Abstract
We recently identified in prostate tumors (PCa) a transcriptional prognostic signature comprising a significant number of genes differentially regulated in patients with worse clinical outcome. Induction of up-regulated genes was due to chromatin remodeling by a combinatorial complex between estrogen receptor (ER)-β and endothelial nitric oxide synthase (eNOS). Here we show that this complex can also repress transcription of prognostic genes that are down-regulated in PCa, such as the glutathione transferase gene GSTP1. Silencing of GSTP1 is a common early event in prostate carcinogenesis, frequently caused by promoter hypermethylation. We validated loss of glutathione transferase (GST) P1-1 expression in vivo, in tissue microarrays from a retrospective cohort of patients, and correlated it with decreased disease-specific survival. Furthermore, we show that in PCa cultured cells ERβ/eNOS causes GSTP1 repression by being recruited at estrogen responsive elements in the gene promoter with consequential remodeling of local chromatin. Treatment with ERβ antagonist or its natural ligand 5α-androstane-3β,17β-diol, eNOS inhibitors or ERβ small interference RNA abrogated the binding and reversed GSTP1 silencing, demonstrating the direct involvement of the complex. In vitro, GSTP1 silencing by ERβ/eNOS was specific for cells from patients with worse clinical outcome where it appeared the sole mechanism regulating GSTP1 expression because no promoter hypermethylation was present. However, in vivo chromatin immunoprecipitation assays on fresh PCa tissues demonstrated that silencing by ERβ/eNOS can coexist with promoter hypermethylation. Our findings reveal that the ERβ/eNOS complex can exert transcriptional repression and suggest that this may represent an epigenetic event favoring inactivation of the GSTP1 locus by methylation. Moreover, abrogation of ERβ/eNOS function by 3β-adiol emphasizes the significance of circulating or locally produced sex steroid hormones or their metabolites in PCa biology with relevant clinical/therapeutic implications.
Prostate cancer (PCa) is the most commonly diagnosed cancer in men in industrialized countries, with the highest incidence in North America (1). PCa, an androgen-dependent tumor, is highly sensitive to perturbation of intratumoral steroid biosynthesis and metabolism of exogenous ligands: androgens but also estrogens and their metabolites. It is now recognized that the combined action, and specifically an imbalance in androgens and estrogens ratio, is critical to PCa development, maintenance, and progression (2, 3). Indeed, a finely tuned balance between estrogens and androgens and the relative expression of the estrogen receptor (ER) subtypes, ERα in the stroma and ERβ in the epithelial compartments of the human prostate (4–8), have been invoked as causative in the etiology of prostate disease (3, 9).
The complexity of PCa pathophysiology is enhanced by other signaling molecules such as nitric oxide (NO) and oxygen. We have recently revealed a novel and pivotal function of ERβ and endothelial NO synthase (eNOS) in the acquisition of an aggressive PCa phenotype (10). Specifically we demonstrated that activation of the ERβ/eNOS pathway is crucial for tumor progression within the prostate microenvironment, highly sensitive to local changes in hormonal levels and oxygen tension.
Estrogens are key signaling molecules regulating various physiological processes, e.g. cell growth, development, and differentiation, and also playing a role in many pathological processes in hormone-dependent diseases. Binding of estrogens to ERs, particularly ERβ in the human prostate epithelium, produces genomic effects (11, 12) that regulate gene transcription. The estrogen-ER complex, once bound to its regulatory site, the estrogen-responsive element (ERE), can interact with adjacent transcription factors and recruit a variety of cofactors, thus inducing modifications of the chromatin resulting in activation or repression of target genes (13–16).
A second key molecule, NO, the product of eNOS, is a free radical involved in many biological processes, among which is angiogenesis. Recently it has been shown that activated eNOS can translocate into the nucleus (17–20) where it binds ERβ (10). Formation of an eNOS/ERβ combinatorial complex determines localized remodeling of chromatin, leading to transcriptional activation of previously identified prognostic genes (e.g.hTERT, MSH2, Cyclin B1, and pS2), all extremely sensitive to estrogen stimulation and/or variations in the intracellular levels of oxygen and NO (10). Of interest, in prostate epithelial cells, other prognostic genes, among which the Pi class of glutathione transferases, are down-regulated.
Glutathione transferase P1 (GSTP1) belongs to a family of isoenzymes that protect cells from cytotoxic and carcinogenic agents. It is highly expressed in embryogenesis and has been associated with preneoplastic and neoplastic changes (21, 22). In PCa, expression of the protein is frequently lost because of promoter hypermethylation, which has been detected in almost 90% of tumors and in approximately 70% of prostatic intraepithelial neoplasias, making it a common and early event in prostate carcinogenesis (23). Regulation of gene expression by DNA methylation results from a series of events that include chromatin modifications and increased density of repressive histone methylations. The regulatory signals triggering these events are virtually unknown and largely depend on the tissue microenvironment.
Although the functional role of the nuclear ER/eNOS complex is not fully characterized, our previous observations indicate that activation of the ERβ/eNOS pathways may represent an early event in the transcriptional program leading to an aggressive phenotype in PCa cells and that nuclear translocation of eNOS affects chromatin remodeling of a subset of prognostic genes and activates their transcription. These findings prompted us to investigate whether and how the same complex may also exert a negative regulatory effect on gene transcription in response to precise microenvironmental conditions.
Results
Expression of GST P1-1 in PCa tissue microarrays (TMA)
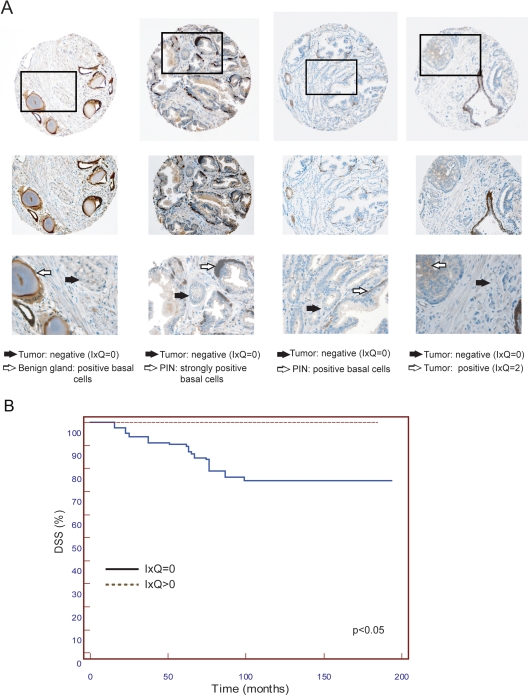
To determine whether the ERβ/eNOS complex, in addition to transcriptional activation (10), is involved in transcriptional silencing, we chose to study its effects on the expression of GSTP1, a gene comprised in the prognostic transcriptional signature we identified in PCa and down-regulated in more aggressive tumors. We first analyzed in vivo expression of the GST P1-1 protein by TMA in a retrospective cohort of PCa patients characterized by very long follow-up (10). We confirmed the loss of GST P1-1, consistent with data in the literature (24, 25), and correlated it with decreased disease-specific survival (DSS; Fig. 1B). Of interest, a limited number of PCa samples (14 of 126) retained GST P1-1 expression (Fig. 1A, rightmost panel) compared with the more abundant and completely negative group (Fig. 1A, left and middle panels).
Fig. 1.
GST P1-1 expression in TMA. A, Representative TMA cores immunostained for GST P1-1 at magnification ×10 (top panels), ×20 (middlepanels) and, for indicated areas, at ×40 (bottom panels). GST P1-1 protein levels (IxQ) in tumoral areas are indicated. Black arrows, Negative tumor; white arrows, positive benign gland, prostatic intraepithelial neoplasia (PIN), and tumor. Basal cell positive staining served as internal control. B, Kaplan-Meier DSS curves for PCa patients with the presence or absence of GSTP 1–1 (I × Q > 0 and I × Q = 0, respectively). The difference between the two groups was statistically significant.
