Abstract
The histone H3-lysine-4 methyltransferase mixed-lineage leukemia 3 (MLL3) belongs to a large complex that functions as a coactivator of multiple transcription factors, including the bile acid (BA)-activated nuclear receptor, farnesoid X receptor (FXR), a critical player in BA homeostasis. BA-activated FXR induces hepatic expression of small heterodimer partner (SHP), which in turn suppresses expression of BA synthesis genes, Cyp7a1 and Cyp8b1. Thus, MLL3Δ/Δ mice that express a catalytically inactive mutant form of MLL3 display increased BA levels. Recently, we have discovered a distinct regulatory pathway for BA homeostasis, in which p53 independently up-regulates SHP expression in the liver. Here, we show that the MLL3 complex is also essential for p53 transactivation of SHP. Although activated p53 signaling in MLL3+/+ mice results in decreased BA levels through hepatic up-regulation of SHP, these changes are abolished in MLL3Δ/Δ mice. For both HepG2 cells and mouse liver, we also demonstrate that p53 directs the recruitment of different components of the MLL3 complex to the p53-response elements of SHP and that p53-dependent H3-lysine-4-trimethylation of SHP requires MLL3. From these results, we conclude that both FXR- and p53-dependent regulatory pathways for SHP expression in BA homeostasis require the MLL3 complex; thus, the MLL3 complex is likely a master regulator of BA homeostasis. Using a common coregulator complex for multiple transcription factors, which independently control expression of the same gene, might be a prevalent theme in gene regulation and may also play critical roles in assigning a specific biological function to a coregulator complex.
Histone H3-lysine-4 (H3K4)-methylation is a mark for active chromatin, which counters the repressive chromatin milieu imposed by H3K9- and H3K27-methylation in higher eukaryotes (1, 2). Interestingly, as opposed to having a single enzyme responsible for H3K4-methylation in lower eukaryotes [e.g. Su(var), enhancer of zeste, Trithorax (Set)1 in yeasts], mammals have multiple H3K4 methyltransferases, including Set1α, Set1β, mixed-lineage leukemia (MLL)1, MLL2, MLL3/HALR, MLL4/ALR, MLL5, Ash1, Set7/9, SMYD3, and PRDM9 (2). Likely, the complexity of mammalian gene regulation directed the evolution of multiple H3K4 methyltransferases in mammals. Therefore, defining target genes and physiological functions for each of these multiple H3K4 methyltransferases, both of which still remain poorly explored, may lead to better understanding of the complexity of mammalian gene regulation.
MLL1–4 and Set1α/β, as well as Set1, form a family of related complexes, which are collectively named Set1-like complexes (1). Each mammal Set1-like complex contains a unique H3K4-methyltransferase, complex-specific subunits, and a common subcomplex, consisting of RBBP5, ASH2L, WDR5, and DPY-30, that facilitates the H3K4-methyltransferase activity of Set1-like complexes (1). Notably, we have reported the first mammal Set1-like complexes (3, 4), validated and further defined by other groups (5, 6). These complexes contain either MLL3 or MLL4 (4) and play essential roles in transactivation by multiple nuclear receptors and transcription factors (1, 3–10). Unique subunits in these complexes include PA1, PTIP, 53BP1, the H3K27-demethylase UTX, and the transcriptional coactivator ASC-2 (3–12). We named these complexes MLL3 complex and MLL4 complex (4).
Because mammals have multiple H3K4-methyltraferases (1, 2), it is reasonable to hypothesize that each H3K4-methyltransferase enzyme or complex has a distinct spectrum of target genes, thereby having different biological functions. For Set1-like complexes, this likely involves specific interactions between unique subunits in each complex and its target transcription factors (3, 4). For example, likely due to the possession of ASC-2, a protein with two LXXLL motifs (L, leucine and X, any amino acid) that interact with nuclear receptors in a ligand-dependent manner, both MLL3 and MLL4 complexes are believed to function as H3K4-methyltransferase complexes for multiple members of the nuclear receptor superfamily (3, 4, 7–9). In line with this argument, our analysis of MLL3Δ/Δ mice expressing a catalytically inactive mutant form of MLL3 (4) revealed that MLL3 has a selective set of biological functions, including bile acid (BA) homeostasis, hepatic lipogenesis and adipogenesis, and tumor suppression (7–10). PTIP, another unique subunit of MLL3/4 complexes, has also been shown to link the transcription factor Pax2 to MLL3/4 complexes (13). The product of the multiple endocrine neoplasia type 1 tumor suppressor gene, menin, an integral component of MLL1/2 complexes, has been reported to present MLL1/2 complexes to estrogen receptor α (14). Similarly, β-catenin associates with MLL1 and MLL2 (15), E2F6 associates with MLL1 (16), and Set1α associates with the viral transcription factor VP16 (17).
One of the most prominent metabolic phenotypes in MLL3Δ/Δ mice was an increase in BA pool size, and we have shown that this is, at least in part, due to the critical roles of the MLL3 complex as a coactivator of the BA-activated nuclear receptor, farnesoid X receptor (FXR) (9). BA-activated FXR induces hepatic expression of small heterodimer partner (SHP), which in turn suppresses expression of the BA synthesis genes Cyp7a1 and Cyp8b1 (18). FXR also regulates a series of other genes involved in BA homeostasis (18). Our results have been independently confirmed by another group (19). Interestingly, this study also found that expression of MLL3/4 is decreased at 1 and 3 d of postcommon bile duct ligation in mice, further suggesting possible roles of MLL3/4 in BA homeostasis (19).
