Abstract
Circadian clock coordinates behavior and physiology in mammals in response to light and feeding cycles. Disruption of normal clock function is associated with increased risk for cardiovascular and metabolic diseases, underscoring the emerging concept that temporal regulation of tissue metabolism is a fundamental aspect of energy homeostasis. We have previously demonstrated that transcriptional coactivator, peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), coordinates circadian metabolic rhythms through simultaneous regulation of metabolic and clock gene expression. In this study, we found that PGC-1α physically interacts with, and is phosphorylated by, casein kinase 1δ (CK1δ), a core component of the circadian pacemaker. CK1δ represses the transcriptional function of PGC-1α in cultured hepatocytes, resulting in decreased gluconeogenic gene expression and glucose secretion. At the molecular level, CK1δ phosphorylation of PGC-1α within its arginine/serine-rich domain enhances its degradation through the proteasome system. Together, these results elucidate a novel mechanism through which circadian pacemaker transduces timing signals to the metabolic regulatory network that controls hepatic energy metabolism.
Many aspects of mammalian behavior and physiology exhibit diurnal rhythms of approximately 24 h, including locomotor activity, sleep-wake cycles, blood pressure, body temperature, and energy metabolism (1–4). These biological rhythms are regulated by body clock that is entrained by external cues, in particular light and feeding cycles. Disruption of clock function has been implicated as a risk factor for sleep disorder, cardiovascular disease, type 2 diabetes, and cancer (5–8). Recent studies demonstrate that circadian misalignment and sleep disruption alter hormonal profiles and lead to insulin resistance in healthy individuals (9, 10). Further, Clock mutant mice develop obesity and display characteristics of metabolic syndrome (11), whereas ablation of peripheral clocks in the liver and islet β-cells impairs hepatic gluconeogenesis and insulin secretion, respectively (12–15). These observations strongly suggest that temporal regulation of tissue metabolism is a fundamental aspect of metabolic homeostasis. Transcriptional profiling studies demonstrate that the expression of numerous genes involved in glucose, lipid, and mitochondrial energy metabolism are rhythmically controlled in the liver, skeletal muscle, and adipose tissue (16–20). However, the physiological and molecular mechanisms that integrate biological clock and energy metabolism remain poorly defined.
The biological clock is organized into positive and negative feedback loops that comprise transcriptional activators and repressors (21–23). These transcriptional regulatory loops are fine tuned through posttranslational mechanisms, such as phosphorylation and acetylation (23, 24). Extensive genetic studies have identified casein kinase 1δ (CK1δ) and CK1ε, members of serine/threonine kinase CK1, as critical regulators of circadian pacemaker function. Mutation of CK1ε causes the τ-mutant phenotype in Syrian hamsters, which has a shortened circadian period (25, 26), whereas a missense mutation (T44A) of CK1δ is responsible for familial advanced sleep-phase syndrome in a subset of patients (27). Recent studies using CK1δ gain- and loss-of-function mouse models provide further evidence for a central role of CK1 in clock function (27–29). At the molecular level, CK1δ exerts its effects, in part, through phosphorylation of the Period proteins, resulting in altered stability and subcellular localization (28–31). Although the role of CK1δ in clock function has been well established, whether CK1δ transmits circadian timing signals to metabolic pathways has not been explored.
Peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) belongs to a small family of transcriptional coactivators that includes PGC-1α, PGC-1β, and PGC-related coactivator (32–34). The expression of PGC-1α is regulated by diverse physiological signals, such as starvation, cold exposure, and exercise (35–37). Through interaction with transcription factors as well as chromatin-remodeling complexes, PGC-1α stimulates mitochondrial biogenesis, fatty acid β-oxidation, hepatic gluconeogenesis, heme biosynthesis, and slow-twitch myofiber formation (37–41). We have previously demonstrated that PGC-1α coactivates retinoid acid receptor-related orphan receptor alpha (RORα) and induces the expression of Bmal1 and Rev-erbα, two core components of the molecular clock (42). In addition, mice deficient in PGC-1α have altered diurnal rhythms of locomotor activity, body temperature, and metabolic rate, suggesting that this coactivator may serve a nodal function in the integration of clock and energy metabolism. In this study, we show that PGC-1α serves as a substrate for CK1δ, thereby transducing circadian timing signals to hepatic metabolic regulation. Phosphorylation of PGC-1α by CK1δ results in decreased PGC-1α protein stability and impairs its induction of the hepatic gluconeogenic program.
Results
PGC-1α interacts with, and is phosphorylated by, CK1δ
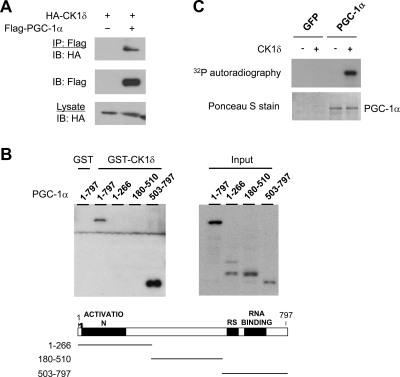
Temporal organization of tissue metabolism is an important aspect of energy homeostasis in mammals. We previously demonstrated that PGC-1α integrates clock and energy metabolism through its regulation of the core components of the clock oscillator (42). Whether PGC-1α transduces timing cues reciprocally from clock to its metabolic targets has not been established. To address this, we examined whether PGC-1α physically interacts with clock components using coimmunoprecipitation assays. These studies led to the identification of casein kinase 1δ (CK1δ), a serine/threonine kinase that plays a central role in clock function, as a novel PGC-1α-interacting protein (Fig. 1A). CK1δ is present in PGC-1α immunocomplexes in transiently transfected cells. We next performed in vitro protein-protein interaction studies to further characterize the interaction between PGC-1α and CK1δ. We incubated recombinant glutathione S-transferase (GST)-CK1δ with various PGC-1α truncation mutants generated using an in vitro transcription/translation kit. Immunoblotting analysis indicates that full-length PGC-1α (1–797) forms a physical complex with GST-CK1δ, but not GST (Fig. 1B). The C-terminal domain of PGC-1α [amino acids (a.a.) 503–797], which contains arginine/serine (RS)-rich domain and RNA recognition motif, strongly associates with CK1δ. We also detected a modest interaction between CK1δ and the N-terminal fragments (a.a. 1–266 and a.a. 180–510) of PGC-1α, which contain transcriptional activation domain and nuclear receptor interaction motifs.