GST P1-1 expression, activity, and promoter methylation in cultured PCa cells
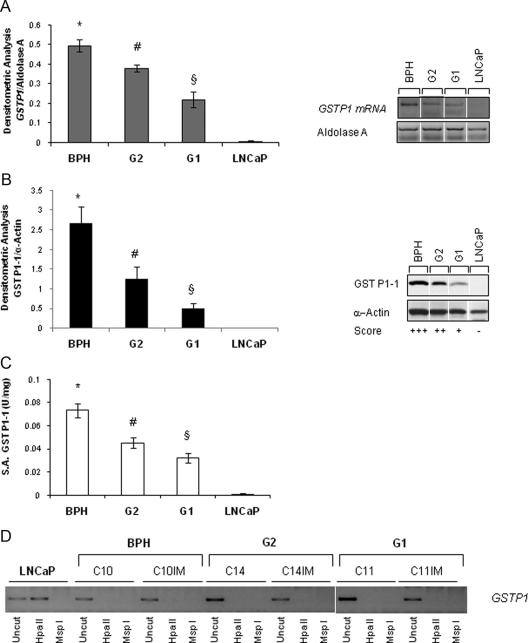
We recapitulated these findings in our ex vivo model of cell lines established from PCa patients (10, 26) by documenting a significant decrease of GSTP1 mRNA and protein expression and enzymatic activity, consistent with data in the literature (Fig. 2, A–C, and Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org) in cells from patients with worse vs. favorable outcome (G1 and G2 cells). As expected, cell lines established from benign prostatic hyperplasia (BPH) showed strong GSTP1 expression and activity. Surprisingly, DNA methylation-sensitive restriction assay (Fig. 2D) revealed that GSTP1 silencing did not involve promoter methylation, unlike the case with LNCaP cells and as generally reported for PCa (24, 27, 28). Lack of hypermethylation was not due to cell immortalization because the parental primary cultures (C10, C14, and C11) exhibited the same pattern as their immortalized derivatives (C10IM, C14IM, and C11IM).
Fig. 2.
GSTP1 expression, activity, and promoter methylation in ex vivo experimental model of PCa. A, Semiquantitative RT-PCR analysis of GSTP1 mRNA levels in immortalized cells derived from BPH, PCa cells of the G1 and G2 groups, and LNCaP cells. Densitometric analysis of GSTP1 normalized to aldolase A (left panel) and representative RT-PCR (right panel). Average values from four experiments for each cell line (BPH, n = 2; G1, n = 3; G2 n = 3) were expressed as mean ± sem. B, Western blot for GST P1-1 and loading control (α-actin) performed in BPH (n = 2); G2 cells (n = 3); G1 cells (n = 3); and LNCaP cells. Densitometric analysis of five experiments expressed as mean ± sem (left panel) and representative gel (rightpanel) is shown. C, GST P1-1 enzymatic activity expressed as units per milligram. Average values from three experiments for each cell line (BPH, n = 2; G1, n = 3; G2, n = 3) expressed as mean ± sem. D, Methylation analysis of the GSTP1 promoter in PCa cells before/after immortalization (IM) using a restriction enzyme specific for methylated DNA (HpaII) in representative cultures of BPH cells (C10 and C10IM) and PCa-derived cells from the G1 (C11 and C11IM) and G2 group (C14 and C14IM). LNCaP cells were positive control. White lines indicate extracts run in noncontiguous lanes of the same gel. *, P < 0.05 vs. G2, G1, and LNCaP; #, P < 0.05 vs. G1 and LNCaP; §, P < 0.05 vs. LNCaP.
Analysis of the GSTP1 promoter by chromatin immunoprecipitation (ChIP), re-ChIP, and EMSA
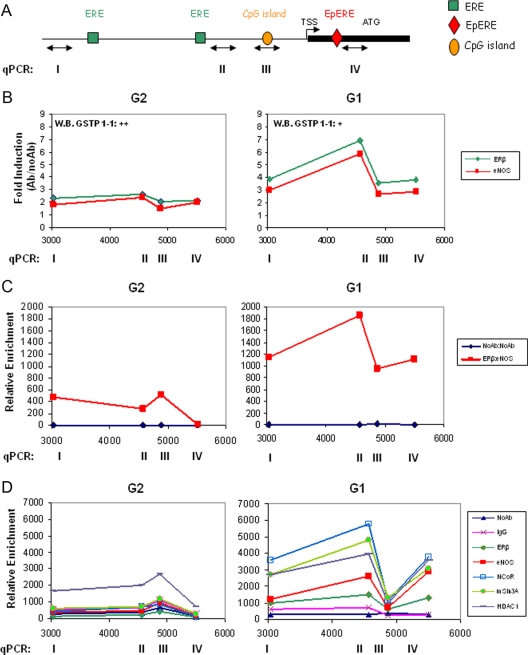
We queried whether GSTP1 silencing could be brought about by a repression mechanism mediated by recruitment of the ERβ/eNOS complex onto the gene-regulatory sequences and performed ChIP assays on a 5-kb region of the GSTP1 promoter using selected G1 and G2 cells (Fig. 3). Chromatins were immunoprecipitated by antibodies to ERβ, eNOS, and antiacetyl-histone H3 (H3Ac) and DNA sequences proximal to or encompassing two ERE, the CpG island and the electrophile ERE (EpERE) (29) were amplified (Fig. 3A). Dynamic occupancy of these sites by ERβ and eNOS was observed in basal conditions almost exclusively in G1 cells (Fig. 3B). ERβ and eNOS recruitment peaked at the EREs (sites I and II) with an increase of 3- to 7-fold over control (NoAb). Furthermore, serial ChIP assays showed recruitment of the ERβ/eNOS combinatorial complex with a similar dynamic, more pronounced in G1 than in G2 cells (Fig. 3C). Of interest, a strong increase (35- to 70-fold over control) in histone H3 acetylation density surrounding the GSTP1 transcription start site (TSS) was detected in PCa (G1 and G2) but not in metastatic LNCaP cells, in agreement with retained vs. lost GST P1-1 expression, respectively (data not shown). Next, we asked whether ERβ and eNOS can repress the GSTP1 expression by recruiting corepressors or deacetylases in a ligand-independent manner. ChIP experiments were therefore performed using specific antibodies to the corepressors nuclear receptor corepressor (NCoR) and mammalian Sin3 homolog A (mSin3A) and to the histone deacetylase 1 (HDAC1). As shown in Fig. 3D, strong recruitment of all three proteins occurred exclusively in G1 cells peaking at the ERE site II in parallel with ERβ and eNOS.
Fig. 3.
Analysis of the GSTP1 promoter by ChIP and re-ChIP. A, Cartoon of the promoter region and partial first exon of the GSTP1 gene (TSS and ATG are indicated). Search for transcription factors binding sites was performed with MatInspector database, and location of ERE, EpRE, and CpG island are indicated; double-headed arrow lines identify the regions I-IV amplified by quantitative PCR (qPCR). B, ChIP in immortalized PCa cells from the G2 (n = 3) and G1 (n = 3) groups. Immunoprecipitations were with antibodies to ERβ, eNOS, or NoAb. Recruitment onto the GSTP1 promoter was detected by qPCR using primers specific for region I, II, III, and IV. Data are represented as fold induction over control (Ab/NoAb) and are the mean of four experiments for each cell line. GST P1-1 expression level is indicated as ++ and + (as in Fig. 2B). C, re-ChIP in G2 (n = 2) and G1 (n = 2) cells with antibodies to ERβ or no antibody followed by antibodies to eNOS or no antibody. The data represent the mean of two experiments. qPCR was as in B. D, ChIP in G2 (n = 2) and G1 (n = 2) cells with antibodies to ERβ, eNOS, NCoR, mSin3A, HDAC1, or NoAb and nonspecific antibody (IgG) as negative controls. The data represent the mean of two experiments. qPCR was as in B.
