In the spleens of mice recovered from experimental autoimmune uveitis is a melanocortin 5 receptor (MC5r)-dependent tolerogenic antigen-presenting cell (APC). Reestablishment of the mechanisms of immunomodulation in the uveitic ocular microenvironment generates a novel MC5r-dependent APC that induces, and stands ready to reactivate, protective ocular autoantigen-specific regulatory T cells.
Abstract
Purpose.
IRBPp-specific regulatory immunity is found in the spleens of mice recovered from experimental autoimmune uveoretinitis (EAU). Induction of this regulatory immunity is dependent on the expression of the melanocortin 5 receptor (MC5r). Therefore, the authors investigated whether dependence on the expression of MC5r was with the T cells or with the APCs mediating protective regulatory immunity in the EAU-recovered mouse spleen.
Methods.
Wild-type and MC5r−/− mice were immunized to induce EAU. The IRBPp-stimulated T-cell response in spleens of wild-type and MC5r−/− mice were compared for surface markers and cytokine production. Spleen APC were isolated and used to stimulate cytokine production and regulatory activity in IRBP-specific T cells from wild-type or MC5r−/− mice assayed in culture by ELISA, by flow cytometry, and in vivo by adoptive transfer into EAU mice.
Results.
IRBPp-specific CD25+CD4+ T cells from spleens of EAU-recovered wild-type mice express a Treg cell phenotype of FoxP3 and TGF-β compared with the effector T-cell phenotype of IFN-γ and IL-17 production in EAU-recovered MC5r−/− mice. APCs from the spleens of wild-type mice recovering from EAU promoted regulatory T-cell activation in IRBP-specific effector T cells from the spleens of EAU-recovering MC5r−/− mice. Spleen APCs from EAU-recovering wild-type, but not MC5r−/−, mice induced TGF-β expression by primed IRBP-specific effector T cells.
Conclusions.
Dependence on MC5r expression is with an APC that promotes or selectively activates IRBP-specific FoxP3+ TGF-β+ CD25+CD4+ Treg cells in the spleens of EAU-recovered mice.
The eye is an immune-privileged tissue that has multiple mechanisms of immunomodulation to suppress the induction of inflammation.1–4 These mechanisms also manipulate immunity to regulate itself. For most, the mechanisms of immune privilege are highly effective in contributing to a lifetime of vision free from inflammation; however, uveitis (intraocular inflammation) does occur and threatens vision. Uveitis is the third leading cause of blindness among Americans; its prevalence is 204 per 100,000 persons per year.5 However, the rate of uveitis may be increasing in our aging population.6 Each year 17.6% of patients with active uveitis experience a transient or permanent loss of vision, and 12.5% of patients with active uveitis will develop glaucoma.7 After an initial episode of uveitis, 11.3% of the patients will within 5 years have at least one recurrence, and 2.5% of the patients will experience two recurrences.8 The mechanisms contributing to the relapsing and remitting nature of chronic autoimmune uveitis are not understood, nor is it well understood how prolonged remission of this disease can be achieved.
Rodent models of experimental autoimmune uveoretinitis (EAU) have greatly helped in exploring the pathology of uveitis.9 Mice immunized with the ocular autoantigen interphotoreceptor retinoid binding-protein (IRBP) show retinal inflammation within 1 to 2 weeks of immunization; untreated B10.RIII mice recover within 30 to 45 days, and untreated C57BL/6 mice recover in 75 to 90 days. The mechanisms of recovery are unclear; however, it must involve the ocular microenvironment reestablishing the mechanisms of immune privilege.10,11 During the resolution of EAU, IRBP-specific CD4+CD25+ Treg cells emerge in the spleens of EAU mice.12 These Treg cells prevent the induction and the expression of memory immunity to IRBP.13
Induction of these Treg cells requires recovery from EAU because naive mice and enucleated mice immunized for EAU do not have this regulatory immunity in their spleens.12 Interestingly, induction of these Treg cells in the spleen is not required for the ocular microenvironment to recover from EAU because melanocortin 5 receptor (MC5r) knockout mice at the resolution of EAU do not express IRBP-specific regulatory immunity in their spleens, but display a tempo and severity of EAU similar to those of wild-type mice.12,13 Moreover, when the MC5r−/− mice are reimmunized with IRBP, they show clinical signs of uveitis within a couple of days and a severity far exceeding levels observed in the first episode.13 In contrast, IRBP-reimmunized wild-type mice have a second episode of EAU with a severity and tempo of a naive mouse. This includes the 9- to 10-day delay in seeing the first symptoms of uveoretinitis. The adoptive transfer of post-EAU spleen Treg cells from wild-type mice suppresses the memory immunity in the IRBP-reimmunized MC5r−/− mice. However, wild-type Treg cells after EAU have to be reactivated by post-EAU spleen APCs before they can adoptively transfer their regulatory activity. Therefore, this suggests that the induction of IRBP-specific Treg cells that reside in the spleens of post-EAU mice is dependent on the expression of MC5r and the activity of an APC that mediates tolerogenic activity. What is not known is whether the expression of MC5r is necessary for the emergence of the Treg cells or for the tolerance-mediating APCs in the spleens of mice recovered from EAU.