We have recently found a unique metabolic regulatory axis that unexpectedly couples p53 to BA homeostasis. We have shown that p53 lowers BA levels under both normal and stressed conditions primarily through up-regulating SHP expression (20). In a separate study, we have also found a direct interaction between p53 and MLL3/4 complexes and discovered that these complexes can act as a p53 coactivator and be required for H3K4-trimethyation and expression of endogenous p53-target genes in response to the DNA-damaging agent doxorubicin (DXR) (10). These results prompted us to test whether MLL3/4 complexes also function as a critical coactivator of p53 in inducing expression of SHP in the liver.
In this report, we show that the MLL3 complex plays essential roles in inducing SHP expression in the liver in response to DXR. These results, along with our previous results that show crucial roles of the MLL3 complex as a coactivator of FXR in BA homeostasis (9), led us to conclude that the MLL3 complex is likely a master regulator of BA homeostasis. Overall, our results raise an interesting possibility that the MLL3 complex has evolved as a specific H3K4-methyltransferase coactivator complex for BA homeostasis due to its ability to act as a common coactivator complex for multiple transcription factors, which independently control expression of SHP. Importantly, this may represent a recurring strategy in evolution in assigning a specific biological function to a coregulator complex.
Results
DXR-dependent expression of SHP in HepG2 cells requires MLL3
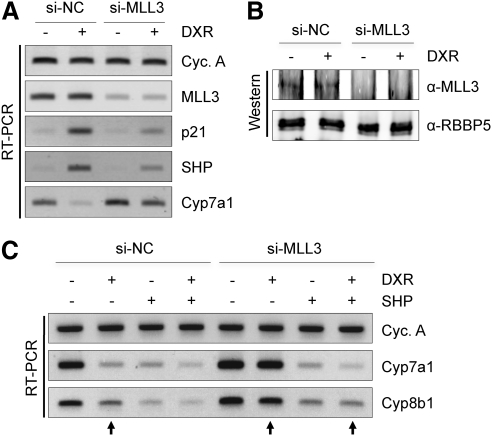
We have recently reported that activation of p53-signaling by DXR results in induction of SHP expression, leading to decreased expression of two key BA synthesis enzymes, Cyp7a1 and Cyp8b1, and therefore a significant decrease in BA pool size (20). Because we have also reported that the MLL3 complex can function as a coactivator of p53 (10), although it is not clear whether the MLL3 complex is needed for all target genes of p53, and that the MLL3 complex has been implicated in BA homeostasis through its coactivator function for FXR (9), we hypothesized that the MLL3 complex may also act as a coactivator of p53 in inducing expression of SHP. To test this idea, we first expressed our previously described si-MLL3 (4) in human hepatoma HepG2 cells. This si-MLL3 readily decreases both MLL3 mRNA and protein levels in HepG2 cells (Fig. 1, A and B). DXR treatment led to up-regulation of p21 and SHP as well as down-regulation of Cyp7a1, as we have reported (Fig. 1A) (20). Interestingly, these responses were significantly blunted by knockdown of MLL3. DXR-induced levels of p21 and SHP in the presence of si-MLL3 were much lower than those in the presence of control small interfering RNA (siRNA) (si-NC) (Fig. 1A). Accordingly, both basal and DXR-induced levels of Cyp7a1 in the presence of si-MLL3 were higher than those in the presence of control siRNA (Fig. 1). To show that SHP is a critical downstream effector of MLL3 in DXR-mediated suppression of Cyp7a1/8b1 levels, we tested whether reexpression of SHP restores the inhibitory effect of DXR on Cyp7a1/8b1 levels in HepG2 cells expressing si-MLL3. Importantly, overexpression of SHP led to suppression of Cyp7a1/8b1 levels even in the presence of DXR and si-MLL3 (compare three arrowed lanes in Fig. 1C). Taken together, these results suggest that the MLL3 complex is likely required for p53 transactivation of SHP in HepG2 cells, leading to up-regulation of SHP and down-regulation of Cyp7a1/8b1 levels.
Fig. 1.
MLL3 is required for DXR to up-regulate expression of SHP in HepG2 cells. A and C, RT-PCR measurement of DXR-induced or DXR-repressed levels of mRNA for cyclophilin A (loading control), MLL3, p21, SHP, Cyp7a1, and Cyp8b1 of HepG2 cells transfected with control siRNA (si-NC) or si-MLL3. Reexpression of SHP led to suppression of Cyp7a1/8b1 levels even in the presence of DXR and si-MLL3 (C). B, RBBP5-associated proteins were isolated from HepG2 cells transfected with control siRNA (si-NC) or si-MLL3 using immunoprecipitation with RBBP5 antibody, followed by immunoblotting analysis with MLL3 and RBBP5 antibodies. All experiments were repeated more than three times with similar results.