To further characterize the interaction of ERβ/eNOS complex with the GSTP1 promoter, EMSA were performed using ERE and EpERE oligonucleotides (sites I, II, and IV) and PCa cells cultured in unstimulated condition (Supplemental Fig. 2). Incubation of nuclear extracts with all probes gave rise to a specific complex that was substantially decreased upon addition of antibodies against ERβ or eNOS, mostly at the ERE site II, supporting a physical interaction between ERβ and eNOS, in parallel with the peak of dynamic occupancy by ERβ/eNOS in ChIP and re-ChIP assays (Fig. 3, B and C). Of note, formation of the complex was partially prevented also upon addition of anti-cFos antibody on ERE II and EpERE (compare lanes 2 and 5 in Supplemental Fig. 2, D–F). This latter finding was confirmed by parallel detection of a specific c-Fos recruitment (2.5-fold increase over control) onto the GSTP1 promoter, most pronounced at the EpERE in the ChIP assay (Supplemental Fig. 2G).
Effects of pharmacological or genetic inhibition of ER and eNOS on chromatin remodeling, GSTP1 mRNA level, and cell invasion
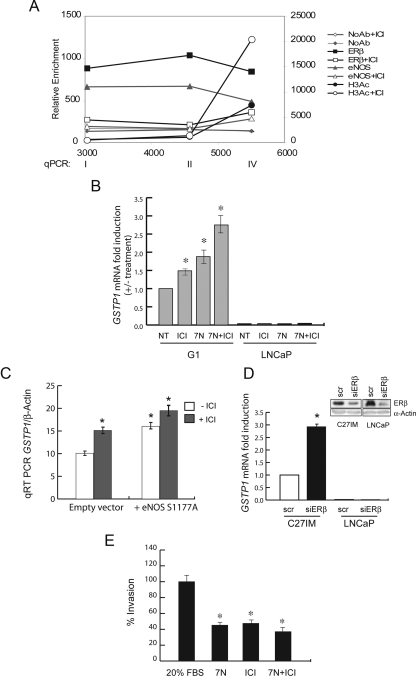
In support of our hypothesis that the ERβ/eNOS acts as a repressive complex on GSTP1 transcription, treatment with a synthetic antagonist of ER [ICI 182,780 (ICI)] abrogated the binding of both proteins to the GSTP1 promoter and relieved GSTP1 mRNA repression in cells from patients with adverse prognosis (Fig. 4, A and B). In agreement with these results, addition of ICI further increased (about 3-fold over control) histone acetylation density at the TSS (Fig. 4A). Rescue of mRNA and protein expression was also obtained using noncompetitive or competitive eNOS inhibitors [7-nitroindazole (7N) or NG-nitro-l-arginine methyl ester (L-NAME)] or by overexpression of a dominant-negative eNOS in the presence or absence of ICI, exclusively in G1 cells (Fig. 4, B and C, and Supplemental Fig. 3A and data not shown). Use of small interference RNA (siRNA) for ERβ confirmed as well its involvement in GSTP1 transcriptional regulation (Fig. 4D). Conversely, a NO donor silenced mRNA and protein expression in cells still expressing GSTP1 (G2 cells; Supplemental Fig. 4). Lastly, pharmacological treatment with ER or eNOS inhibitors, singly or in combination, impaired the invasion capability of PCa cells (Fig. 4E), suggesting that reexpression of GSTP1 through removal of the ERβ/eNOS complex may be associated with the acquisition of a less aggressive phenotype (see also Fig. 6 below).
Fig. 4.
Effects of pharmacological or genetic inhibition of ER and eNOS on chromatin remodeling, GSTP1 mRNA level, and cell invasion. A, ChIP assay onto the GSTP1 promoter was performed as in Fig. 3 using antibody to ERβ, eNOS, H3Ac, or NoAb. PCa cells from the G1 group (n = 2) were treated with/without ICI (10−7 m). The data represent the mean of two experiments. B, GSTP1 mRNA levels were assessed by quantitative PCR (qRT-PCR) in G1 (n = 2) and LNCaP cells after 24 h of treatment with ICI (10−7 m), the NO synthase inhibitors 7N (0.5 mm), alone or in combination. Results, plotted as fold induction (+/- treatment), represent the mean ± sem of three experiments in duplicate. *, P < 0.05 vs. untreated (NT). C, GSTP1 mRNA was analyzed by qRT-PCR in G1 cells (n = 2) transfected with a dominant-negative mutant of eNOS (S1177A) or empty vector with or without ICI (10−7 m). The data represent the mean ± sem of two experiments in duplicate. *, P < 0.05 vs. empty vector. D, C27IM and LNCaP cells transfected with small interference to ERβ (siERβ) or control (scr) were analyzed for ERβ expression by immunoblot (upper panel) and for GSTP1 mRNA by qRT-PCR (lowerpanel). Data, expressed as fold induction, represent the mean ± sem of three experiments. *, P < 0.05. E, The capability of G1 cells (n = 2) invasion was assessed by migration in matrigel under basal conditions (20% FBS) or upon treatment with ICI, 7N, or combination of both as in B. Data represent the mean ± sem of three experiments in quadruplicate. *, P < 0.05 vs. basal condition.
Fig. 6.
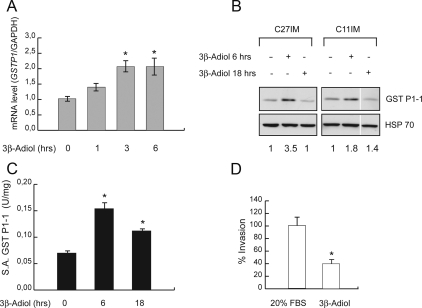
Effects of 3β-adiol on GSTP1 expression, enzymatic activity, and cell invasion. A, GSTP1 mRNA levels assessed by quantitative RT-PCR in G1 cells (n = 2) after 1, 3, or 6 h of treatment with 3β-adiol (10−6 m). The results, normalized to the housekeeping gene GAPDH, are plotted as fold induction (+/- treatment) and represent the mean ± sem of three experiments performed in duplicate. *, P < 0.05 vs. untreated (NT). B, Western blot of GST P1-1 in G1 cells (C27IM and C11IM) in basal condition and after treatment with 3β-adiol (10−6 m) for 6 and 18 h. Heat shock protein 70 (HSP70) was the loading control. Ratio (+/- treatment), after normalization to the HSP70 signal by densitometric analysis, is indicated in the bottom row. C, GST P1-1 enzymatic activity before and after treatment with 3β-adiol as in B was evaluated as described in Fig. 2C. D, The capability of G1 cell (n = 2) invasion assessed by migration in matrigel under basal conditions (20% FBS) or upon treatment with 3β-adiol (10−7 m) for 7 h. The data represent the mean ± sem of three experiments in quadruplicate. White lines indicate extracts run in noncontiguous lanes of the same gel.