Fig. 1.
Phosphorylation of PGC-1α by CK1δ. A, Physical interaction between PGC-1α and CK1δ. Immunoblotting was performed on total lysates and immunocomplexes from HEK293 cells transiently transfected with HA-CK1δ in the presence or absence of Flag-PGC-1α. IP, Immunoprecipitation; IB, immunoblotting. B, Mapping of PGC-1α domains that interact with CK1δ. In vitro transcribed and translated PGC-1α truncation mutants were incubated with GST or GST-CK1δ before binding to glutathione-agarose beads followed by immunoblotting with Flag antibody. C, In vitro kinase assay. Immunocomplexes from HepG2 cells transduced with GFP and Flag-PGC-1α adenoviruses were incubated with or without CK1δ in the presence of [γ-32P]ATP. Ponceau S stain indicates immunoprecipitated PGC-1α protein.
The physical interaction between CK1δ and PGC-1α raises the possibility that PGC-1α may serve as a substrate for CK1δ and potentially transduce circadian signals to the metabolic regulatory pathways. To test this, we performed an in vitro kinase assay using PGC-1α affinity purified from HepG2 cells transduced with adenovirus expressing Flag-tagged PGC-1α. Control and PGC-1α immunocomplexes were incubated with purified recombinant CK1δ in the presence of [γ32P]ATP. Although no phosphorylation occurred in the absence of CK1δ, we observed robust PGC-1α phosphorylation when CK1δ was added to the kinase reaction (Fig. 1C). These studies identified PGC-1α as a novel interacting protein and substrate of CK1δ.
CK1δ modulates the activation of hepatic gluconeogenesis by PGC-1α
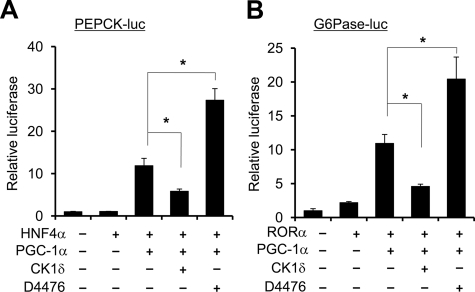
The expression of PGC-1α is induced in the liver in response to starvation through Crtc2-mediated glucagon signaling and cAMP response element-binding protein activation (37, 43, 44). PGC-1α stimulates the expression of gluconeogenic genes and enhances hepatic glucose production, thereby contributing to steady blood glucose levels during fasting. Two of the key enzymes involved in fasting glucose production are phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) (45, 46). The induction of their expression is mediated through the coactivation of several transcription factors by PGC-1α, particularly glucocorticoid receptor, hepatocyte nuclear factor 4α (HNF4α), FoxO1, and RORα (37, 47, 48). We next performed coactivation assays using reporter constructs for PEPCK and G6Pase genes to determine whether CK1δ modulates the transcriptional function of PGC-1α. Similar to previous studies, PGC-1α coactivates hepatocyte nuclear factor 4α on the PEPCK promoter in transiently transfected HepG2 hepatoma cells (Fig. 2A). Compared with control, CK1δ significantly decreased the induction of PEPCK-luciferase reporter activity by PGC-1α. Consistently, inhibition of CK1δ activity by D4476, a cell-permeable inhibitor of CK1δ, further augmented PGC-1α activity by approximately 2-fold (49). D4476 has been shown to be a highly selective inhibitor for CK1δ and CK1ε with IC50 in the nanomolar and low micromolar range, respectively (49–51). Similar results were observed for the G6Pase-luciferase reporter construct (Fig. 2B). In this case, PGC-1α coactivation of RORα is significantly reduced by CK1δ, whereas D4476 has the opposite effect on reporter gene activity. Thus, CK1δ appears to exert an inhibitory effect on the activation of gluconeogenic gene expression by PGC-1α.
Fig. 2.
CK1δ inhibits transcriptional coactivation function of PGC-1α. Luciferase gene assay on PEPCK (A) and G6Pase (B) reporters was performed in transiently transfected HepG2 cells using indicated plasmids. Data represent mean ± sd of one representative experiment performed in triplicate. *, P < 0.05.
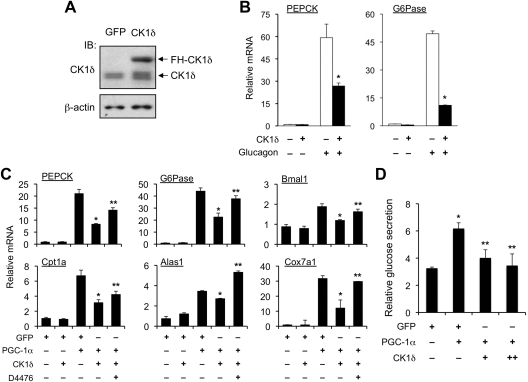
To further address the role of CK1δ in hepatic glucose metabolism, we transduced primary hepatocytes with recombinant adenoviruses expressing green fluorescent protein (GFP) or CK1δ (Ad-CK1δ) and examined the expression of endogenous gluconeogenic genes as well as glucose secretion. Compared with control, transduction of Ad-CK1δ led to an approximately 2- to 3-fold increase in CK1δ protein expression in hepatocytes (Fig. 3A). Adenoviral-mediated overexpression of CK1δ significantly blunted the induction of G6Pase and PEPCK mRNA expression in response to glucagon (Fig. 3B). Glucagon transduces its stimulatory effects on gluconeogenic gene expression in part through PGC-1α. To determine the effects of CK1δ on the induction of gluconeogenic gene expression by PGC-1α, we transduced the hepatocytes with GFP or PGC-1α adenoviruses in the presence or absence of Ad-CK1δ. Gene expression analysis indicated that although CK1δ alone had modest effects on basal gluconeogenic gene expression, it significantly diminished the induction of PEPCK and G6Pase mRNA expression by PGC-1α (Fig. 3C). The induction of Bmal1, Cpt1a, Alas1, and Cox7a1 mRNA expression by PGC-1α was also blunted by CK1δ. D4476 treatment partially restored the induction of target genes by PGC-1α. Consistent with these results, CK1δ significantly dampened the ability of PGC-1α to augment glucose secretion in transduced hepatocytes (Fig. 3D). These studies suggest that phosphorylation of PGC-1α by CK1δ inhibits its stimulatory effects on gluconeogenic and clock gene expression.