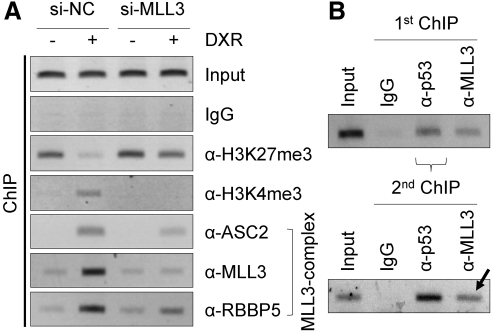
To further test this possibility, we decided to carry out chromatin immunoprecipitation (ChIP) experiments in HepG2 cells and determine whether the MLL3 complex is recruited to p53-response elements of SHP (SHP-p53REs) (20). In support of our hypothesis that the MLL3 complex is a coactivator of p53 for transactivating SHP, we observed that three subunits of the MLL3 complex (ASC-2, MLL3, and RBBP5) are recruited to SHP-p53REs in response to DXR treatment in the presence of control siRNA (Fig. 2A). Interestingly, knockdown of MLL3 using si-MLL3 decreased the amount of not only MLL3 but of ASC-2 and RBBP5 recruited to SHP-p53REs as well (Fig. 2A). These results suggest two interesting possibilities that are not mutually exclusive. First, MLL3 may play an important role for the structural integrity of the MLL3 complex. In support of this possibility, Set1/MLL1–4 proteins are known to interact with multiple core subunits of Set1-like complexes (21–25). Second, it is also possible that p53 recruits the MLL3 complex via direct contact(s) with MLL3, in addition to our previously described interactions between p53 and 53BP1, the p53-interacting subunit of MLL3/4 complexes (10). Consistent with the impaired recruitment of the MLL3 complex, DXR-induced H3K4-trimethylation was abolished by si-MLL3 (Fig. 2A). Moreover, basal levels of H3K27-trimethylation were increased by si-MLL3 and, although H3K27-trimethylation levels were reduced by DXR, the decrease in the presence of si-MLL3 was not as prominent as that in the presence of control siRNA (Fig. 2A). To demonstrate that p53 and the MLL3 complex coexist on SHP-p53REs, we carried out double ChIP experiments in HepG2 cells. We first immunopurified chromatin associated with p53 using p53 antibody (Fig. 2B). We used IgG and MLL3 antibody as negative and positive controls, respectively. Next, the p53-associated chromatin was subjected to a second ChIP for IgG as well as p53 and MLL3 antibodies. Importantly, MLL3 was readily detected in the second ChIP (Fig. 2B, arrow). These results demonstrate that MLL3 is bound to SHP-p53REs together with p53. Taken together, these results strongly suggest that the MLL3 complex functions as a coactivator of p53 in inducing expression of SHP in HepG2 cells by occupying SHP-p53REs in a p53-dependent manner.
Fig. 2.
MLL3 complex is a coactivator of p53 in regulating SHP expression. A, ChIP analysis of HepG2 cells treated with vehicle or 0.5 μm DXR for 24 h to examine establishment of active chromatin and recruitment of the MLL3 complex to SHP-p53REs. B, ChIP analysis of HepG2 cells treated with 0.5 μm DXR for 24 h, followed by second ChIP of p53-associated chromatin by IgG and p53 and MLL3 antibodies to examine coassociation of p53 and MLL3 with SHP-p53REs. All experiments were repeated more than three times with similar results.
Requirement of MLL3 in DXR-dependent expression of SHP in the mouse liver
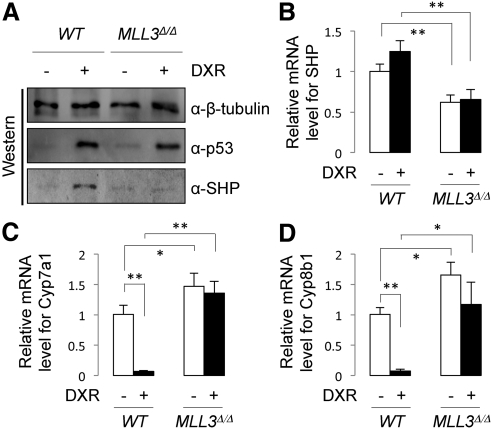
To test whether our HepG2 cell results can be extended to the liver, we turned to our previously described MLL3Δ/Δ mice (4). As we have reported (20), SHP proteins were readily observed in wild-type mice treated with DXR for 24 h (Fig. 2A). In support of our results with HepG2 cells (Fig. 1), this induction was abolished in MLL3Δ/Δ mice, although comparable amounts of p53 proteins were observed between wild-type and MLL3Δ/Δ mice (Fig. 3A). We have previously demonstrated that the observation of SHP proteins at 24 h after DXR treatment involves at least two mechanisms. An initial p53-dependent increase in SHP mRNA levels through SHP-p53REs, which peaks at 2 h after DXR treatment, followed by stabilization of SHP proteins by p53 signaling (20). Consistent with this report (20), there was not a statistically significant response in SHP mRNA levels to DXR at 24 h after treatment. However, we still observed much lower levels of SHP mRNA in MLL3Δ/Δ livers than in wild-type livers (Fig. 3B), which is consistent with the requirement of MLL3 in basal hepatic expression levels of SHP (9). More importantly, in support of our immunoblotting results (Fig. 3A), we observed significantly higher levels of Cyp7a1 and Cyp8b1 mRNA in MLL3Δ/Δ mice relative to wild-type mice, and DXR failed to suppress Cyp7a1/8b1 mRNA levels in MLL3Δ/Δ mice (Fig. 3, C and D). These results provide in vivo evidence for our finding in HepG2 cells that MLL3 is required for DXR-dependent expression of SHP, which in turn leads to negative modulation of Cyp7a1 and Cyp8b1 expression (Fig. 1).
Fig. 3.
