type 2 diabetes. Horm Metab Res 39:717–721 [DOI] [PubMed] [Google Scholar]
- 4. Yokomori N, Tawata M, Onaya T. 1999. DNA demethylation during the differentiation of 3T3–L1 cells affects the expression of the mouse GLUT4 gene. Diabetes 48:685–690 [DOI] [PubMed] [Google Scholar]
- 5. Nilsson S, Gustafsson JA. 2002. Biological role of estrogen and estrogen receptors. Crit Rev Biochem Mol Biol 37:1–28 [DOI] [PubMed] [Google Scholar]
- 6. Brown LM, Clegg DJ. 2010. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol 122:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pettersson K, Gustafsson JA. 2001. Role of estrogen receptor β in estrogen action. Annu Rev Physiol 63:165–192 [DOI] [PubMed] [Google Scholar]
- 8. Barros RP, Gabbi C, Morani A, Warner M, Gustafsson JA. 2009. Participation of ERα and ERβ in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab 297:E124–E133 [DOI] [PubMed] [Google Scholar]
- 9. Lin Z, Shen H, Huang J, Chen S, Chen L, Chen J, Liu G, Jiang H, Shen X. 2008. Butyl 4-(butyryloxy)benzoate functions as a new selective estrogen receptor β agonist and induces GLUT4 expression in CHO-K1 cells. J Steroid Biochem Mol Biol 110:150–156 [DOI] [PubMed] [Google Scholar]
- 10. Huang K, Fan G. 2010. DNA methylation in cell differentiation and reprogramming: an emerging systematic view. Regen Med 5:531–544 [DOI] [PubMed] [Google Scholar]
- 11. Karimi M, Johansson S, Stach D, Corcoran M, Grandér D, Schalling M, Bakalkin G, Lyko F, Larsson C, Ekström TJ. 2006. LUMA (LUminometric Methylation Assay)–a high throughput method to the analysis of genomic DNA methylation. Exp Cell Res 312:1989–1995 [DOI] [PubMed] [Google Scholar]
- 12. Creusot F, Acs G, Christman JK. 1982. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2′-deoxycytidine. J Biol Chem 257:2041–2048 [PubMed] [Google Scholar]
- 13. Rettino A, Rafanelli F, Genovese G, Goracci M, Cifarelli RA, Cittadini A, Sgambato A. 2009. Identification of Sp1 and GC-boxes as transcriptional regulators of mouse Dag1 gene promoter. Am J Physiol Cell Physiol 297:C1113–C1123 [DOI] [PubMed] [Google Scholar]
- 14. Harrington MA, Jones PA, Imagawa M, Karin M. 1988. Cytosine methylation does not affect binding of transcription factor Sp1. Proc Natl Acad Sci USA 85:2066–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G, Gustafsson JA, Safe S. 2000. Ligand-, cell-, and estrogen receptor subtype (α/β)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem 275:5379–5387 [DOI] [PubMed] [Google Scholar]
- 16. Ray R, Snyder RC, Thomas S, Koller CA, Miller DM. 1989. Mithramycin blocks protein binding and function of the SV40 early promoter. J Clin Invest 83:2003–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thymiakou E, Zannis VI, Kardassis D. 2007. Physical and functional interactions between liver X receptor/retinoid X receptor and Sp1 modulate the transcriptional induction of the human ATP binding cassette transporter A1 gene by oxysterols and retinoids. Biochemistry 46:11473–11483 [DOI] [PubMed] [Google Scholar]
- 18. Swedenborg E, Rüegg J, Mäkelä S, Pongratz I. 2009. Endocrine disruptive chemicals: mechanisms of action and involvement in metabolic disorders. J Mol Endocrinol 43:1–10 [DOI] [PubMed] [Google Scholar]
- 19. Michelotti GA, Brinkley DM, Morris DP, Smith MP, Louie RJ, Schwinn DA. 2007. Epigenetic regulation of human α1d-adrenergic receptor gene expression: a role for DNA methylation in Sp1-dependent regulation. FASEB J 21:1979–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meyer K, Zhang H, Zhang L. 2009. Direct effect of cocaine on epigenetic regulation of PKCϵ gene repression in the fetal rat heart. J Mol Cell Cardiol 47:504–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Batistuzzo de Medeiros SR, Krey G, Hihi AK, Wahli W. 1997. Functional interactions between the estrogen receptor and the transcription activator Sp1 regulate the estrogen-dependent transcriptional activity of the vitellogenin A1 io promoter. J Biol Chem 272:18250–18260 [DOI] [PubMed] [Google Scholar]
- 22. Porter W, Saville B, Hoivik D, Safe S. 1997. Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol Endocrinol 11:1569–1580 [DOI] [PubMed] [Google Scholar]