Fig. 3.
CK1δ decreases the induction of gluconeogenic genes and glucose production by PGC-1α in primary hepatocytes. A, Adenoviral-mediated expression of CK1δ in transduced primary hepatocytes. B, qPCR analyses of gluconeogenic gene expression in transduced hepatocytes treated with vehicle or 40 nm glucagon for 4 h. C, qPCR analyses of gene expression in transduced hepatocytes. Data represent mean ± sd of one representative experiment performed in triplicate. *, P < 0.01 CK1δ vs. GFP; **, P < 0.05 D4476 vs. vehicle. D, Glucose secretion assay in transduced primary hepatocytes. Relative glucose production was calculated after normalization to protein content. Data represent mean ± sd of one representative experiment performed in triplicate. *, P < 0.05 PGC-1α vs. GFP; **, P < 0.05 CK1δ vs. control. IB, Immunoblotting.
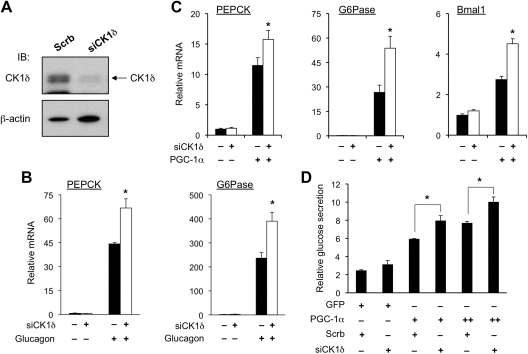
We next examined whether inhibition of endogenous CK1δ augments the transcriptional activity of PGC-1α. We generated a recombinant adenovirus that expressed short hairpin RNA directed toward CK1δ [small interfering CK1δ (siCK1δ)]. Immunoblotting analysis indicates that transduction of primary hepatocytes with siCK1δ adenovirus markedly reduced the expression of endogenous CK1δ (Fig. 4A). Compared with control, the induction of PEPCK and G6Pase mRNA expression after glucagon treatments was significantly augmented in primary hepatocytes transduced with siCK1δ adenovirus (Fig. 4B). Similarly, the ability of PGC-1α to stimulate PEPCK and G6Pase expression was also enhanced by RNA interference knockdown of CK1δ in hepatocytes (Fig. 4C). Measurements of gluconeogenic activity in transduced hepatocytes indicate that CK1δ knockdown further increased glucose production in primary hepatocytes transduced by PGC-1α adenovirus (Fig. 4D). Together, these gain- and loss-of-function studies suggest that CK1δ exerts its effects on gluconeogenic gene expression and glucose secretion through modulating PGC-1α transcriptional activity.
Fig. 4.
RNA interference knockdown of CK1δ augments the induction of gluconeogenesis by PGC-1α. A, Immunoblotting (IB) of total lysates of primary hepatocytes transduced with control (Scrb) or CK1δ short hairpin RNA adenoviruses (siCK1δ). B, qPCR analyses of gluconeogenic gene expression in transduced hepatocytes with or without glucagon treatments. C, qPCR analyses of mRNA expression in primary hepatocytes transduced with PGC-1α adenoviruses together with control (filled bars) or siCK1δ (open bars) adenoviruses. Data represent mean ± sd of one representative experiment performed in triplicate. *, P < 0.05 siCK1δ vs. control. D, Glucose secretion assay in transduced primary hepatocytes. Relative glucose production was calculated after normalization to protein content. Data represent mean ± sd of one representative experiment performed in triplicate. *, P < 0.05.
CK1δ regulates PGC-1α protein stability
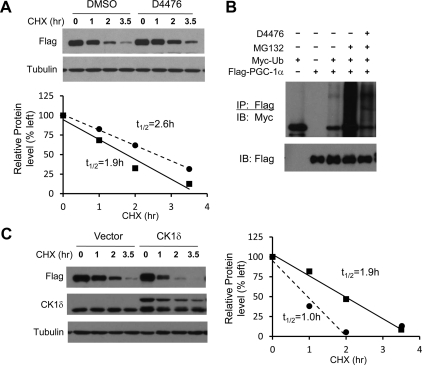
Previous studies have established PGC-1α as a protein that undergoes rapid turnover in the cell (52). The short half-life potentially allows PGC-1α to respond to various physiological signals through altering its protein stability. Because CK1δ appears to affect the expression of multiple targets of PGC-1α, including gluconeogenic and clock genes, we postulated that it might control PGC-1α transcriptional function through modulating its degradation. To test this, we examined the effects of CK1δ on the half-life of PGC-1α in cells treated with cycloheximide, an inhibitor of protein synthesis. Similar to previous studies (53), PGC-1α undergoes rapid turnover with a half-life of approximately 1.9 h. Inhibition of CK1δ activity by D4476 led to stabilization of PGC-1α and increased its half-life to approximately 2.6 h (Fig. 5A). PGC-1α is degraded by the proteasome system after ubiquitination (54). We next performed an in vivo ubiquitination assay to determine whether CK1δ regulates PGC-1α stability through modulating its ubiquitination. We examined the accumulation of ubiquitinated PGC-1α in transiently transfected human embryonic kidney (HEK)293 cells after treatments with MG132, a proteasome inhibitor. As expected, we observed marked accumulation of ubiquitinated PGC-1α in the cells treated with MG132 (Fig. 5B). The degree of PGC-1α ubiquitination was significantly reduced by D4476 treatments. We next asked whether CK1δ accelerates the turnover of PGC-1α. Compared with vector, cotransfection of CK1δ resulted in more rapid disappearance of PGC-1α protein in the presence of cycloheximide. In fact, the half-life of PGC-1α was reduced from 1.9 h to approximately 1.0 h (Fig. 5C). These data strongly suggest that CK1δ modulates the ubiquitination and proteasomal degradation of PGC-1α.