MLL3 is required for DXR to up-regulate expression of SHP in the liver. A, Western blot analysis of hepatic expression of β-tubulin (loading control), p53, and SHP in wild-type (WT) and MLL3Δ/Δ mice killed 24 h after ip injection of saline or DXR. B–D, qRT-PCR measurement of mRNA levels for SHP (B), Cyp7a1 (C), and Cyp8b1 (D) of wild-type and MLL3Δ/Δ male mice (n = 3–4), 24 h after ip injection of saline or DXR.
Recruitment of the MLL3 complex to SHP-p53REs in the mouse liver
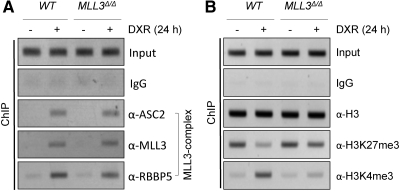
To determine whether DXR-dependent induction of SHP expression in wild-type mice (Fig. 3A) involves hepatic recruitment of the MLL3 complex to SHP-p53REs, we performed ChIP for liver lysates of wild-type mice. All three subunits of the MLL3 complex that we have tested, ASC-2, MLL3, and RBBP5, were indeed recruited to SHP-p53REs upon DXR treatment (Fig. 4A). Consistent with these results, DXR treatment increased H3K4-trimethylation levels of the region containing SHP-p53REs in wild-type mice while decreasing H3K27-trimethylation levels (Fig. 4B). In MLL3Δ/Δ mice, these DXR-dependent alterations in H3K4- and H3K27-trimethylation levels were blunted (Fig. 4B). Interestingly, recruitment of ASC-2, MLL3Δ (the mutant MLL3 protein in MLL3Δ/Δ mice; see Ref. 4), and RBBP5 was not significantly affected in MLL3Δ/Δ mice (Fig. 4A), unlike the dramatic decrease in recruitment of the MLL3 complex in HepG2 cells treated with si-MLL3 (Fig. 2A). These results support our previous finding that expression of MLL3Δ protein is not impaired and MLL3Δ protein is readily incorporated into the complex in MLL3Δ/Δ mice (4). In contrast, a reduction in MLL3 protein may cause problems in assembly of the complex and/or interfere with recruitment of the MLL3 complex to p53 (Fig. 2A). Overall, these results provide in vivo evidence that the MLL3 complex is recruited to SHP-p53REs in a p53-dependent manner and likely plays important roles in inducing SHP expression by p53 via contributing to formation of active chromatin in SHP.
Fig. 4.
MLL3 directs DXR-dependent regulation of the SHP promoter in the liver. A and B, ChIP analysis of liver tissues of wild-type (WT) and MLL3Δ/Δ mice killed 24 h after ip injection of saline or DXR to examine recruitment of the MLL3 complex to SHP-p53REs (A) and establishment of active chromatin (B). All experiments were repeated more than three times with similar results.
DXR-mediated BA homeostasis is impaired in MLL3Δ/Δ mice
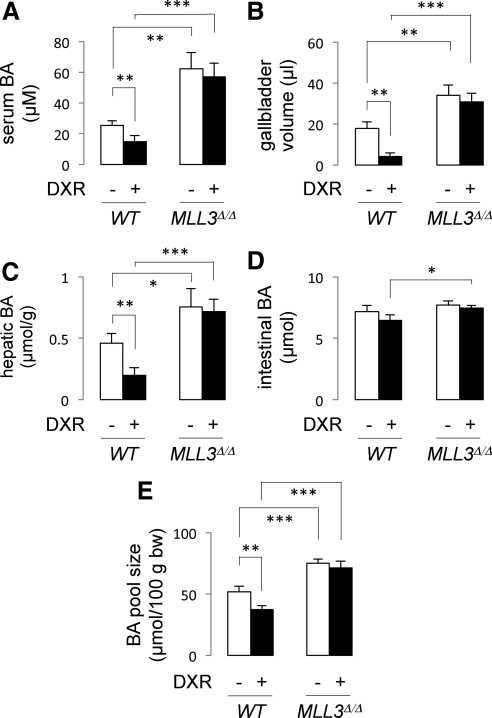
We have recently shown that p53 lowers the BA pool size in wild-type mice through inducing expression of SHP and down-regulating expression of Cyp7a1 and Cyp8b1 (20). Because our results suggest that this regulation requires the MLL3 complex, we expect to observe less responsiveness of MLL3Δ/Δ mice to DXR in BA homeostasis. To test this possibility, we measured BA levels in serum, gallbladder, liver, and intestines of both wild-type and MLL3Δ/Δ mice. As we have reported (20), DXR treatment significantly lowered BA levels in serum, gallbladder, and liver but not in intestines (Fig. 5, A–D). As a consequence, DXR treatment lowered the overall BA pool size in wild-type mice (Fig. 5E). In MLL3Δ/Δ mice, two prominent features were observed. First, BA levels in serum, gallbladder, and liver, but not in intestine, were significantly higher in comparison with wild-type mice (Fig. 5, A–D). Second, DXR-dependent decreases of BA levels in serum, gallbladder, and liver were no longer observed. Correspondingly, the BA pool size of MLL3Δ/Δ mice was significantly higher than that of wild-type mice and did not decrease upon DXR treatment (Fig. 5E). These results clearly demonstrate that MLL3 plays essential roles in BA homeostasis directed by p53.
Fig. 5.