In vivo ChIP assays
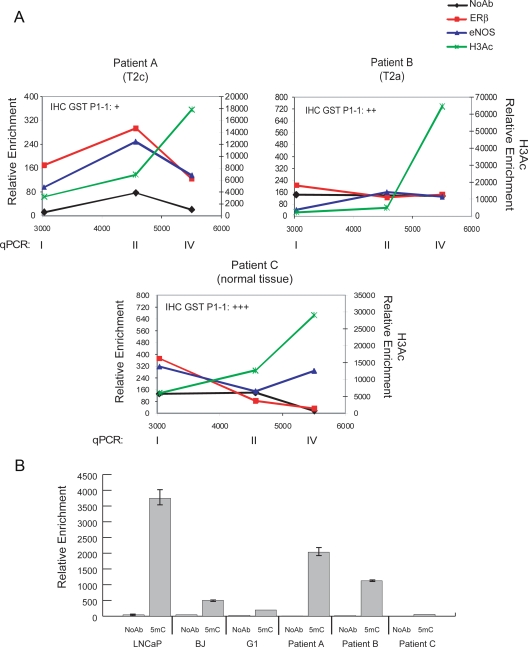
We next asked whether the dynamic occupancy of the GSTP1 promoter by ERβ and eNOS and the local increase in histone H3 acetylation occur also in vivo. ChIP assays on fresh surgical tissues explanted from PCa patients were performed as proof of principle to validate the data from PCa cell cultures. As shown in Fig. 5A, a recruitment profile of ERβ and eNOS, comparable with that in G1 cells (Fig. 3B), was observed in patient A, with a tumor at pathological stage T2c and an immunohistochemistry (IHC) scoring for GST P1-1 of +. No recruitment was seen, as expected, in normal/hyperplastic tissue (patient C with an IHC scoring of +++) or patient B at an earlier pathological stage (T2a) than patient A. The H3 acetylation pattern revealed a direct correlation between acetylation density at the TSS in ChIP assays and protein levels detected by IHC, both being less pronounced in patient A. Cytosine methylation at the promoter CpG island was investigated in the same fresh specimens by methylated DNA immunoprecipitation (Fig. 5B). Methylation, at levels intermediate between that of the positive (LNCaP) and negative (G1 and BJ cells) controls, was observed in PCa patients A and B but not control patient C. Of interest, the presence of methylation in patient A, combined with the observed recruitment of ERβ and eNOS, indicated the existence in vivo of both mechanisms of gene silencing.
Fig. 5.
In vivo ChIP assays. A, In vivo ChIP assays were performed using freshly explanted PCa samples (n = 3). Immunoprecipitations were with antibodies to ERβ, eNOS, H3Ac, or NoAb. Recruitment onto the GSTP1 promoter was detected by quantitative PCR (qPCR) using primers for region I, II, and IV. The pathological stage of each tumor is indicated. Data represent the mean of two experiments. GST P1-1 expression was evaluated by IHC, and its quantification is reported in each panel. B, ChIP assays were performed with antibody to 5methylcytidine (5mC) or NoAb in G1 cells (n = 2), freshly explanted PCa samples (patient A, patient B, and patient C), human foreskin fibroblast (BJ), and LNCaP cells (positive control). Immunoprecipitated fragments were amplified by qPCR using primers for region III of the GSTP1 promoter (Fig. 3). Data represent the mean ± SEM of two experiments.
Effects of 5α-androstane-3β,17β-diol (3β-adiol) on GSTP1 expression and activity and ERβ and eNOS nuclear localization
The critical involvement of ERβ in PCa progression is mainly related to the observation that retained expression of ERβ in recurrent PCa is associated with increased mortality and metastases (7, 30, 31). In addition, recent data indicate that ERβ is highly sensitive to variations in the prostate microenvironment (10, 32–35) and particularly affected by the relative abundance of its natural ligand 5α-androstane-3β,17β-diol (36, 37). Therefore, we asked whether 3β-adiol-activated ERβ would contribute to the rescue of GSTP1 expression and to the reversion of the aggressive phenotype. Indeed, 3β-adiol restored GSTP1 expression (mRNA and protein) and activity (Fig. 6, A–C). Of note, 3β-adiol also significantly reduced the invasion capability of G1 cells (Fig. 6D), correlating the rescue of GSTP1 expression (through destabilization of the ERβ/eNOS repressing complex) and loss of its protective effect against PCa progression.
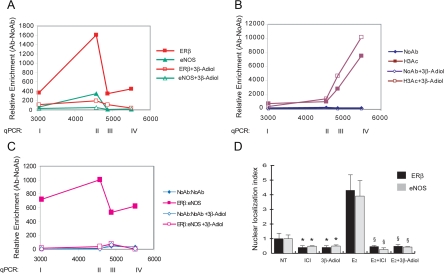
With this ligand, we observed no occupancy of the GSTP1 promoter by ERβ and eNOS, individually or as a complex, and a modest but reproducible increase in histone acetylation near the TSS (Fig. 7, A–C). Mechanistically, the natural ligand is likely to cause detachment of ERβ (and consequently eNOS), rather than prevent its binding as may be the case with the ERβ antagonist ICI (see Fig. 4). Interestingly, 3β-adiol is capable of reversing recruitment of ERβ and eNOS also on other estrogen-regulated target genes such us the classical pS2 promoter and the catalytic subunit of human telomerase (hTERT) that are both activated by the complex (Supplemental Fig. 5).
Fig. 7.
GSTP1 promoter analysis and nuclear localization of ERβ and eNOS in G1 cells treated with 3β-adiol. ChIP assays (A and B) and re-ChIP assays (C) of the GSTP1 promoter were performed as described in Fig. 3 using antibody to ERβ and eNOS (A and C) and H3Ac (B). NoAb was used as control. PCa cells from the G1 group (n = 2) were treated with or without 3β-adiol (10−6 m). The data represent the mean ± sem of three experiments. D, G1 cells were cultured for 72 h in stripped serum before treatment with the agonist E2, the antagonist (ICI), or the ERβ-specific ligand 3β-adiol, alone or in combination. Cells were stained with antibody to ERβ or eNOS and examined by confocal microscopy. Nuclei were stained with Topro 3. Original magnification was ×80 (see Supplemental Fig. 6). Images were digitally transformed to quantify the mean fluorescence intensity on selected areas for single eNOS- or ERβ-positive cells. The resulting histograms reveal the presence or accumulation of the eNOS or ERβ proteins in the nuclei of prostate cells (nuclear localization index). Data, expressed as fold induction, represent the mean ± sem of two independent experiments, each performed in duplicate. *, P < 0.05 vs. untreated (NT); §, P < 0.05 vs. E2.
To understand the effects mediated by small molecules such as ICI and 3β-adiol on the ERβ/eNOS complex signaling, we examined by confocal microscopy the localization of both ERβ and eNOS in basal condition as well as upon addition of estradiol (E2), ICI, or 3β-adiol, alone or in combination (Fig. 7D and Supplemental Fig. 6). ERβ and eNOS are present in G1 cells in both nuclear and cytoplasmatic compartments, in agreement with our previous data (10). Interestingly, the addition of the sintetic or natural ligands, ICI and 3β-adiol, respectively, resulted in significant relocalization of both proteins in the cytoplasm and a significant reduction of the mean fluorescence in the nuclei. Most interestingly, pretreatment with ICI or 3β-adiol prevented estradiol-induced nuclear translocation, supporting the concept that, at least in this context, 3β-adiol may act as an antagonist indistinguishable from the synthetic antagonist ICI (E2+ICI and E2+3β-adiol vs. E2, P <0.05).
Discussion
In the present paper, we provide evidence in favor of an active repressive mechanism on gene expression in PCa mediated by the complex ERβ/eNOS.
We previously demonstrated that in response to the combination of constitutive hypoxia-inducible factor (HIF)-1α expression and nuclear translocation of eNOS, ERβ and eNOS form a complex that induces transcription of prognostic genes activated in PCa (10, 26). This effect was enhanced by treatment with E2, alone or in combination with hypoxic conditions. These findings led us to query whether upon variations in the cell microenvironment the same complex may exert an opposite function and repress transcription of prognostic genes down-regulated in PCa (26).
Here we demonstrate that in PCa cells featuring a constitutively hypoxic phenotype, as are cells of the G1 group (10), the ERβ/eNOS complex is recruited onto the regulatory regions of GSTP1 and represses transcription of the gene. This is the first evidence of a repressive function mediated by ERβ in a ligand-independent fashion.