Greater understanding of the melanocortin pathway in EAU recovery has the potential to lead to more effective therapies with long-term benefits not only for uveitis but also for other immunologic diseases needing the induction of immune tolerance.14 This article reports that the dependence on MC5r expression occurs with an APC that mediates regulatory activity in CD4+ effector T cells found in the post-EAU spleen.
Materials and Methods
Induction of EAU
EAU was induced in C57BL/6 mice purchased from Jackson Laboratories (Bar Harbor, ME). The MC5r−/− mice, on a C57BL/6 background, were obtained from Roger D. Cone (Oregon Health Sciences University, Portland, OR) and propagated within our animal facility. All mouse use was approved by our Institutional Animal Care and Use Committee and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. EAU was induced by immunizing the mice with an emulsion of 2 mg/mL human IRBP peptide amino acids 1 to 20 (IRBPp; Genscript, Piscataway, NJ) in PBS with complete Freund's adjuvant fortified with 5 mg/mL desiccated Mycobacterium tuberculosis (Difco Laboratories, Detroit, MI).12,15 The emulsion (100 μL per injection) was injected subcutaneously into two sites on the lower back of the mice. This was followed by intraperitoneal injection of 0.3 μg pertussis toxin. EAU progress was evaluated by fundus examinations every 3 to 4 days. Clinical scoring was a 0 to 5 scoring system based on observable infiltration and vasculitis in the retina, as previously described.15 The ocular fundus was examined using a slit lamp microscope by flattening the cornea with a glass coverslip and dilating the iris with 0.5% tropicamide.
In Vitro Spleen Cell Cultures
Spleens were removed from EAU-recovered mice and placed in RPMI 1640 supplemented with 5% FBS, 10 μg/mL gentamicin (Sigma, St. Louis, MO), 10 mM HEPES (BioWhittaker, Walkersville, MD), 1 mM sodium pyruvate (BioWhittaker), and nonessential amino acids (BioWhittaker). Spleens were made into a single-cell suspension, depleted of red blood cells using RBC lysis buffer (Sigma), washed, and suspended in serum-free media (SFM), RPMI 1640 supplemented with 10 μg/mL gentamicin (Sigma), 10 mM HEPES, 1 mM sodium pyruvate (BioWhittaker), nonessential amino acids 0.2%, ITS+1 (Sigma), and 0.1% BSA.16 Cells (4 × 105 cells/well) were incubated with 50 μg IRBPp at 37°C and 5% CO2 for 48 hours before their supernatant was assayed for IFN-γ, IL-17, and TGF-β. Supernatants from cultured cells were collected after a 48-hour incubation. The concentrations of IFN-γ and IL-17 were measured by ELISA (R&D Systems, Minneapolis, MN), and the concentration of TGF-β was measured using the standard Mv1Lu bioassay.17
Adoptive Transfer
To assay for specific APC and T-cell activity, the spleen cells at a concentration of 1 × 106 cells/well were seeded onto a flat-bottomed 96-well plate in SFM and were incubated for 90 minutes at 37°C and 5% CO2. Nonadherent cells were removed by washing wells twice with SFM. Adherent cells were used as APCs, pulsed with IRBPp (50 μg) in SFM, and incubated for 24 hours at 37°C and 5% CO2 and then used to stimulate T cells from the spleens of other EAU-recovered mice. T cells (1 × 106 cells) from the spleens of EAU-recovered mice were added to the wells of adherent spleen APCs. Cultures were incubated at 37°C and 5% CO2 for 24 hours to collect cells for flow cytometry analysis and for adoptive transfer, or they were incubated for 48 hours to assay the supernatant for cytokines. Restimulated nonadherent T cells (1 × 106 cells) were given to recipient mice for adoptive transfer experiments.