Fig. 5.
CK1δ regulates PGC-1α protein stability. A, Half-life of PGC-1α in the presence of vehicle (solid lines) or D4476 (dashed lines). Transiently transfected HEK293 cells were treated with vehicle or 25 μm D4476 for 4 h before treatment with 40 μm cycloheximide (CHX) for indicated time points. Protein abundance was quantified and normalized to tubulin for the calculation of half-life. B, Ubiquitination of PGC-1α. HEK293 cells transiently transfected with Flag PGC-1α and Myc-ubiquitin were incubated with D4476 for 4 h before treatment with 10 μm MG132 for 4 h. Total cell lysates were immunoprecipitated using anti-Flag agarose beads followed by immunblotting (IB) with anti-Myc antibody. C, Half-life of PGC-1α in the presence of vector (solid lines) or CK1δ (dashed lines). Immunoblots of Flag-PGC-1α in HEK293 cells cotransfected with vector or CK1δ after CHX treatments for the indicated time points. Because PGC-1α level at t =2.0 h is close to baseline (lane 7), the data point at t = 3.5 h (lane 8) was not included for the calculation of the half-life (circle). DMSO, Dimethylsulfoxide; IP, immunoprecipitation.
CK1δ phosphorylates the RS domain of PGC-1α
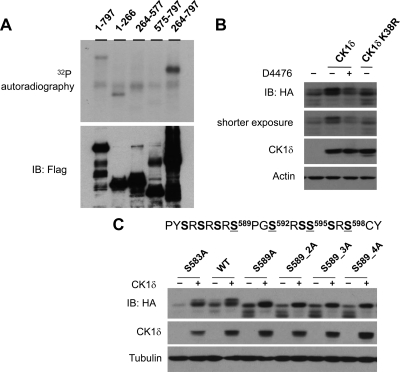
To identify CK1δ phosphorylation sites on PGC-1α, we performed an in vitro kinase assay on various PGC-1α fragments. We found that CK1δ is able to phosphorylate PGC-1α fragments corresponding to a.a. 1–266 and a.a. 264–797 (Fig. 6A), suggesting that CK1δ may phosphorylate multiple sites on PGC-1α. Interestingly, fragments containing a.a. 264–577 and a.a. 575–797 served as poor substrates for CK1δ phosphorylation, suggesting that residues in proximity to the junction site may contain potential CK1δ phosphorylation sites. To facilitate the identification of phosphorylation sites within this section, we constructed a shorter truncation mutant (a.a. 505–797) of PGC-1α, which contains the RS domain and exhibits a mobility shift on SDS-PAGE in response to CK1δ phosphorylation (Fig. 6B). D4476 treatment reduces the migration shift caused by CK1δ expression. Further, this mobility shift is largely absent when a kinase-dead CK1δ (K38R) mutant was included in transient transfection, suggesting that the shift is likely caused by CK1δ phosphorylation.
Fig. 6.
CK1δ phosphorylates the RS domain of PGC-1α. A, In vitro kinase assay. Various truncation mutants of PGC-1α were transiently transfected into HEK293 cells before immunoprecipitation using anti-Flag agarose followed by in vitro kinase assay in the presence of [γ32P]ATP and recombinant CK1δ. B, Mobility shift of PGC-1α in response to CK1δ. Flag/HA-PGC-1α (a.a. 503–797) plasmid was cotransfected with vector, CK1δ, or CK1δ K38R plasmid into HEK293 cells. Cells were harvested after 4 h of treatments with D4476 or vehicle. C, Effects of CK1δ on mobility shift of wild-type (WT), S583A, S589A, S589_2A, S589_3A, and S589_4A PGC-1α mutants. IB, Immunoblotting.
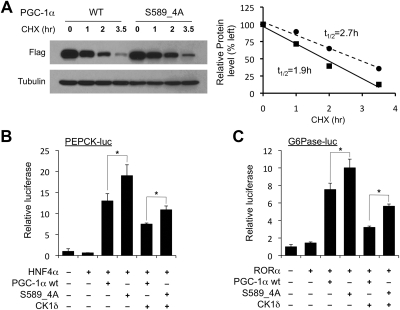
Known substrates for CK1δ frequently contain S/T-X-X-S/T, with N-terminal phosphoserine or threonine serving as a priming site for CK1δ phosphorylation (55, 56). Recent studies have revealed a new substrate motif for CK1δ (K/R-X-K/R-X-X-S/T) (57). Interestingly, the residues N-terminal to S589 (S-R-S-R-S-R-S589)-P-G-S) conform to this new consensus motif. We performed site-directed mutagenesis on S589 and found that mutation of this single residue to alanine (S589A) completely abolished the mobility shift caused by CK1δ (Fig. 6C). As CK1δ typically phosphorylates consecutive serine and/or threonine residues spaced by two amino acids, we generated serine to alanine PGC-1α mutants containing two (S589/S592, S589_2A), three (S589/S592/S595, S589_3A), or all four residues (S589/S592/S595/S598, S589_4A). Mobility analyses of these mutants indicated that they migrate similarly to the S589A mutant after phosphorylation by CK1δ (Fig. 6C), suggesting that S589 phosphorylation is likely critical for the subsequent phosphorylation of S592, S595, and S598. To examine the significance of these CK1δ phosphorylation sites on PGC-1α protein stability, we introduced serine to alanine mutations into the full-length PGC-1α and evaluated its stability in HEK293 cells. Compared with wild-type PGC-1α, the S589_4A mutant had a significantly increased half-life from 1.9 h to approximately 2.7 h (Fig. 7A). Consistently, the S589_4A mutation enhanced the transcriptional activation of the PEPCK and G6Pase reporter genes compared with wild-type PGC-1α (Fig. 7, B and C). In addition, the mutant protein appeared to be partially resistant to the inhibition by CK1δ, suggesting that CK1δ exerts its effects on PGC-1α in part through its phosphorylation of these sites.