Mechanistically our finding is supported by parallel occupancy of the GSTP1 promoter by the corepressors NCoR and mSin3A as well as by HDCA1 (Fig. 3D), indicating a critical role of these proteins in suppressing GSTP1 gene expression in the nonactivated condition. Occupancy of the GSTP1 promoter, primarily at ERE sites, was abolished by the synthetic antiestrogen ICI, resulting in local chromatin changes such as an increase in the histone H3 acetylation density surrounding the GSTP1 TSS. Ligand-dependent chromatin remodeling was associated with modulation of gene transcription and ultimately with rescue of GSTP1 expression. Of interest, recruitment of ERβ/eNOS also was abrogated by the addition of the androgen metabolite 3β-adiol, a natural ERβ ligand, whereas no effect was obtained with the more potent natural agonist E2 or the antagonist of ER action, 4-hydroxitamoxifen (data not shown). In this regard, our results are in agreement with those of Dondi et al. (32), who demonstrated that ERβ activated by 3β-adiol, but not by E2, exerts antiproliferative and antiinvasion effects in the PC3 cell line and in xenografts in vivo. We expanded on this aspect and analyzed by confocal microscopy the nuclear/cytoplasmic distribution of ERβ and eNOS in cells untreated or treated with ICI or 3β-adiol. Interestingly, both small molecules significantly reduced the nuclear localization of both proteins in basal conditions and virtually abrogated the E2-induced nuclear translocations, supporting the antagonistic role of the selective ERβ ligand (with a magnitude undistinguishable from that of the synthetic antagonist ICI) (see Fig. 7D and Supplemental Fig. 6). Detachment of the ERβ/eNOS complex by the same small molecules also was observed onto target genes such as hTERT or pS2 that are positively regulated by estrogen, indicating that the reversal of the ERβ/eNOS binding may represent a common mechanism of ERβ signaling mediated by the classical antagonist ICI as well as by 3β-adiol.
Thus, silencing of GSTP1, a prognostic gene commonly repressed in PCa, may represent a novel consequence of an abnormal ER signaling, essentially ascribable to a specific perturbation of intratumoral steroid biosynthesis and metabolism. An age-dependent imbalance in the estrogens to androgens ratio in favor of estrogens has been invoked as the main trait in the etiology of PCa (38–43). In particular, it is becoming evident that in the PCa microenvironment, the traditional sex steroid hormone pathways, such as androgen-activated androgen receptor and E2-activated ER, are accompanied by alternative estrogenic signaling mediated by the 3β-adiol-ERβ complex (10, 32, 33). More importantly, each ligand-activated pathway appears to affect, with significant differences, tumor cells behavior. Specifically, recent literature (32–34) has indicated that 3β-adiol-activated ERβ plays a pivotal role in suppressing the acquisition of mesenchymal characteristics and invasive behavior of PCa cells in vitro and in vivo.
In line with the above, abrogation of ERβ/eNOS recruitment by ICI or 3β-adiol, resulted in rescue of GSTP1 expression and activity and impairment of the invasion capability of PCa cells. Thus reexpression of GSTP1 is associated with partial reversion of the aggressive phenotype. Overall these data strongly indicate that the nuclear steroid receptors, differentially activated by circulating or locally produced steroids or their metabolites, greatly affects the fine equilibrium governing the androgenic and estrogenic pathways in the human prostate.
In addition, pharmacological inhibition of the NO/eNOS pathway (Fig. 4B) or disruption of the eNOS/calmodulin complex by the calmodulin inhibitor W-7 (44) (Supplemental Fig. 3) relieved the repression of GSTP1 mRNA in cells from patients with adverse prognosis. Similar results were obtained by genetic approaches (siRNA to ERβ and also overexpression of a dominant negative eNOS), confirming the crucial role of the ERβ/eNOS complex in GSTP1 repression. Conversely, a NO donor silenced this mRNA in cells still expressing GSTP1 (G2; Supplemental Fig. 4), suggesting that also NO signaling contributes to the fine-tuned regulation of GSTP1 transcription.
Of interest, a positive correlation between NO biosynthesis and grade of malignancy, specifically in terms of increase in tumor blood flow has been reported also in breast cancer (45). It has been further suggested that GST, specifically GST P1-1, can act as an NO carrier or scavenger when the intracellular NO concentration is greatly increased (46). Our results are consistent with this role and with the down-regulation of GSTP1 being part of the overall strategy by PCa cells to maintain NO bioavailability and favor tumor progression.
An additional outcome of our study is the demonstration of the coexistence of two repressive mechanisms in the prostate tumor cells: the already reported promoter methylation and the repression by the combinatorial ERβ/eNOS complex mediated by chromatin remodeling. Although silencing of GSTP1 did not appear to involve methylation of its promoter in our cell lines, unlike the case for LNCaP cells and generally for PCa, analysis of fresh PCa tissues revealed in one patient the presence of both ERβ/eNOS recruitment and a significant level of methylated cytosines in the GSTP1 regulatory regions. Of interest, in a second sample (patient B, at an earlier pathological stage), we observed appreciable level of methylation of GSTP1 but no promoter occupancy by ERβ/eNOS. The presence or absence of ERβ/eNOS in the two samples inversely correlated with levels of GST P1-1 expression and acetylation density at the GSTP1 TSS, an epigenetic mark of transcriptionally permissive chromatin. These in vivo data suggest that the repressive function exerted by the ERβ/eNOS complex may be an epigenetic event favoring inactivation of the GSTP1 locus and representing an alternative or reinforcing mechanism to DNA methylation (compare methylation profile of G1 cells, patients A and B).
Overall our findings strongly support the concept that in the physiopathology of the human prostate exists a very complex signaling pathway mediated by ERβ and its cofactor eNOS, highly sensitive to variations in the tissue microenvironment (androgens, estrogens and their metabolites, oxygen tension, and NO) with major effects on the acquisition of a malignant phenotype by human prostate epithelial cells.
Materials and Methods
Cell cultures and treatments
PCa cells, primary and immortalized, were cultured and characterized as described (10, 26). Metastatic prostate cancer cells LNCaP were obtained from Aria Baniahmad (University of Giessen, Giessen, Germany) and cultured in RPMI 1640 (Invitrogen, Carlsbad, CA) plus 20% fetal bovine serum (FBS; Cambrex, Charles City, IA). At least 72 h before experimental use, cells were switched to a medium with hormone-deprived serum (35) and treated with 7-nitroindazole (Biomol, Farmingdale, NY), ICI 182,780 (ICI; Sigma-Aldrich, St. Louis, MO), diethylenetriamine/NO adduct (DETA-NO; Sigma-Aldrich), NG-nitro-l-arginine methyl ester (L-NAME; Alexis, Lorrach, Germany), 3β-adiol (Sigma-Aldrich), or N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride (W-7; Santa Cruz Biotechnology, Santa Cruz, CA) for the concentrations and the times indicated in the figure legends.
Antibodies
Anti-ERβ (L-20; Santa Cruz Biotechnology; and GTX110607; GeneTex, Irvine, CA), anti-eNOS (BD Biosciences, Franklin Lakes, NJ), anti-IgG (Santa Cruz Biotechnology), anti-NCoR (H303; Santa Cruz Biotechnology), anti-c-Fos (4 and H-125; Santa Cruz Biotechnology), anti-HDAC1 (Sigma-Aldrich), anti-mSin3A (Abcam, Cambridge, UK), antiacetyl-histone H3 (Upstate-Millipore, Billerica, MA), anti-5-methylcytidine (Eurogentec, Seraing, Belgium), anti-α-actin (Sigma-Aldrich), and anti-HSP70 (StressGen Biotechnologies, San Diego, CA). The polyclonal anti-GST P1-1 was derived from rabbits immunized against purified human GSTp.
GST P1-1 enzymatic activity and promoter methylation
Enzymatic activity was determined spectrophotometrically as described (47). Enzymatic restriction and analysis of GSTP1 promoter methylation were performed as described (48).