MLL3Δ/Δ mice are not as responsive as wild-type mice to DXR in regulating BA homeostasis. A–D, Quantitation of serum (A), hepatic (C), and intestinal (D) BA levels as well as gallbladder volume (B) of wild-type (WT) and MLL3Δ/Δ mice (n = 3–4) measured 24 h after ip injection of saline or DXR. E, BA levels in the intestine, gallbladder, and liver were combined together to assess the BA pool size.
Discussion
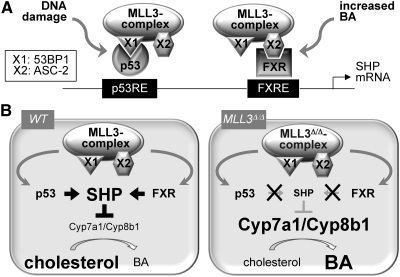
We (9) and more recently Ananthanarayanan et al. (19) have reported that the MLL3 complex functions as a coactivator of FXR in the liver, regulating expression of SHP and other hepatic target genes of FXR involved in BA homeostasis. Our studies included characterization of MLL3Δ/Δ mice expressing a catalytically inactive mutant form of MLL3 (4), and surprisingly, one of the major phenotypes in these mice was a dramatic increase in BA levels (9). Interestingly, we have recently found another independent regulatory pathway in BA homeostasis, which involves direct regulation of SHP mRNA expression by p53 through SHP-p53REs followed by subsequent stabilization of the SHP protein by p53 signaling (20). In this report, we presented our findings that this p53 regulation of SHP expression in the liver also requires the MLL3 complex (Fig. 6A). As a consequence, MLL3Δ/Δ mice failed to respond to DXR in decreasing BA levels (Fig. 6B). These findings, together with the results showing critical roles of the MLL3 complex in FXR-mediated BA homeostasis (9, 19) and the identification of deregulated BA homeostasis as a major phenotype in MLL3Δ/Δ mice (9), suggest that the MLL3 complex is likely a master regulator of BA homeostasis (Fig. 6B). Importantly, our results raise an interesting possibility that regulation of multiple transcription factors, which independently modulate expression of the same gene, by a common coregulator complex could be a prevalent theme in gene regulation that plays key roles in assigning a specific function to a coregulator complex. Because expression of SHP is highly regulated by diverse signaling pathways (18), it will be interesting to determine whether the MLL3 complex also functions as a coactivator of other transcription factors that link those signaling pathways to regulation of SHP expression and BA homeostasis. Notably, we have previously reported that the MLL3 complex is likely a tumor suppressor (10), which is consistent with recent reports of frequent somatic mutations in MLL3, MLL4, and UTX in human cancers (26–29), and that this complex can function as a coactivator of p53 in inducing expression of the cell cycle inhibitor p21 (10). Because p21 is also subjected to multiple regulatory pathways, including positive regulation by two nuclear receptors, retinoic acid receptor (RAR) and vitamin D receptor (30), it will be interesting to test whether the MLL3 complex also serves as a common coactivator of p53, RAR, and vitamin D receptor in inducing expression of p21. Of note, the MLL3 complex plays critical roles in RAR transactivation (3, 4).
Fig. 6.
Model for MLL3 complex action in BA homeostasis. A, In response to a DNA-damaging agent, such as DXR, and increased BA levels, the MLL3 complex is recruited to p53 and FXR, respectively, via specific adaptor proteins: X1/53BP1 for p53 and X2/ASC-2 for FXR. B, In wild-type (WT) mice, the MLL3 complex mediates p53 and FXR transactivation of SHP leading to decreased levels of Cyp7a1/8b1 in the liver and a reduction in BA pool size. In MLL3Δ/Δ mice, up-regulation of SHP is impaired due to the inactivating mutation in MLL3, leading to an increase in Cyp7a1/8b1 levels and BA pool size.
We have previously shown that, although SHP mRNA peaks at 2 h after DXR treatment in the mouse liver, SHP protein is highest at 24 h after DXR treatment, because the initial increase in SHP mRNA levels by p53 transactivation of SHP is coupled to stabilization of SHP protein by p53 signaling (20). As a consequence, Cyp7a1/8b1 levels are lowest at 24 h after DXR treatment (20). It is interesting to note that SHP is not detected even at 24 h after DXR treatment in MLL3Δ/Δ mice (Fig. 3A). These results suggest that the initial transcriptional activation of SHP by p53 is essential for detecting SHP protein at 24 h after DXR treatment, unless MLL3, a transcriptional coactivator, is also involved with stabilization of SHP protein by p53 signaling.
Our previous results with HepG2 cells and mouse embryonic fibroblasts cells revealed that MLL3 appears to be important for p53 transactivation of two p53 target genes: p21 and Mdm2 (10). Interestingly, in the liver of MLL3Δ/Δ mice, expression of p21, but not Mdm2 expression, was affected (Fig. 1A and data not shown). In addition, apart from binding directly to p53 and effecting p53-dependent H3K4 methylation of chromatin templates, the MLL1 complex stimulates p53-dependent transcription from a chromatin template in a reconstituted in vitro transcription system (16). Taken together, these results suggest that the MLL3 complex is not likely to regulate all p53 target genes but regulates them in a cell-type specific manner.