Fig. 7.
PGC-1α S589_4A mutant has increased stability and transcriptional function. A, Half-life of wild type (WT) (solid) and S589_4A mutant (dashed) PGC-1α. HEK293 cells transiently transfected with WT or S589_4A mutant PGC-1α were treated with cycloheximide (CHX) for indicated time points. Protein abundance was quantified and normalized to tubulin for the calculation of half-life. B and C, Luciferase reporter gene assay using PEPCK (B) and G6Pase (C) in the presence of WT or S589_4A PGC-1α in transiently transfected HepG2 cells. Data represent mean ± sd of one representative experiment performed in triplicate. *, P < 0.05.
Discussion
It has become increasingly evident that circadian regulation of nutrient and energy metabolism is critical for metabolic homeostasis in mammals. The temporal restriction of metabolic activities to certain times of the day is likely mediated through reciprocal crosstalk between the circadian timing and metabolic regulatory networks. However, the signaling mechanisms underlying the integration of clock and metabolism remain elusive. In this study, we identified PGC-1α as a target of circadian signaling that links the clock to metabolic regulation. PGC-1α physically interacts with, and is phosphorylated at, multiple sites by CK1δ, a key component of the circadian pacemaker. At the functional level, CK1δ represses the induction of gluconeogenic genes and glucose secretion by PGC-1α in primary hepatocytes. Finally, we found that CK1δ phosphorylation of PGC-1α at S589/592/595/598 reduces its half-life and enhances proteasome-mediated degradation.
CK1 family members, particularly CK1δ and CK1ε, play an evolutionarily conserved role in the regulation of clock function. Recent studies have identified a new substrate motif for CK1δ (K/R-X-K/R-X-X-S/T) (57). Because the residues N-terminal to S589 (S-R-S-R-S-R-S589-P-G-S) of PGC-1α conform to this consensus motif, it is likely that S589 phosphorylation may initiate subsequent phosphorylation of C-terminal serine residues (S592, S595, and S598) by CK1δ. Although CK1δ is thought to be a constitutively active kinase, its nuclear localization exhibits diurnal regulation (30, 58). As such, cyclic access to PGC-1α in the nucleus may allow CK1δ to phosphorylate and modulate the turnover of PGC-1α in a rhythmic manner. We cannot exclude the possibility that a priming kinase may be engaged in the regulation of PGC-1α phosphorylation by CK1δ. A potential candidate is Cdc2-like kinase, which is a conserved kinase recently found to phosphorylate the RS domain of PGC-1α and antagonize PGC-1α activity on hepatic gluconeogenesis (59). Because Cdc2-like kinase is regulated by nutritional status, it is possible that its kinase activity may modulate subsequent phosphorylation of PGC-1α by CK1δ.
Phosphorylation of PGC-1α by CK1δ appears to destabilize PGC-1α and shorten its half-life. Consistently, inhibition of endogenous CK1δ by D4476 increased the stability of PGC-1α. Although both N- and C-terminal fragments of PGC-1α appear to contain CK1δ phosphorylation sites, mutation of serine residues within the RS domain (S589/592/595/598) was sufficient to stabilize the protein and render the mutant protein partially resistant to CK1δ inhibition in reporter assays. In contrast to CK1δ, phosphorylation of PGC-1α by p38 MAPK (T262, S265, and T298) stabilizes PGC-1α and augments its stimulatory effects on mitochondrial biogenesis and respiration (53). Similarly, AMP-activated protein kinase phosphorylation augments the transcriptional activity of PGC-1α (60). As such, different phosphorylation signals may generate distinct biological readouts. Interestingly, AKT2-mediated phosphorylation of PGC-1α alters the association of PGC-1α with its target promoters and impairs the induction of hepatic genes involved in gluconeogenesis and fatty acid β-oxidation (61). More recently, PGC-1α was identified as a substrate for S6K1 (62). In this case, the phosphorylation event appears to selectively affect the regulation of gluconeogenesis, but not mitochondrial biogenesis, by PGC-1α. These results underscore the emerging role of PGC-1α as an integrator of circadian and nutritional signals, which fine tune the biological function of PGC-1α in a context- dependent manner.
In hepatocytes, CK1δ represses the induction of gluconeogenic gene expression in response to glucagon, which induces gluconeogenesis in part through PGC-1α (37, 44, 63). Our gain- and loss-of-function studies indicate that CK1δ plays a significant role in modulating the activation of gluconeogenic gene expression and glucose secretion in response to PGC-1α. These data strongly suggest that a major target of CK1δ in the regulation of hepatic metabolism is mediated through its effects on PGC-1α. However, we cannot exclude the possibility that other substrates of CK1δ may also be involved. Of note, previous studies have demonstrated that FoxO1 is a substrate for CK1δ phosphorylation (49). Because FoxO1 regulates several aspects of hepatic glucose and lipid metabolism, it is possible that phosphorylation of this protein may also contribute to the inhibition of gluconeogenesis by CK1δ. In addition, we previously demonstrated that the expression of PGC-1β, a closely related homolog of PGC-1α, is also rhythmically regulated in the liver (42). Similar to PGC-1α, PGC-1β is phosphorylated by CK1δ (our unpublished data), suggesting that the crosstalk between CK1δ and PGC-1 coactivators may be one of mechanisms that link circadian timing cues to metabolic regulation. PGC-1β regulates hepatic triglyceride synthesis and very low density lipoprotein secretion (64, 65). As such, it is likely that CK1δ may also modulate lipoprotein metabolism through its phosphorylation of PGC-1β. CK1δ mutation in humans leads in familial advanced sleep phase syndrome. To date, whether these patients have altered glucose and lipid metabolism has not been reported. Together, our studies have identified a novel pathway that mediates crosstalk between clock and metabolic control.
