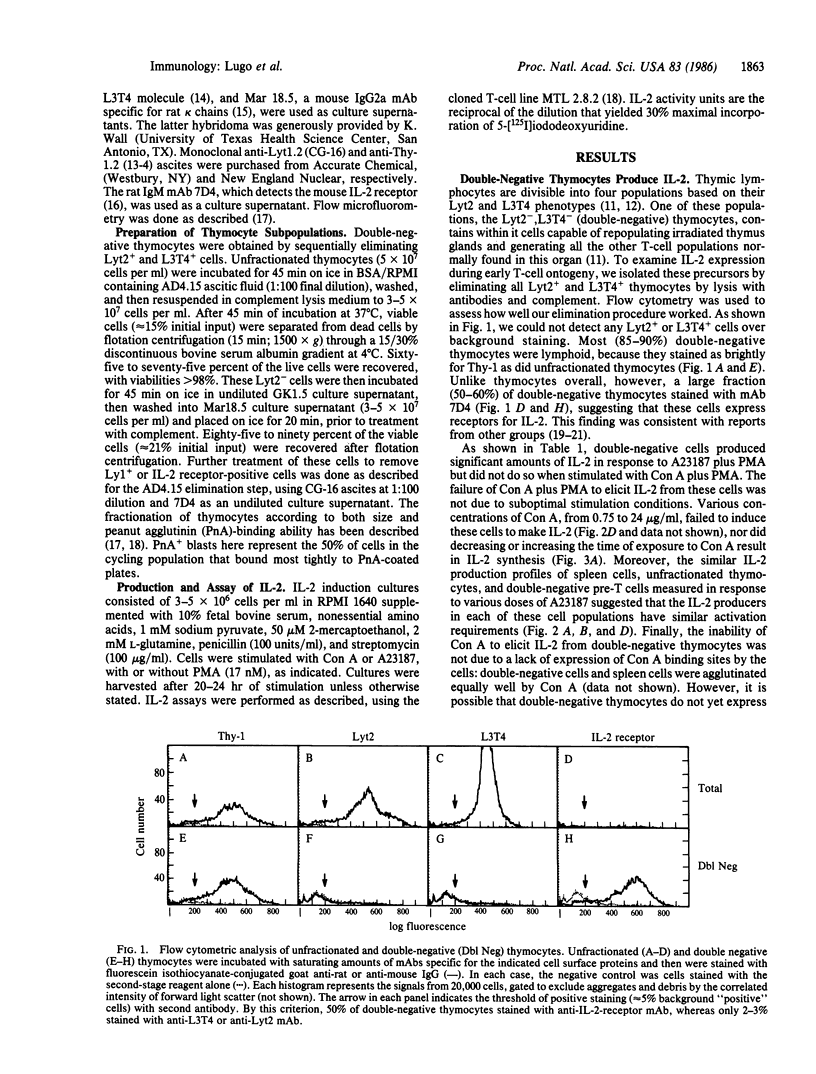
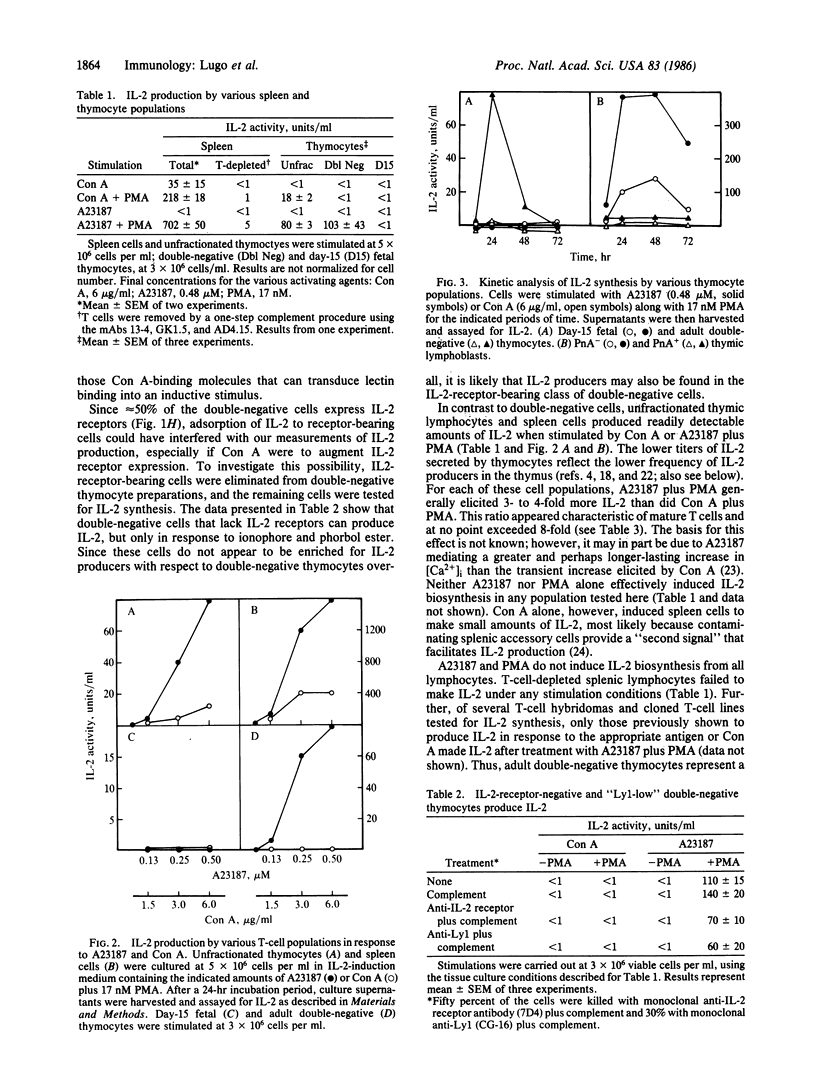
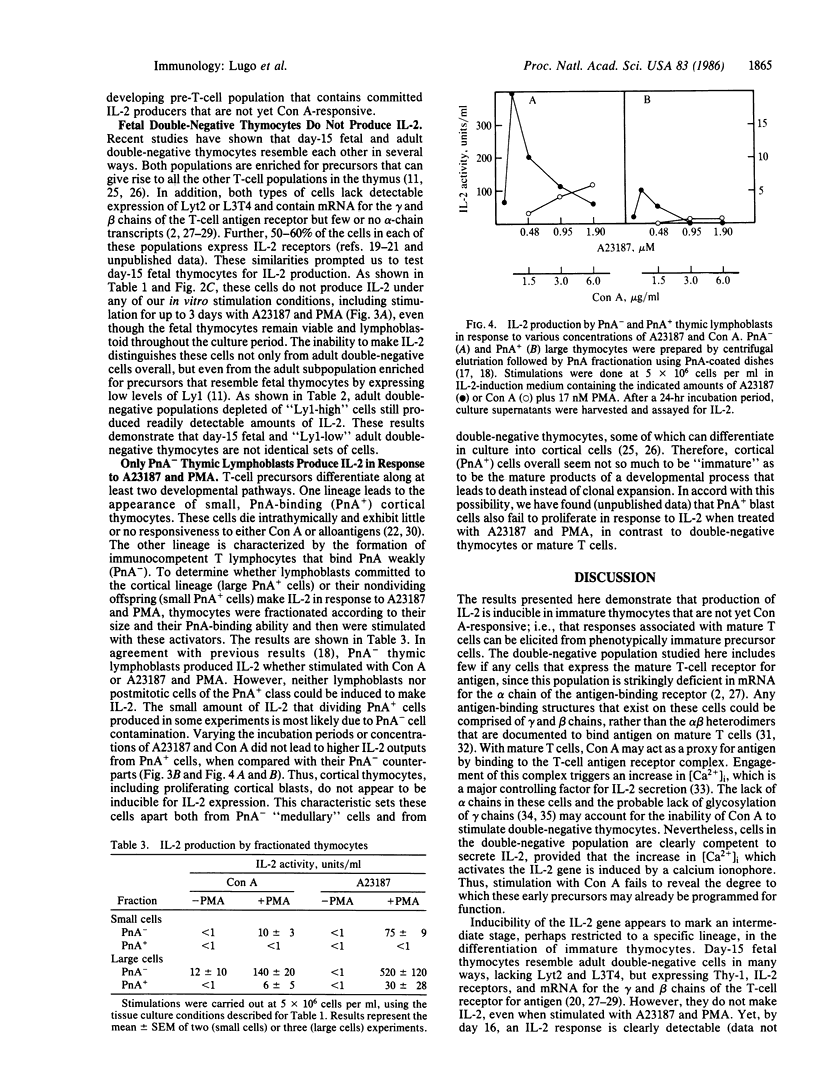
Abstract
T-cell precursors were stimulated with a conventional T-cell mitogen or with the calcium ionophore A23187 in order to determine whether pre-T cells acquire the ability to produce interleukin 2 (IL-2) before they acquire the ability to respond to antigen or mitogenic lectins. Immature T cells were obtained by eliminating mouse thymocytes that expressed the Lyt2 and L3T4 cell surface proteins. The remaining Lyt2-, L3T4- cells were stimulated for IL-2 production by using concanavalin A (Con A) or A23187, together with phorbol 12-myristate 13-acetate (PMA). We found that these "double-negative" thymocytes were unresponsive to Con A plus PMA but produced substantial amounts of IL-2 when stimulated with A23187 plus PMA. In contrast, both stimulation regimens induced more mature T-lymphocyte populations to produce IL-2. This implies that developing T cells acquire the ability to make IL-2 upon induction before they acquire the ability to be triggered by Con A. Day-15 fetal and cortical thymocytes were also tested for their ability to make IL-2. Both populations failed to synthesize this growth factor, even when stimulated with A23187 and PMA. For cortical thymocytes, this result, together with the finding that A23187 plus PMA fails to activate these cells, suggests that this population is immunologically inert rather than immature. On the other hand, the inability of day-15 fetal thymocytes to produce IL-2 indicates that these T-cell precursors are developmentally distinct from adult Lyt2-, L3T4- thymocytes, which they phenotypically resemble.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert F., Hua C., Truneh A., Pierres M., Schmitt-Verhulst A. M. Distinction between antigen receptor and IL 2 receptor triggering events in the activation of alloreactive T cell clones with calcium ionophore and phorbol ester. J Immunol. 1985 Jun;134(6):3649–3655. [PubMed] [Google Scholar]