Transfections, cell extracts, and Western blot
Transient transfections were performed as described (10). For Western blots, cells were lysed in 10 mm Tris-HCl (pH 7.4) and 0.1 m phenylmethylsulfonyl fluoride buffer; 30 μg of extracts were resolved on 12% SDS-PAGE gel and transferred onto a polyvinylidene difluoride membranes (Millipore).
RNA extraction and real-time PCR
RNA isolation, cDNA preparation, and PCR were performed as described (19). Quantitative RT-PCR was performed using SYBR master mix (Applied Biosystems Inc., Foster City, CA) and primers for GSTP1 mRNA. The housekeeping genes aldolase, GAPDH, and β-actin (Applied Biosystems) served as controls (10). The following primers were used: GSTP1 mRNA forward, 5′-GGA GAC CTC ACC CTG TAC CA-3′; reverse, 5′-GGA CAG CAG GGT CTC AAA AG-3′; GSTP1 mRNA for quantitative PCR forward, 5′-ATC AGG GCC AGA GCT GGA A-3′; reverse, 5′-ATA GGC AGG AGG CTT TGA GTG A-3′.
Chromatin immunoprecipitations
ChIP and re-ChIP assays from cultured cells were performed as described (10) using specific antibodies to ERβ, eNOS, H3Ac, NCoR, mSin3A, HDAC1, and c-Fos. Negative controls were absence of antibody (NoAb) or unrelated antibody, normal rabbit IgG. In vivo ChIP assays were performed on freshly explanted prostate tissues, obtained after informed consent from three patients undergoing radical prostatectomy (Department of Urology, Catholic University, Rome, Italy). After fixation with formaldehyde and blockage as described (49), the tissues were chopped, dounced in hypotonic buffer, and chromatin prepared as described (10). Immunoprecipitations were performed with 20 ng of sonicated chromatin for each antibody and subsequent ChIP steps were carried out as described (10). DNA fragments were recovered and analyzed as described (10) using the following primers for GSTP1 promoter by quantitative PCR: 3034/3044 (site I), 5′-AAT TCC AGC CTG GCA AAT TCT-3′ and 5′-CGC ACT GTC AGG GTT CAA GA-3′; 4568/4632 (site II), 5′-TGG CAC GCA CCT ATA ATT CCA-3′ and 5′-TCT CGG GTT CAA GCA ATT CTG-3′; 4872/4929 (site III), 5′-GCG CGC CAG TTC GCT-3′ and 5′-AGT AAA CAG ACA GCA GGA AGA GGA C-3′; 5505/5573 (site IV), 5′-GGG CTC CAG CAA ACT TTT CTT-3′ and 5′-CCT ACC TCG AAC TGG GAA ATA GAC-3′. Primers for the hTERT and pS2 gene promoters are as described (10).
Methylation analysis by DNA immunoprecipitation
The protocol was as described (50) with modifications. Briefly, cultured cells or tissue samples were cross-linked and sonicated, as for the ChIP protocol, digested by proteinase K and reverse cross-linked overnight. DNA was recovered by phenol-chloroform extraction, ethanol precipitation, and ribonuclease digestion. Four micrograms of purified DNA was denaturated (10 min at 95 C), placed on ice, and immunoprecipitated (2 h at 4 C) with 10 μg of 5-methylcytidine antibody in 500 μl immunoprecipitation buffer. After incubation with 30 μl of Protein G (Pierce, Rockford, IL) overnight at 4 C, samples were washed three times and treated with proteinase K (3 h at 50 C). Methylated DNA, recovered by phenol-chloroform extraction and ethanol precipitation, was analyzed by PCR, using primers as described (48), and by quantitative PCR, using primers for site III of the GSTP1 promoter.
siRNA methods
Small interference to ERβ was obtained using an optimized combination of oligonucleotides: small interference to ERβ (siERβ) custom stealth (51) plus predesigned stealth RNA interference oligos (set of three, no. 1299003; Invitrogen). Cells were transfected consecutively two times every 72 h, according to the manufacturer's instructions (Lipofectamine RNAiMAX; Invitrogen). Negative control was a set of three oligos (no. 12935-100; Invitrogen).
Cell invasion
Assay was performed using a 48-well modified Boyden's chamber (NeuroProbe Inc., Biomap, Gaithersburg, MD) and 8 μm pore polyvinyl pyrrolidone-free polycarbonate Nucleopore (Carlo Erba Reagenti, Milano, Italy). The filter was coated with a thin layer of 2 mg/ml Cultrex BME (Space Import Export, Milan, Italy). PCa cells were treated as described in the figure legends. Cells that invaded were fixed and stained with Diff Quick (Biomap) and counted at ×40 magnification in five fields per sample.
TMA construction, immunohistochemistry, scoring, and outcome analysis
Tissue microarrays were generated, stained, and scored as described (10). Briefly, TMA sections were scored semiquantitatively based on the proportion of tumor cells stained [quantity (Q)] and the staining intensity (I) to obtain the final score as the product of I × Q relative to the tumoral area. The scoring system (52, 53) was as follows: Q, 0, negative; 1, 1–9% positive cells; 2, 10–39% positive cells; 3, 40–69% positive cells; 4, 70–100% positive cells; and I, 0, negative; 1, low; 2, moderate; 3, high. Immunostaining was assessed by a histopathologist and reviewed independently by a second histopathologist. Both individuals were blinded to the patients' outcome. Only representative tissue cores containing at least 20% of tumor cells were scored.
Confocal microscopy analysis
PCa cells were grown on glass slides and after treatments fixed in ice-cold acetone 80% for 1 h. Samples were incubated for 1 h with 10% BSA/PBS to block nonspecific protein-binding sites and overnight at 4 C with primary antibodies (ERβ, 1:100; eNOS, 1:50). After a brief rinse, slides were incubated for 1 h at 37 C with fluorescein isothiocyanate secondary antibodies (dilution 1:150; Dylight, Jackson Immunoresearch Lab. Inc., Mill Valley, CA). Nuclei were stained with TOPRO3 (1:200; Invitrogen). The quantification of eNOS and ERβ nuclear localization, expressed as an index, was determined measuring the relative mean fluorescence intensity on selected regions using an LSM 500 Meta analyzer program (Zeiss, Oberkochen, Germany). Values are expressed as fold induction over control considered equal to 1. About 300 cells/well were evaluated for each experimental condition. Lasers' power, beam splitters, filter settings, pinhole diameters, and scan mode were the same for all examined fields of each sample. Negative immunofluorescence control was performed using normal rabbit IgG instead of the rabbit primary antibody.
Electrophoretic mobility shift assays
The experimental procedure was performed as described (35) with modifications. The EMSA reaction was in a final volume of 20 μl: 5 μg of nuclear extracts, prepared from G1 cells as described (10); binding buffer (100 mm HEPES, 5 mm dithiothreitol, 10 mm MgC12, 50% glycerol, 0.5 mg/ml BSA); 1 μg of poly(deoxyinosine-deoxycytosine); 0.5 mm spermidine. Two micrograms of specific antibodies to ERβ, eNOS, cFos, and an unrelated antibody (normal rabbit IgG) were added after the addition of labeled probes. The following probes were used: 3132/3161 (ERE site I), 5′-TTCGCCGTGACCTTCTGCCCTGTGATCTTT-3′ (sense); 4482/4516 (ERE site II), 5′-CAGATCACCTAAGGTCAGGAGTTCGAGACCAGCC-3′ (sense); and 5140/5161 (EpERE), 5′-CGGCCGGCGCCGTGACTCAGCACTGGGGCGGA-3′ (sense).
For competition binding experiments, increasing concentrations of unlabeled specific (see above) or unrelated (5′-GGGCTGAGCTAGAGGCAGAAGGGGAAATCCC-3′, sense) oligonucleotides were added to the binding mixture immediately before the addition of the 32P-labeled probes.