Materials and Methods
Plasmid constructs
The truncation mutants of PGC-1α were generated using Gateway cloning system into pcDNA3 destination vector containing N-terminal Flag and hemagglutinin (HA) tags. Mutagenesis PCR was performed to create serine to alanine PGC-1α mutants. For the generation of recombinant adenoviruses, CK1δ was cloned into pAdTrack destination construct using the Gateway method. Short-hairpin RNA construct targeting mouse CK1δ (5′-CCATTGAAGTGCTGTGCAA-3′) was cloned downstream of the H1 promoter. The generation, amplification, and transduction of recombinant adenoviruses were carried out as previously described (66).
Cell culture, transient transfection, and Western blotting
HEK293 and HepG2 cells were cultured in DMEM supplemented with 5% and 10% fetal bovine serum, respectively. For transient transfection, HEK293 cells were plated and transfected using Polyethylenimine when they reached approximately 80% confluence. Transfected cells were harvested 40 h after treatments with vehicle, 25 μm D4476 (Tocris Bioscience, Ellisville, MO), 40 μm cycloheximide (Sigma Chemical Co., St Louis, MO) or 10 μm MG132 (Cayman Chemical Co., Ann Arbor, MI) for indicated time before lysis in immunoprecipitation buffer [50 mm Tris-HCl, pH 7.8; 137 mm NaCl; 10 mm NaF; 10 mm Na4P2O7; 1 mm Na3VO4; 1 mm EDTA; 1% Triton X-100; 10% glycerol; and protease inhibitors (Roche, Indianapolis, IN)]. For coimmunoprecipitation studies, total lysates from transfected cells were incubated with anti-Flag agarose beads (Sigma). Protein complexes and total lysates were analyzed by immunoblotting using specific antibodies, including rat monoclonal anti-HA (3F10), mouse anti-CK1δ (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), M2 anti-Flag, rabbit polyclonal anti-Myc, and mouse antiactin (Sigma). To calculate the half-life of PGC-1α, the amount of each protein band was quantified using Image J followed by normalization to tubulin through repeated Western blotting.
Kinase assays
In vitro kinase assays were performed as described previously (67). Briefly, PGC-1α was immunoprecipitated using anti-Flag agarose beads from transiently transfected HEK293 cells, and incubated at 30 C for 20 min in a buffer containing 50 mm 3[N-morpholino]propane sulfonic acid at pH 7.2, 50 mm sodium chloride, 25 mm β-glycerolphosphate, 10 mm magnesium chloride, 1 mm sodium vanadate, 1 mm sodium fluoride, and 100 μm ATP supplemented with 1–5 μCi of [γ-32P]ATP. Purified recombinant CK1δ was purchased from Millipore Corp. (Milford, MA). Kinase reactions were stopped by adding 4× SDS-PAGE sample buffer and boiling at 95 C for 5 min. After SDS-PAGE, the proteins were transferred to nitrocellulose membranes and detected by autoradiograph and immunoblotting.
Primary hepatocyte studies
The maintenance and adenoviral transduction of primary hepatocytes was performed as previously described (42). Total RNA was isolated using Trizol reagents (Invitrogen, Carlsbad, CA) 48 h after transduction, reverse transcribed, and analyzed by quantitative PCR (qPCR) using Sybr-Green (ABI 7300, Applied Biosystems, Foster City, CA). For the measurements of glucose secretion, transduced hepatocytes were incubated with glucose-free DMEM supplemented with 20 mm sodium lactate and 2 mm sodium pyruvate for 3 h before harvesting for glucose assay (Sigma).
qPCR primers is as below:
G6Pase: ACACCGACTACTACAGCAACAG and CCTCGAAAGATAGCAAGAGTAG
PEPCK: CATATGCTGATCCTGGGCATAAC and CAAACTTCATCCAGGCAATGTC
Bmal1: AAAGAGGCGTCGGGACAAA and CCATCCTTAGCACGGTGAGTTT
Cpt1a: GAGAAATACCCTGACTATGTG and TGTGAGTCTGTCTCAGGGCTAG
Alas1: GGCGGATGACTACACGGACTC and GCACCATGCTGTTTCACAGTC
Cox7a1: GTCTCCCAGGCTCTGGTCCG and CTGTACAGGACGTTGTCCATTC
36B4 (control for normalization): GAAACTGCTGCCTCACATCCG and GCTGGCACAGTGACCTCACACG.
Acknowledgments
We thank Shengjuan Gu for technical assistance and members of the laboratory for discussions. We thank Drs. Eisuki Nishida at Kyoto University for providing CK1δ expression constructs. We thank the core support provided by the Michigan Diabetes Research and Training Center (P60 DK020572).
This work was supported by National Institutes of Health Grants DK077086 and HL097738 (to J.D.L.). S.L. and X.W.C. are supported by a Scientist Development Grant and postdoctoral fellowship from the American Heart Association, respectively.
Disclosure Summary: The authors declare no conflict of interest.
NURSA Molecule Pages†:
Coregulators: PGC-1.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- a.a.
- Amino acids
- CK1δ
- casein kinase 1δ
- GFP
- green fluorescent protein
- G6Pase
- glucose-6-phosphatase
- GST
- glutathione S-transferase
- HA
- hemagglutinin
- HEK
- human embryonic kidney
- HNF4α
- hepatocyte nuclear factor 4 alpha
- PEPCK
- phosphoenolpyruvate carboxykinase
- PGC-1α
- peroxisome proliferator-activated receptor γ coactivator 1α
- qPCR
- quantitative PCR
- RORα
- retinoid acid receptor-related orphan receptor alpha
- RS
- arginine/serine
- siCK1δ
- small interfering CK1δ.