- 23. Macleod D, Charlton J, Mullins J, Bird AP. 1994. Sp1 sites in the mouse aprt gene promoter are required to prevent methylation of the CpG island. Genes Dev 8:2282–2292 [DOI] [PubMed] [Google Scholar]
- 24. Brandeis M, Frank D, Keshet I, Siegfried Z, Mendelsohn M, Nemes A, Temper V, Razin A, Cedar H. 1994. Sp1 elements protect a CpG island from de novo methylation. Nature 371:435–438 [DOI] [PubMed] [Google Scholar]
- 25. Marin M, Karis A, Visser P, Grosveld F, Philipsen S. 1997. Transcription factor Sp1 is essential for early embryonic development but dispensable for cell growth and differentiation. Cell 89:619–628 [DOI] [PubMed] [Google Scholar]
- 26. Dickson J, Gowher H, Strogantsev R, Gaszner M, Hair A, Felsenfeld G, West AG. 2010. VEZF1 elements mediate protection from DNA methylation. PLoS Genet 6:e1000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen D, Lucey MJ, Phoenix F, Lopez-Garcia J, Hart SM, Losson R, Buluwela L, Coombes RC, Chambon P, Schar P, Ali S. 2003. T:G mismatch-specific thymine-DNA glycosylase potentiates transcription of estrogen-regulated genes through direct interaction with estrogen receptor α. J Biol Chem 278:38586–38592 [DOI] [PubMed] [Google Scholar]
- 28. Metivier R, Gallais R, Tiffoche C, Le Peron C, Jurkowska RZ, Carmouche RP, Ibberson D, Barath P, Demay F, Reid G, Benes V, Jeltsch A, Gannon F, Salbert G. 2008. Cyclical DNA methylation of a transcriptionally active promoter. Nature [Erratum (2010) 463:384] 452:45–50 [DOI] [PubMed] [Google Scholar]
- 29. Su AI, Cook MP, Ching KA, Haka Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB. 2002. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci USA 99: 4465–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pikulkaew S, De Nadai A, Belvedere P, Colombo L, Dalla Valle L. 2010. Expression analysis of steroid hormone receptor mRNAs during zebrafish embryogenesis. Gen Comp Endocrinol 165:215–220 [DOI] [PubMed] [Google Scholar]
- 31. Lassiter CS, Kelley B, Linney E. 2002. Genomic structure and embryonic expression of estrogen receptor βa (ERβa) in zebrafish (Danio rerio). Gene 299:141–151 [DOI] [PubMed] [Google Scholar]
- 32. Kangaspeska S, Stride B, Métivier R, Polycarpou-Schwarz M, Ibberson D, Carmouche RP, Benes V, Gannon F, Reid G. 2008. Transient cyclical methylation of promoter DNA. Nature 452:112–115 [DOI] [PubMed] [Google Scholar]
- 33. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. 2000. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA 97:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, Gustafsson JA, Unger T, Kintscher U. 2008. Metabolic actions of estrogen receptor β (ERβ) are mediated by a negative cross-talk with PPARγ. PLoS Genet 4:e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. 1998. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karimi M, Johansson S, Ekström TJ. 2006. Using LUMA: a Luminometric-based assay for global DNA-methylation. Epigenetics 1:45–48 [DOI] [PubMed] [Google Scholar]
- 37. Swedenborg E, Rüegg J, Hillenweck A, Rehnmark S, Faulds MH, Zalko D, Pongratz I, Pettersson K. 2008. 3-Methylcholanthrene displays dual effects on estrogen receptor (ER) α and ER β signaling in a cell-type specific fashion. Mol Pharmacol 73:575–586 [DOI] [PubMed] [Google Scholar]
- 38. Cai W, Rambaud J, Teboul M, Masse I, Benoit G, Gustafsson JA, Delaunay F, Laudet V, Pongratz I. 2008. Expression levels of estrogen receptor β are modulated by components of the molecular clock. Mol Cell Biol 28:784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rüegg J, Swedenborg E, Wahlström D, Escande A, Balaguer P, Pettersson K, Pongratz I. 2008. The transcription factor aryl hydrocarbon receptor nuclear translocator functions as an estrogen receptor β-selective coactivator, and its recruitment to alternative pathways mediates antiestrogenic effects of dioxin. Mol Endocrinol 22:304–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hardeland U, Kunz C, Focke F, Szadkowski M, Schär P. 2007. Cell cycle regulation as a mechanism for functional separation of the apparently redundant uracil DNA glycosylases TDG and UNG2. Nucleic Acids Res 35:3859–3867 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.