The MLL4 complex appears to be identical to the MLL3 complex except for the presence of MLL4 instead of MLL3. These two complexes share ASC-2, an adaptor protein that recruits MLL3/4 complexes to many members of the nuclear receptor superfamily, including RAR, FXR, liver X receptor, and peroxisome proliferator-activated receptor-γ, through its two LXXLL motifs (12). Consistent with these results, ASC-2 (indicated as X2 in Fig. 6) appears to be involved in recruiting both MLL3 and MLL4 complexes to FXR (9). ASC-2 also appears to play important roles in recruiting MLL3 and MLL4 to p53, but this likely involves interactions between ASC-2 and 53BP1, the p53-binding subunit of MLL3/4 complexes (indicated as X1 in Fig. 6) (10). However, it is also possible that other unique subunits of MLL3/4 complexes, including the MLL3/4 subunits themselves, could make additional contacts with FXR and p53 and that these interactions may play additional, important roles in recruiting MLL3/4 complexes to FXR/p53. Because MLL3 and MLL4 appear to function redundantly with FXR and p53 (9, 10), any additional contact of FXR/p53 with MLL3/4 may involve the conserved regions between MLL3 and MLL4. Overall, these results suggest that both MLL3- and MLL4 complexes are likely to function redundantly with inducing expression of SHP in response to DXR or other activators of p53 signaling. Although this notion remains to be formally determined in vivo, our newly established mutant mouse line for MLL4 is expected to facilitate this study.
In conclusion, we have found that the MLL3 complex is a pivotal hepatic coactivator of p53 in inducing expression of SHP to impinge on BA homeostasis. These results, coupled with the previous findings that implicate the MLL3 complex as a key coactivator of FXR in independently inducing expression of SHP (9, 19), identify the MLL3 complex as a likely master regulator of BA homeostasis. These results are consistent with our initial observation regarding deregulated BA homeostasis as a major phenotype in MLL3Δ/Δ mice (9). Notably, expression of MLL3 and MLL4 are down-regulated in an animal cholestasis model (19), suggesting that MLL3/4, which are critical regulators of BA homeostasis, are targeted for deregulation in pathogenic states. It will be interesting to examine whether other subunits of MLL3/4 complexes also show altered hepatic expression patterns in diseased states. Our results suggest that MLL3, MLL4, and UTX, three enzymes in MLL3/4 complexes, may serve as novel targets in development of drugs to treat diseases resulting from deregulated BA homeostasis, including cholestasis.
Materials and Methods
RT/quantitative RT-PCR (qRT-PCR)
HepG2 cells were transfected with control siRNA or si-MLL3 (sense strand, GCUUAUCUUCCUGUCCAGU) using calcium phosphate method. At 48 h after transfection, cells were treated with 0.5 μm DXR and incubated for 24 h before isolating total RNA. Total RNA from HepG2 cells and liver samples were isolated after lysis in TRIzol reagent according to the manufacturer's protocol (Invitrogen, Carlsbad, CA), and RT-PCR/qRT-PCR was performed as described previously (31). The primers for human p21 were 5′-ACTTCCTCCTCCCCACTTGT-3′ and 5′-AGGTGAGGGGACTCCAAAGT-3′. The primers for mouse Cyp8b1 were 5′-GGACAGCCTATCCTTGGTGA-3′ and 5′-CGGAACTTCCTGAACAGCTC-3′. The primers for human Cyp8b1 were 5′-TCATTGCTGGATACCTGTGC-3′ and 5′-GGTCCATGACGAAGGTGAAG-3′. The primers for human MLL3, SHP, Cyp7a1, and mouse SHP and Cyp7a1 were as previously described (9).
ChIP/re-ChIP
ChIP and re-ChIP assays for HepG2 cells and mouse livers were performed as described (20, 32). The antibodies used for ChIP/re-ChIP assays were α-H3K27me3 antibody (Ab6002; Abcam, Cambridge, MA), α-H3K4me3 (ab8580; Abcam), and p53 antibody (FL393; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The antibodies against ASC-2, MLL3, and RBBP5 were as described (8). The primers for human SHP-p53REs were 5′-TGGAGTGCAGTCGCACTATC-3′ and 5′-CCTGAGGTCAGGAGTTCGAG-3′. The primers for mouse SHP-p53REs were 5′-GGTGGTGCCATCTCTGGGCT-3′ and 5′-GCAAGTTGGGGAGGAAAGGGT-3′.
Animals
Mice were housed in a pathogen-free animal facility under a standard 12-h light, 12-h dark cycle. Mice were allowed to ad libitum access to water and regular rodent chow. Eight- to 10-wk-old male C57BL/6–129S6 mice (MLL3+/+ or MLL3Δ/Δ) were used for all experiments, and 10 mg/kg of DXR was administered by ip injection in a constant volume of saline.
Measurements of BA levels and gallbladder volume
To extract BA from the liver, the gallbladder, including the region of liver immediately surrounding the gallbladder or the entire small intestine, was weighed and homogenized in 75% ethanol. The resulting homogenate was incubated at 50 C for 2 h to extract BA and centrifuged at 6000 × g for 10 min at 4 C. The BA content of all samples, including serum, was measured using a Colormetric Total Bile Acid Assay kit (Diazyme Laboratories, Poway, CA). For the volume of the gallbladder, the weight of the contents squeezed out from each gallbladder was measured.