References
- 1. Asher G, Schibler U. 2011. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab 13:125–137 [DOI] [PubMed] [Google Scholar]
- 2. Green CB, Takahashi JS, Bass J. 2008. The meter of metabolism. Cell 134:728–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reppert SM, Weaver DR. 2002. Coordination of circadian timing in mammals. Nature 418:935–941 [DOI] [PubMed] [Google Scholar]
- 4. Rutter J, Reick M, McKnight SL. 2002. Metabolism and the control of circadian rhythms. Annu Rev Biochem 71:307–331 [DOI] [PubMed] [Google Scholar]
- 5. Copinschi G, Spiegel K, Leproult R, Van Cauter E. 2000. Pathophysiology of human circadian rhythms. Novartis Found Symp 227:143–157; discussion 157–162 [DOI] [PubMed] [Google Scholar]
- 6. Cutolo M, Otsa K, Aakre O, Sulli A. 2005. Nocturnal hormones and clinical rhythms in rheumatoid arthritis. Ann NY Acad Sci 1051:372–381 [DOI] [PubMed] [Google Scholar]
- 7. Fu L, Lee CC. 2003. The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer 3:350–361 [DOI] [PubMed] [Google Scholar]
- 8. Jones CR, Campbell SS, Zone SE, Cooper F, DeSano A, Murphy PJ, Jones B, Czajkowski L, Ptácek LJ. 1999. Familial advanced sleep-phase syndrome: a short-period circadian rhythm variant in humans. Nat Med 5:1062–1065 [DOI] [PubMed] [Google Scholar]
- 9. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. 2009. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 106:4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spiegel K, Tasali E, Leproult R, Van Cauter E. 2009. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol 5:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. 2005. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308:1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lamia KA, Storch KF, Weitz CJ. 2008. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 105:15172–15177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. 2010. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466:627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. 2004. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2:e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. 2011. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia 54:120–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, Takahashi JS. 2007. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci USA 104:3342–3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. 2002. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109:307–320 [DOI] [PubMed] [Google Scholar]
- 18. Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. 2002. Extensive and divergent circadian gene expression in liver and heart. Nature 417:78–83 [DOI] [PubMed] [Google Scholar]
- 19. Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, Hashimoto S. 2002. A transcription factor response element for gene expression during circadian night. Nature 418:534–539 [DOI] [PubMed] [Google Scholar]
- 20. Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. 2006. Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55:962–970 [DOI] [PubMed] [Google Scholar]
- 21. Doherty CJ, Kay SA. 2010. Circadian control of global gene expression patterns. Annu Rev Genet 44:419–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lowrey PL, Takahashi JS. 2000. Genetics of the mammalian circadian system: Photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu Rev Genet 34:533–562 [DOI] [PubMed] [Google Scholar]
- 23. Reppert SM, Weaver DR. 2001. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol 63:647–676 [DOI] [PubMed] [Google Scholar]
- 24. Gallego M, Virshup DM. 2007. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8:139–148 [DOI] [PubMed] [Google Scholar]
- 25. Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS. 2000. Positional syntenic cloning and functional characterization of the mammalian circadian mutation τ. Science 288:483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ralph MR, Menaker M. 1988. A mutation of the circadian system in golden hamsters. Science 241:1225–1227 [DOI] [PubMed] [Google Scholar]
- 27. Xu Y, Padiath QS, Shapiro RE, Jones CR, Wu SC, Saigoh N, Saigoh K, Ptácek LJ, Fu YH. 2005. Functional consequences of a CKIδ mutation causing familial advanced sleep phase syndrome. Nature 434:640–644 [DOI] [PubMed] [Google Scholar]
- 28. Meng QJ, Logunova L, Maywood ES, Gallego M, Lebiecki J, Brown TM, Sládek M, Semikhodskii AS, Glossop NR, Piggins HD, Chesham JE, Bechtold DA, Yoo SH, Takahashi JS, Virshup DM, Boot-Handford RP, Hastings MH, Loudon AS. 2008. Setting clock speed in mammals: the CK1 ϵ τ mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron 58:78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu Y, Toh KL, Jones CR, Shin JY, Fu YH, Ptácek LJ. 2007. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell 128:59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. 2001. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107:855–867 [DOI] [PubMed] [Google Scholar]
- 31. Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptácek LJ, Fu YH. 2001. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291:1040–1043 [DOI] [PubMed] [Google Scholar]
- 32. Finck BN, Kelly DP. 2006. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 116:615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hock MB, Kralli A. 2009. Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol 71:177–203 [DOI] [PubMed] [Google Scholar]
- 34. Lin J, Handschin C, Spiegelman BM. 2005. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370 [DOI] [PubMed] [Google Scholar]
- 35. Goto M, Terada S, Kato M, Katoh M, Yokozeki T, Tabata I, Shimokawa T. 2000. cDNA Cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun 274:350–354 [DOI] [PubMed] [Google Scholar]
- 36. Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. 1998. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92:829–839 [DOI] [PubMed] [Google Scholar]
- 37. Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. 2001. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413:131–138 [DOI] [PubMed] [Google Scholar]