Statistical methods
Statistical analysis was with Prism 2.01 statistical software (GraphPad, San Diego, CA) and R Software (54). Differences among subject groups were assessed by a two-tailed Mann-Whitney U test. For TMA data analysis, the DSS was calculated using the Kaplan-Meier method from the time of prostatectomy (10). The statistical significance level was set at P < 0.05.
Supplementary Material
Acknowledgments
We thank Carlo Gaetano for constructive discussions and critical reading of the manuscript and Marcella Mottolese for advice on tissue microarray immunostaining. We are greatly indebted to Ada Sacchi for her encouragement and support. Funding from the Associazione Italiana Ricerca sul Cancro and the Italian Ministry of Health and the Italian Ministry of Education, University, and Research is acknowledged.
This work was supported by the Associazione Italiana Ricerca sul Cancro and the Italian Ministry of Health (RF 07, Programma Strategico IMA) (to A.F.) and the Italian Ministry of Education, University, and Research (Grant PRIN 2008NY72SJ) (to A.F. and M.L.B.). V.B. is the recipient of a Federazione Italiana Ricerca sul Cancro Fellowship.
Disclosure Summary: The authors declare no conflict of interest.
NURSA Molecule Pages†:
Nuclear Receptors: ER-β;
Ligands: 17β-estradiol.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- 3β-adiol
- 5α-Androstane-3β,17β-diol
- BPH
- benign prostatic hyperplasia
- ChIP
- chromatin immunoprecipitation
- DSS
- disease-specific survival
- E2
- estradiol
- eNOS
- endothelial NO synthase
- EpERE
- electrophile ERE
- ER
- estrogen receptor
- ERE
- estrogen-responsive element
- FBS
- fetal bovine serum
- GSTP1
- glutathione transferase P1
- H3Ac
- acetyl-histone H3
- HDAC1
- histone deacetylase 1
- HIF
- hypoxia-inducible factor
- I
- intensity
- ICI
- ICI 182,780
- IHC
- immunohistochemistry
- 7N
- 7-nitroindazole
- mSin3A
- mammalian Sin3 homolog A
- NCoR
- nuclear receptor corepressor
- NO
- nitric oxide
- NoAb
- absence of antibody
- PCa
- prostate cancer
- Q
- quantity
- siRNA
- small interference RNA
- TMA
- tissue microarray
- TSS
- transcription start site.
References
- 1. Parkin DM, Bray F, Ferlay J, Pisani P. 2005. Global cancer statistics, 2002. CA Cancer J Clin 55:74–108 [DOI] [PubMed] [Google Scholar]
- 2. Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. 2006. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res 66:5624–5632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Risbridger GP, Davis ID, Birrell SN, Tilley WD. 2010. Breast and prostate cancer: more similar than different. Nat Rev Cancer 10:205–212 [DOI] [PubMed] [Google Scholar]
- 4. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. 1997. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138:863–870 [DOI] [PubMed] [Google Scholar]
- 5. Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. 1996. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matthews J, Gustafsson JA. 2003. Estrogen signaling: a subtle balance between ER α and ER β. Mol Interv 3:281–292 [DOI] [PubMed] [Google Scholar]
- 7. Weihua Z, Lathe R, Warner M, Gustafsson JA. 2002. An endocrine pathway in the prostate, ERβ, AR, 5α-androstane-3β,17β-diol, and CYP7B1 regulates prostate growth. Proc Natl Acad Sci USA 99:13589–13594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weihua Z, Warner M, Gustafsson JA. 2002. Estrogen receptor β in the prostate. Mol Cell Endocrinol 193:1–5 [DOI] [PubMed] [Google Scholar]
- 9. Ellem SJ, Risbridger GP. 2009. The dual, opposing roles of estrogen in the prostate. Ann NY Acad Sci 1155:174–186 [DOI] [PubMed] [Google Scholar]
- 10. Nanni S, Benvenuti V, Grasselli A, Priolo C, Aiello A, Mattiussi S, Colussi C, Lirangi V, Illi B, D'Eletto M, Cianciulli AM, Gallucci M, De Carli P, Sentinelli S, Mottolese M, Carlini P, Strigari L, Finn S, Mueller E, Arcangeli G, Gaetano C, Capogrossi MC, Donnorso RP, Bacchetti S, Sacchi A, Pontecorvi A, Loda M, Farsetti A. 2009. Endothelial NOS, estrogen receptor β, and HIFs cooperate in the activation of a prognostic transcriptional pattern in aggressive human prostate cancer. J Clin Invest 119:1093–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. 2007. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87:905–931 [DOI] [PubMed] [Google Scholar]
- 12. Katzenellenbogen BS, Montano MM, Ediger TR, Sun J, Ekena K, Lazennec G, Martini PG, McInerney EM, Delage-Mourroux R, Weis K, Katzenellenbogen JA. 2000. Estrogen receptors: selective ligands, partners, and distinctive pharmacology. Rec Prog Horm Res 55:163–193; discussion 194–195 [PubMed] [Google Scholar]
- 13. Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. 2006. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- 14. Métivier R, Penot G, Hübner MR, Reid G, Brand H, Kos M, Gannon F. 2003. Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751–763 [DOI] [PubMed] [Google Scholar]
- 15. Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. 2000. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852 [DOI] [PubMed] [Google Scholar]
- 16. Smith CL, O'Malley BW. 2004. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev 25:45–71 [DOI] [PubMed] [Google Scholar]
- 17. Gobeil F, Jr, Zhu T, Brault S, Geha A, Vazquez-Tello A, Fortier A, Barbaz D, Checchin D, Hou X, Nader M, Bkaily G, Gratton JP, Heveker N, Ribeiro-da-Silva A, Peri K, Bard H, Chorvatova A, D'Orléans-Juste P, Goetzl EJ, Chemtob S. 2006. Nitric oxide signaling via nuclearized endothelial nitric-oxide synthase modulates expression of the immediate early genes iNOS and mPGES-1. J Biol Chem 281:16058–16067 [DOI] [PubMed] [Google Scholar]
- 18. Goetz RM, Thatte HS, Prabhakar P, Cho MR, Michel T, Golan DE. 1999. Estradiol induces the calcium-dependent translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci USA 96:2788–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grasselli A, Nanni S, Colussi C, Aiello A, Benvenuti V, Ragone G, Moretti F, Sacchi A, Bacchetti S, Gaetano C, Capogrossi MC, Pontecorvi A, Farsetti A. 2008. Estrogen receptor-α and endothelial nitric oxide synthase nuclear complex regulates transcription of human telomerase. Circ Res 103:34–42 [DOI] [PubMed] [Google Scholar]
- 20. Klinz FJ, Schmidt A, Schinköthe T, Arnhold S, Desai B, Popken F, Brixius K, Schwinger R, Mehlhorn U, Staib P, Addicks K, Bloch W. 2005. Phospho-eNOS Ser-114 in human mesenchymal stem cells: constitutive phosphorylation, nuclear localization and upregulation during mitosis. Eur J Cell Biol 84:809–818 [DOI] [PubMed] [Google Scholar]
- 21. Sato K. 1989. Glutathione transferases as markers of preneoplasia and neoplasia. Adv Cancer Res 52:205–255 [DOI] [PubMed] [Google Scholar]
- 22. Hayes JD, Flanagan JU, Jowsey IR. 2005. Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88 [DOI] [PubMed] [Google Scholar]
- 23. Nakayama M, Gonzalgo ML, Yegnasubramanian S, Lin X, De Marzo AM, Nelson WG. 2004. GSTP1 CpG island hypermethylation as a molecular biomarker for prostate cancer. J Cell Biochem 91:540–552 [DOI] [PubMed] [Google Scholar]