- Born W., Yagüe J., Palmer E., Kappler J., Marrack P. Rearrangement of T-cell receptor beta-chain genes during T-cell development. Proc Natl Acad Sci U S A. 1985 May;82(9):2925–2929. doi: 10.1073/pnas.82.9.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan B., Rothenberg E. High-level secretion of interleukin 2 by a subset of proliferating thymic lymphoblasts. J Immunol. 1984 Oct;133(4):1983–1991. [PubMed] [Google Scholar]
- Ceredig R., Dialynas D. P., Fitch F. W., MacDonald H. R. Precursors of T cell growth factor producing cells in the thymus: ontogeny, frequency, and quantitative recovery in a subpopulation of phenotypically mature thymocytes defined by monoclonal antibody GK-1.5. J Exp Med. 1983 Nov 1;158(5):1654–1671. doi: 10.1084/jem.158.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R., Glasebrook A. L., MacDonald H. R. Phenotypic and functional properties of murine thymocytes. I. Precursors of cytolytic T lymphocytes and interleukin 2-producing cells are all contained within a subpopulation of "mature" thymocytes as analyzed by monoclonal antibodies and flow microfluorometry. J Exp Med. 1982 Feb 1;155(2):358–379. doi: 10.1084/jem.155.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Sekaly R. P., MacDonald H. R. Differentiation in vitro of Lyt 2+ thymocytes from embryonic Lyt 2- precursors. Nature. 1983 May 19;303(5914):248–250. doi: 10.1038/303248a0. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Gillis S., Mizel S. B. T-Cell lymphoma model for the analysis of interleukin 1-mediated T-cell activation. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1133–1137. doi: 10.1073/pnas.78.2.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday A. C., Saito H., Gillies S. D., Kranz D. M., Tanigawa G., Eisen H. N., Tonegawa S. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985 Feb;40(2):259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J., Kubo R., Haskins K., Hannum C., Marrack P., Pigeon M., McIntyre B., Allison J., Trowbridge I. The major histocompatibility complex-restricted antigen receptor on T cells in mouse and man: identification of constant and variable peptides. Cell. 1983 Nov;35(1):295–302. doi: 10.1016/0092-8674(83)90232-5. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Gutman G. A., Lewis D. E., Griswold S. T., Warner N. L. Monoclonal antibodies against rat immunoglobulin kappa chains. Hybridoma. 1982;1(2):125–131. doi: 10.1089/hyb.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Lugo J. P., Krishnan S. N., Sailor R. D., Koen P., Malek T., Rothenberg E. Proliferation of thymic stem cells with and without receptors for interleukin 2. Implications for intrathymic antigen recognition. J Exp Med. 1985 May 1;161(5):1048–1062. doi: 10.1084/jem.161.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieson B. J., Fowlkes B. J. Cell surface antigen expression on thymocytes: development and phenotypic differentiation of intrathymic subsets. Immunol Rev. 1984 Dec;82:141–173. doi: 10.1111/j.1600-065x.1984.tb01121.x. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills G. B., Cheung R. K., Grinstein S., Gelfand E. W. Increase in cytosolic free calcium concentration is an intracellular messenger for the production of interleukin 2 but not for expression of the interleukin 2 receptor. J Immunol. 1985 Mar;134(3):1640–1643. [PubMed] [Google Scholar]
- Ortega G., Robb R. J., Shevach E. M., Malek T. R. The murine IL 2 receptor. I. Monoclonal antibodies that define distinct functional epitopes on activated T cells and react with activated B cells. J Immunol. 1984 Oct;133(4):1970–1975. [PubMed] [Google Scholar]
- Raulet D. H. Expression and function of interleukin-2 receptors on immature thymocytes. Nature. 1985 Mar 7;314(6006):101–103. doi: 10.1038/314101a0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Reichert R. A., Gallatin W. M., Butcher E. C., Weissman I. L. A homing receptor-bearing cortical thymocyte subset: implications for thymus cell migration and the nature of cortisone-resistant thymocytes. Cell. 1984 Aug;38(1):89–99. doi: 10.1016/0092-8674(84)90529-4. [DOI] [PubMed] [Google Scholar]
- Roehm N., Herron L., Cambier J., DiGuisto D., Haskins K., Kappler J., Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells: distribution on thymus and peripheral T cells. Cell. 1984 Sep;38(2):577–584. doi: 10.1016/0092-8674(84)90512-9. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Lugo J. P. Differentiation and cell division in the mammalian thymus. Dev Biol. 1985 Nov;112(1):1–17. doi: 10.1016/0012-1606(85)90114-9. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. 1984 Jun 28-Jul 4Nature. 309(5971):757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Lindsten T., Fowlkes B. J., van den Elsen P., Terhorst C., Davis M. M., Germain R. N., Schwartz R. H. Expression of genes of the T-cell antigen receptor complex in precursor thymocytes. 1985 Jun 27-Jul 3Nature. 315(6022):765–768. doi: 10.1038/315765a0. [DOI] [PubMed] [Google Scholar]
- Scollay R., Shortman K. Identification of early stages of T lymphocyte development in the thymus cortex and medulla. J Immunol. 1985 Jun;134(6):3632–3642. [PubMed] [Google Scholar]
- Snodgrass H. R., Dembić Z., Steinmetz M., von Boehmer H. Expression of T-cell antigen receptor genes during fetal development in the thymus. Nature. 1985 May 16;315(6016):232–233. doi: 10.1038/315232a0. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Kisielow P., Kiefer M., Steinmetz M., von Boehmer H. Ontogeny of the T-cell antigen receptor within the thymus. Nature. 1985 Feb 14;313(6003):592–595. doi: 10.1038/313592a0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Lesley J., Trotter J., Hyman R. Thymocyte subpopulation enriched for progenitors with an unrearranged T-cell receptor beta-chain gene. Nature. 1985 Jun 20;315(6021):666–669. doi: 10.1038/315666a0. [DOI] [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985 Jan 24;313(6000):318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Wei-Feng C., Scollay R., Shortman K. The functional capacity of thymus subpopulations: limit-dilution analysis of all precursors of cytotoxic lymphocytes and of all T cells capable of proliferation in subpopulations separated by the use of peanut agglutinin. J Immunol. 1982 Jul;129(1):18–24. [PubMed] [Google Scholar]
- Weiss A., Imboden J., Shoback D., Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Crisanti A., Kisielow P., Haas W. Absence of growth by most receptor-expressing fetal thymocytes in the presence of interleukin-2. Nature. 1985 Apr 11;314(6011):539–540. doi: 10.1038/314539a0. [DOI] [PubMed] [Google Scholar]