Western blotting
Liver tissues were homogenized in lysis buffer consisting of 50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1 mm DTT, 0.5 mm EDTA, 1% Nonidet P-40, 10% glycerol, and a protease inhibitor cocktail tablet (Complete EDTA-free; Roche, Indianapolis, IN). Western blot analyses were carried out using antibodies against β-tubulin (sc-5274; Santa Cruz Biotechnology, Inc.), p53 (FL-393; Santa Cruz Biotechnology, Inc.), SHP (SC-30169; Santa Cruz Biotechnology, Inc.), RBBP5 (A300-109A, Bethyl), and MLL3 (8).
Statistical analysis
Statistical differences were determined by Student's t test. Statistical significance is displayed as P < 0.05 (one asterisk), P < 0.01 (two asterisks), or P < 0.001 (three asterisks).
Acknowledgments
We thank Alexandria Harrold for critically reading the manuscript.
This work was supported by the National Institutes of Health Grant DK064678 (to J.W.L.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BA
- Bile acid
- ChIP
- chromatin immunoprecipitation
- DXR
- doxorubicin
- FXR
- farnesoid X receptor
- H3K4
- H3-lysine-4
- MLL
- mixed-lineage leukemia
- qRT-PCR
- quantitative RT-PCR
- Set
- Su(var), enhancer of zeste, Trithorax
- SHP
- small heterodimer partner
- SHP-p53REs
- p53-response elements of SHP
- RAR
- retinoic acid receptor
- siRNA
- small interfering RNA.
References
- 1. Shilatifard A. 2006. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem 75:243–269 [DOI] [PubMed] [Google Scholar]
- 2. Malik S, Bhaumik SR. 2010. Mixed lineage leukemia: histone H3 lysine 4 methyltransferases from yeast to human. FEBS J 277:1805–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goo YH, Sohn YC, Kim DH, Kim SW, Kang MJ, Jung DJ, Kwak E, Barlev NA, Berger SL, Chow VT, Roeder RG, Azorsa DO, Meltzer PS, Suh PG, Song EJ, Lee KJ, Lee YC, Lee JW. 2003. Activating signal cointegrator 2 belongs to a novel steady-state complex that contains a subset of trithorax group proteins. Mol Cell Biol 23:140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee S, Lee DK, Dou Y, Lee J, Lee B, Kwak E, Kong YY, Lee SK, Roeder RG, Lee JW. 2006. Coactivator as a target gene specificity determinant for histone H3 lysine 4 methyltransferases. Proc Natl Acad Sci USA 103:15392–15397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cho YW, Hong T, Hong S, Guo H, Yu H, Kim D, Guszczynski T, Dressler GR, Copeland TD, Kalkum M, Ge K. 2007. PTIP associates with MLL3- and MLL4-containing histone H3 lysine 4 methyltransferase complex. J Biol Chem 282:20395–20406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Issaeva I, Zonis Y, Rozovskaia T, Orlovsky K, Croce CM, Nakamura T, Mazo A, Eisenbach L, Canaani E. 2007. Knockdown of ALR (MLL2) reveals ALR target genes and leads to alterations in cell adhesion and growth. Mol Cell Biol 27:1889–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee S, Lee J, Lee SK, Lee JW. 2008. Activating signal cointegrator-2 is an essential adaptor to recruit histone H3 lysine 4 methyltransferases MLL3 and MLL4 to the liver X receptors. Mol Endocrinol 22:1312–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee J, Saha PK, Yang QH, Lee S, Park JY, Suh Y, Lee SK, Chan L, Roeder RG, Lee JW. 2008. Targeted inactivation of MLL3 histone H3-lysine-4 methyltransferase activity in the mouse reveals vital roles for MLL3 in adipogenesis. Proc Natl Acad Sci USA 105:19229–19234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim DH, Lee J, Lee B, Lee JW. 2009. ASCOM controls farnesoid X receptor transactivation through its associated histone H3 lysine 4 methyltransferase activity. Mol Endocrinol 23:1556–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee J, Kim DH, Lee S, Yang QH, Lee DK, Lee SK, Roeder RG, Lee JW. 2009. A tumor suppressive coactivator complex of p53 containing ASC-2 and histone H3-lysine-4 methyltransferase MLL3 or its paralogue MLL4. Proc Natl Acad Sci USA 106:8513–8518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swigut T, Wysocka J. 2007. H3K27 demethylases, at long last. Cell 131:29–32 [DOI] [PubMed] [Google Scholar]
- 12. Lee SK, Anzick SL, Choi JE, Bubendorf L, Guan XY, Jung YK, Kallioniemi OP, Kononen J, Trent JM, Azorsa D, Jhun BH, Cheong JH, Lee YC, Meltzer PS, Lee JW. 1999. A nuclear factor, ASC-2, as a cancer-amplified transcriptional coactivator essential for liganddependent transactivation by nuclear receptors in vivo. J Biol Chem 274:34283–34293 [DOI] [PubMed] [Google Scholar]
- 13. Patel SR, Kim D, Levitan I, Dressler GR. 2007. The BRCT-domain containing protein PTIP links PAX2 to a histone H3, lysine 4 methyltransferase complex. Dev Cell 13:580–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dreijerink KM, Mulder KW, Winkler GS, Hoppener JW, Lips CJ, Timmers HT. 2006. Menin links estrogen receptor activation to histone H3K4 trimethylation, Cancer Res 66:4929–4935 [DOI] [PubMed] [Google Scholar]