- 24. Lee WH, Morton RA, Epstein JI, Brooks JD, Campbell PA, Bova GS, Hsieh WS, Isaacs WB, Nelson WG. 1994. Cytidine methylation of regulatory sequences near the PI-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc Natl Acad Sci USA 91:11733–11737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin X, Tascilar M, Lee WH, Vles WJ, Lee BH, Veeraswamy R, Asgari K, Freije D, van Rees B, Gage WR, Bova GS, Isaacs WB, Brooks JD, DeWeese TL, De Marzo AM, Nelson WG. 2001. GSTP1 CpG island hypermethylation is responsible for the absence of GSTP1 expression in human prostate cancer cells. Am J Pathol 159:1815–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nanni S, Priolo C, Grasselli A, D'Eletto M, Merola R, Moretti F, Gallucci M, De Carli P, Sentinelli S, Cianciulli AM, Mottolese M, Carlini P, Arcelli D, Helmer-Citterich M, Gaetano C, Loda M, Pontecorvi A, Bacchetti S, Sacchi A, Farsetti A. 2006. Epithelial-restricted gene profile of primary cultures from human prostate tumors: a molecular approach to predict clinical behavior of prostate cancer. Mol Cancer Res 4:79–92 [DOI] [PubMed] [Google Scholar]
- 27. Hopkins TG, Burns PA, Routledge MN. 2007. DNA methylation of GSTP1 as biomarker in diagnosis of prostate cancer. Urology 69:11–16 [DOI] [PubMed] [Google Scholar]
- 28. Jerónimo C, Henrique R, Hoque MO, Mambo E, Ribeiro FR, Varzim G, Oliveira J, Teixeira MR, Lopes C, Sidransky D. 2004. A quantitative promoter methylation profile of prostate cancer. Clin Cancer Res 10:8472–8478 [DOI] [PubMed] [Google Scholar]
- 29. Montano MM, Deng H, Liu M, Sun X, Singal R. 2004. Transcriptional regulation by the estrogen receptor of antioxidative stress enzymes and its functional implications. Oncogene 23:2442–2453 [DOI] [PubMed] [Google Scholar]
- 30. Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, Makela S, Delprado W, Golovsky D, Brenner PC, O'Neill G, Kooner R, Stricker PD, Grygiel JJ, Gustafsson JA, Sutherland RL. 2001. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res 61:5331–5335 [PubMed] [Google Scholar]
- 31. Ho SM, Leung YK, Chung I. 2006. Estrogens and antiestrogens as etiological factors and therapeutics for prostate cancer. Ann NY Acad Sci 1089:177–193 [DOI] [PubMed] [Google Scholar]
- 32. Dondi D, Piccolella M, Biserni A, Della Torre S, Ramachandran B, Locatelli A, Rusmini P, Sau D, Caruso D, Maggi A, Ciana P, Poletti A. 2010. Estrogen receptor β and the progression of prostate cancer: role of 5α-androstane-3β,17β-diol. Endocr Relat Cancer 17:731–742 [DOI] [PubMed] [Google Scholar]
- 33. Guerini V, Sau D, Scaccianoce E, Rusmini P, Ciana P, Maggi A, Martini PG, Katzenellenbogen BS, Martini L, Motta M, Poletti A. 2005. The androgen derivative 5α-androstane-3β,17β-diol inhibits prostate cancer cell migration through activation of the estrogen receptor β subtype. Cancer Res 65:5445–5453 [DOI] [PubMed] [Google Scholar]
- 34. Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA, Gouvin LM, Sharma VM, Mercurio AM. 2010. ERβ impedes prostate cancer EMT by destabilizing HIF-1α and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell 17:319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nanni S, Narducci M, Della Pietra L, Moretti F, Grasselli A, De Carli P, Sacchi A, Pontecorvi A, Farsetti A. 2002. Signaling through estrogen receptors modulates telomerase activity in human prostate cancer. J Clin Invest 110:219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA. 1998. The estrogen receptor β subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol 19:253–286 [DOI] [PubMed] [Google Scholar]
- 37. Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. 2001. Mechanisms of estrogen action. Physiol Rev 81:1535–1565 [DOI] [PubMed] [Google Scholar]
- 38. Baulieu EE. 2002. Androgens and aging men. Mol Cell Endocrinol 198:41–49 [DOI] [PubMed] [Google Scholar]
- 39. Culig Z, Hobisch A, Cronauer MV, Cato AC, Hittmair A, Radmayr C, Eberle J, Bartsch G, Klocker H. 1993. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol Endocrinol (Baltimore, Md) 7:1541–1550 [DOI] [PubMed] [Google Scholar]
- 40. Ellem SJ, Risbridger GP. 2010. Aromatase and regulating the estrogen:androgen ratio in the prostate gland. J Steroid Biochem Mol Biol 118:246–251 [DOI] [PubMed] [Google Scholar]
- 41. Gray A, Berlin JA, McKinlay JB, Longcope C. 1991. An examination of research design effects on the association of testosterone and male aging: results of a meta-analysis. J Clin Epidemiol 44:671–684 [DOI] [PubMed] [Google Scholar]
- 42. Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I. 2002. Estradiol in elderly men. Aging Male 5:98–102 [PubMed] [Google Scholar]
- 43. Vermeulen A, Rubens R, Verdonck L. 1972. Testosterone secretion and metabolism in male senescence. J Clin Endocrinol Metab 34:730–735 [DOI] [PubMed] [Google Scholar]
- 44. Fleming I. 2010. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch 459:793–806 [DOI] [PubMed] [Google Scholar]
- 45. Thomsen LL, Miles DW, Happerfield L, Bobrow LG, Knowles RG, Moncada S. 1995. Nitric oxide synthase activity in human breast cancer. Br J Cancer 72:41–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cesareo E, Parker LJ, Pedersen JZ, Nuccetelli M, Mazzetti AP, Pastore A, Federici G, Caccuri AM, Ricci G, Adams JJ, Parker MW, Lo Bello M. 2005. Nitrosylation of human glutathione transferase P1-1 with dinitrosyl diglutathionyl iron complex in vitro and in vivo. J Biol Chem 280:42172–42180 [DOI] [PubMed] [Google Scholar]
- 47. Habig WH, Jakoby WB. 1981. Assays for differentiation of glutathione S-transferases. Methods Enzymol 77:398–405 [DOI] [PubMed] [Google Scholar]
- 48. Bernardini S, Miano R, Iori R, Finazzi-Agrò E, Palmieri G, Ballerini S, Angeloni C, Orlandi A, Bellincampi L, Cortese C, Federici G. 2004. Hypermethylation of the CpG islands in the promoter region of the GSTP1 gene in prostate cancer: a useful diagnostic and prognostic marker? Clin Chim Acta 350:181–188 [DOI] [PubMed] [Google Scholar]
- 49. Schmidt D, Wilson MD, Spyrou C, Brown GD, Hadfield J, Odom DT. 2009. ChIP-seq: using high-throughput sequencing to discover protein-DNA interactions. Methods (San Diego, Calif) 48:240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weber M, Davies JJ, Wittig D, Oakeley EJ, Haase M, Lam WL, Schübeler D. 2005. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet 37:853–862 [DOI] [PubMed] [Google Scholar]
- 51. Arnold JT, Liu X, Allen JD, Le H, McFann KK, Blackman MR. 2007. Androgen receptor or estrogen receptor-β blockade alters DHEA-, DHT-, and E(2)-induced proliferation and PSA production in human prostate cancer cells. Prostate 67:1152–1162 [DOI] [PubMed] [Google Scholar]
- 52. Signoretti S, Di Marcotullio L, Richardson A, Ramaswamy S, Isaac B, Rue M, Monti F, Loda M, Pagano M. 2002. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J Clin Invest 110:633–641 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53. Ogino S, Nosho K, Meyerhardt JA, Kirkner GJ, Chan AT, Kawasaki T, Giovannucci EL, Loda M, Fuchs CS. 2008. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol 26:5713–5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. R Development Core Team 2008. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2008 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.