- 38. Handschin C, Lin J, Rhee J, Peyer AK, Chin S, Wu PH, Meyer UA, Spiegelman BM. 2005. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1α. Cell 122:505–515 [DOI] [PubMed] [Google Scholar]
- 39. Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. 2002. Transcriptional co-activator PGC-1 α drives the formation of slow-twitch muscle fibres. Nature 418:797–801 [DOI] [PubMed] [Google Scholar]
- 40. Vega RB, Huss JM, Kelly DP. 2000. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol 20:1868–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. 1999. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124 [DOI] [PubMed] [Google Scholar]
- 42. Liu C, Li S, Liu T, Borjigin J, Lin JD. 2007. Transcriptional coactivator PGC-1α integrates the mammalian clock and energy metabolism. Nature 447:477–481 [DOI] [PubMed] [Google Scholar]
- 43. Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. 2001. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183 [DOI] [PubMed] [Google Scholar]
- 44. Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. 2005. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437:1109–1111 [DOI] [PubMed] [Google Scholar]
- 45. Chakravarty K, Cassuto H, Reshef L, Hanson RW. 2005. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit Rev Biochem Mol Biol 40:129–154 [DOI] [PubMed] [Google Scholar]
- 46. Hutton JC, O'Brien RM. 2009. Glucose-6-phosphatase catalytic subunit gene family. J Biol Chem 284:29241–29245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chopra AR, Louet JF, Saha P, An J, Demayo F, Xu J, York B, Karpen S, Finegold M, Moore D, Chan L, Newgard CB, O'Malley BW. 2008. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science 322:1395–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. 2003. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423:550–555 [DOI] [PubMed] [Google Scholar]
- 49. Rena G, Bain J, Elliott M, Cohen P. 2004. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep 5:60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Knippschild U, Milne DM, Campbell LE, DeMaggio AJ, Christenson E, Hoekstra MF, Meek DW. 1997. p53 is phosphorylated in vitro and in vivo by the δ and epsilon isoforms of casein kinase 1 and enhances the level of casein kinase 1 δ in response to topoisomerase-directed drugs. Oncogene 15:1727–1736 [DOI] [PubMed] [Google Scholar]
- 51. Mashhoon N, DeMaggio AJ, Tereshko V, Bergmeier SC, Egli M, Hoekstra MF, Kuret J. 2000. Crystal structure of a conformation-selective casein kinase-1 inhibitor. J Biol Chem 275:20052–20060 [DOI] [PubMed] [Google Scholar]
- 52. Puigserver P, Spiegelman BM. 2003. Peroxisome proliferator-activated receptor-γ coactivator 1 α (PGC-1 α): transcriptional coactivator and metabolic regulator. Endocr Rev 24:78–90 [DOI] [PubMed] [Google Scholar]
- 53. Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. 2001. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARγ coactivator-1. Mol Cell 8:971–982 [DOI] [PubMed] [Google Scholar]
- 54. Sano M, Tokudome S, Shimizu N, Yoshikawa N, Ogawa C, Shirakawa K, Endo J, Katayama T, Yuasa S, Ieda M, Makino S, Hattori F, Tanaka H, Fukuda K. 2007. Intramolecular control of protein stability, subnuclear compartmentalization, and coactivator function of peroxisome proliferator-activated receptor γ coactivator 1α. J Biol Chem 282:25970–25980 [DOI] [PubMed] [Google Scholar]
- 55. Flotow H, Graves PR, Wang AQ, Fiol CJ, Roeske RW, Roach PJ. 1990. Phosphate groups as substrate determinants for casein kinase I action. J Biol Chem 265:14264–14269 [PubMed] [Google Scholar]
- 56. Flotow H, Roach PJ. 1989. Synergistic phosphorylation of rabbit muscle glycogen synthase by cyclic AMP-dependent protein kinase and casein kinase I. Implications for hormonal regulation of glycogen synthase. J Biol Chem 264:9126–9128 [PubMed] [Google Scholar]
- 57. Kawakami F, Suzuki K, Ohtsuki K. 2008. A novel consensus phosphorylation motif in sulfatide- and cholesterol-3-sulfate-binding protein substrates for CK1 in vitro. Biol Pharm Bull 31:193–200 [DOI] [PubMed] [Google Scholar]
- 58. Etchegaray JP, Machida KK, Noton E, Constance CM, Dallmann R, Di Napoli MN, DeBruyne JP, Lambert CM, Yu EA, Reppert SM, Weaver DR. 2009. Casein kinase 1 δ regulates the pace of the mammalian circadian clock. Mol Cell Biol 29:3853–3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rodgers JT, Haas W, Gygi SP, Puigserver P. 2010. Cdc2-like kinase 2 is an insulin-regulated suppressor of hepatic gluconeogenesis. Cell Metab 11:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jäger S, Handschin C, St-Pierre J, Spiegelman BM. 2007. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci USA 104:12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li X, Monks B, Ge Q, Birnbaum MJ. 2007. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1α transcription coactivator. Nature 447:1012–1016 [DOI] [PubMed] [Google Scholar]
- 62. Lustig Y, Ruas JL, Estall JL, Lo JC, Devarakonda S, Laznik D, Choi JH, Ono H, Olsen JV, Spiegelman BM. 2011. Separation of the gluconeogenic and mitochondrial functions of PGC-1α through S6 kinase. Genes Dev 25:1232–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jäger S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. 2004. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1α null mice. Cell 119:121–135 [DOI] [PubMed] [Google Scholar]
- 64. Hernandez C, Molusky M, Li Y, Li S, Lin JD. 2010. Regulation of hepatic ApoC3 expression by PGC-1β mediates hypolipidemic effect of nicotinic acid. Cell Metab 12:411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, Newgard CB, Spiegelman BM. 2005. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1β coactivation of SREBP. Cell 120:261–273 [DOI] [PubMed] [Google Scholar]
- 66. Li S, Liu C, Li N, Hao T, Han T, Hill DE, Vidal M, Lin JD. 2008. Genome-wide coactivation analysis of PGC-1α identifies BAF60a as a regulator of hepatic lipid metabolism. Cell Metab 8:105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen XW, Leto D, Xiao J, Goss J, Wang Q, Shavit JA, Xiong T, Yu G, Ginsburg D, Toomre D, Xu Z, Saltiel AR. 2011. Exocyst function is regulated by effector phosphorylation. Nat Cell Biol 13:580–588 [DOI] [PMC free article] [PubMed] [Google Scholar]