- 15. Sierra J, Yoshida T, Joazeiro CA, Jones KA. 2006. The APC tumor suppressor counteracts β-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev 20:586–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dou Y, Milne TA, Tackett AJ, Smith ER, Fukuda A, Wysocka J, Allis CD, Chait BT, Hess JL, Roeder RG. 2005. Physical association and coordinate function of the H3 K4 methyltransferase MLL1 and the H4 K16 acetyltransferase MOF. Cell 121:873–885 [DOI] [PubMed] [Google Scholar]
- 17. Wysocka J, Herr W. 2003. The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem Sci 28:294–304 [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Hagedorn CH, Wang L. 2011. SHP review. Role of nuclear receptor SHP in metabolism and cancer. Biochim Biophys Acta 1812:893–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ananthanarayanan M, Li Y, Surapureddi S, Balasubramaniyan N, Ahn J, Goldstein JA, Suchy FJ. 2011. Histone H3K4 trimethylation by MLL3 as part of ASCOM complex is critical for NR activation of bile acid transporter genes and is downregulated in cholestasis. Am J Physiol Gastrointest Liver Physiol 300:G771–G781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim DH, Lee JW. 2011. The tumor suppressor p53 regulates bile acid homeostasis via small heterodimer partner. Proc Natl Acad Sci USA 108:12266–12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, Allis CD, Roeder RG. 2006. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol 13:713–719 [DOI] [PubMed] [Google Scholar]
- 22. Patel A, Vought VE, Dharmarajan V, Cosgrove MS. 2008. A conserved arginine-containing motif crucial for the assembly and enzymatic activity of the mixed lineage leukemia protein-1 core complex. J Biol Chem 283:32162–32175 [DOI] [PubMed] [Google Scholar]
- 23. Patel A, Dharmarajan V, Vought VE, Cosgrove MS. 2009. On the mechanism of multiple lysine methylation by the human mixed lineage leukemia protein-1 (MLL1) core complex. J Biol Chem 284:24242–24256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel A, Dharmarajan V, Cosgrove MS. 2008. Structure of WDR5 bound to mixed lineage leukemia protein-1 peptide. J Biol Chem 283:32158–32161 [DOI] [PubMed] [Google Scholar]
- 25. Song JJ, Kingston RE. 2008. WDR5 interacts with mixed-lineage leukemia (MLL) protein via the histone H3 binding pocket. J Biol Chem 283:35258–35264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parsons DW, Li M, Zhang X, Jones S, Leary RJ, Lin JC, Boca SM, Carter H, Samayoa J, Bettegowda C, Gallia GL, Jallo GI, Binder ZA, Nikolsky Y, Hartigan J, Smith DR, Gerhard DS, Fults DW, VandenBerg S, Berger MS, Marie SK, Shinjo SM, Clara C, Phillips PC, Minturn JE, Biegel JA, Judkins AR, Resnick AC, Storm PB, Curran T, He Y, Rasheed BA, Friedman HS, Keir ST, McLendon R, Northcott PA, Taylor MD, Burger PC, Riggins GJ, Karchin R, Parmigiani G, Bigner DD, Yan H, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. 2011. The genetic landscape of the childhood cancer medulloblastoma. Science 331:435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang XX, Fu L, Li X, Wu X, Zhu Z, Fu L, Dong JT. 2011. Somatic mutations of the mixed-lineage leukemia 3 (MLL3) gene in primary breast cancers. Pathol Oncol Res 17:429–433 [DOI] [PubMed] [Google Scholar]
- 28. Balakrishnan A, Bleeker FE, Lamba S, Rodolfo M, Daniotti M, Scarpa A, van Tilborg AA, Leenstra S, Zanon C, Bardelli A. 2007. Novel somatic and germline mutations in cancer candidate genes in glioblastoma, melanoma, and pancreatic carcinoma. Cancer Res 67:3545–3550 [DOI] [PubMed] [Google Scholar]
- 29. van Haaften G, Dalgliesh GL, Davies H, Chen L, Bignell G, Greenman C, Edkins S, Hardy C, O'Meara S, Teague J, Butler A, Hinton J, Latimer C, Andrews J, Barthorpe S, Beare D, Buck G, Campbell PJ, Cole J, Forbes S, Jia M, Jones D, Kok CY, Leroy C, Lin ML, McBride DJ, Maddison M, Maquire S, McLay K, Menzies A, Mironenko T, Mulderrig L, Mudie L, Pleasance E, Shepherd R, Smith R, Stebbings L, Stephens P, Tang G, Tarpey PS, Turner R, Turrell K, Varian J, West S, Widaa S, Wray P, Collins VP, Ichimura K, Law S, Wong J, Yuen ST, Leung SY, Tonon G, DePinho RA, Tai YT, Anderson KC, Kahnoski RJ, Massie A, Khoo SK, Teh BT, Stratton MR, Futreal PA. 2009. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat Genet 41:521–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jung YS, Qian Y, Chen X. 2010. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signal 22:1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen WD, Wang YD, Zhang L, Shiah S, Wang M, Yang F, Yu D, Forman BM, Huang W. 2010. Farnesoid X receptor alleviates age-related proliferation defects inregenerating mouse livers by activating forkhead box m1b transcription. Hepatology 51:953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kanamaluru D, Xiao Z, Fang S, Choi SE, Kim DH, Veenstra TD, Kemper JK. 2011. Arginine methylation by PRMT5 at a naturally occurring mutation site is critical for liver metabolic regulation by small heterodimer partner. Mol Cell Biol 31:1540–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]