Activation of Primed T Cells by EAU-Recovered APCs
Adherent spleen cells, prepared as described, were used to stimulate primed T cells from popliteal lymph nodes and were not pulsed with IRBPp. T cells from popliteal lymph nodes of mice immunized 7 days earlier with an injection into the footpad with 50 μL of 1 mg/mL IRBPp, emulsified with 5 mg/mL heat-killed desiccated M. tuberculosis in mineral oil, were enriched using a mouse CD3+ T-cell enrichment column (R&D Systems) and seeded into the wells containing adherent spleen cells (1 × 106/well) prepared as described. Cultures were incubated at 37°C and 5% CO2 for 24 hours and then collected for flow cytometry analysis.
Flow Cytometry Analysis
Brefeldin A was added to the cell cultures (1 μg/mL) for 4 hours. Cells were washed and incubated in fixation buffer (Imgenex, San Diego, CA) for 10 minutes. Fixed cells were washed in staining buffer (PBS containing 1% BSA and 1.6% azide) and blocked with mouse IgG. Cells were then stained with anti-CD4-AF700–conjugated antibody (BioLegend, San Diego, CA). Intracellular staining was performed sequentially and in permeabilization buffer (Imgenex). The following conjugated antibodies were used: anti-TGF-β-APC (R&D Systems), mouse IgG1-APC (R&D Systems), anti-IL-17-PE (BioLegend), rat IgG1-PE (BioLegend), anti-FoxP3-FITC (eBioscience, San Diego, CA), rat IgG2a-FITC (BioLegend), anti-IFN-γ-PE/Cy7 (BioLegend), and rat IgG1-PE/Cy7 (BioLegend). After intracellular staining, cells were washed, and CD25-APC/Cy7 (BioLegend) or rat IgG1-APC/Cy7 (BioLegend) staining was performed in staining buffer. Cells were then resuspended in PBS and analyzed on a flow cytometer (BD LSRII; BD Biosciences, San Jose, CA). The flow data were analyzed using flow cytometry analysis software (FlowJo; Tree Star, Inc., Ashland, OR).
Statistical Analysis
Experimental results for the EAU scoring used the nonparametric Mann-Whitney U test for statistical differences in the EAU scores between groups of mice. In addition, changes in the tempo of disease between the groups of treated EAU mice were analyzed by two-way ANOVA. Comparisons of flow cytometry results and cytokine concentrations were statistically analyzed by Student's unpaired t-test. A statistical difference was determined when P ≤ 0.05.
Results
RBPp-Stimulated Spleen T-Cell Response between EAU-Recovered Wild-Type and MC5r−/− Mice
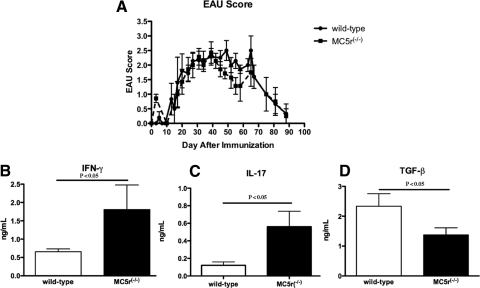
Previous results showed that the course of IRBPp-induced EAU in wild-type and MC5r−/− mice was similar (Fig. 1A).13 Despite the similar disease severity and tempo, when the mice recovered from EAU there were EAU-suppressing IRBPp-specific Treg cells in the spleens of wild-type mice, but not in the spleens of EAU-recovered MC5r−/− mice.12,13 Therefore, the spleen cells in EAU-recovered mice were assayed for the production of IFN-γ, IL-17, and TGF-β. The spleen cells were collected 80 days after immunization and were IRBPp-stimulated in culture. The levels of IFN-γ and IL-17 produced were significantly lower in the wild-type T cells than in the MC5r−/− T cells (Figs. 1B, 1C). In contrast, the production of TGF-β was significantly higher in wild-type T cells than in MC5r−/− T cells (Fig. 1D). Therefore, the cytokine pattern of the T-cell response to IRBP in the spleens of wild-type mice recovered from EAU corresponded with the previous finding that post-EAU wild-type mice have Treg cells in their spleens. In contrast, the spleens of EAU-recovered MC5r−/− mice have effector T cells.13
Figure 1.
Cytokines produced by IRBPp-stimulated spleen T cells from EAU-recovered mice. (A) The severity of EAU in wild-type and MC5r−/− mice was evaluated by scoring the extent of inflammation observed by fundus examinations every 2 to 3 days. Presented are the mean ± SEM scores of each group (7 mice) on a given day. There was no statistical difference in the severity of inflammation between EAU scores of wild-type and MC5r−/− mice or in the tempo of inflammation and resolution. Spleens were collected from EAU wild-type and MC5r−/− mice on day 80. Spleen cells were restimulated with IRBPp for 48 hours, and culture supernatants were assayed for (B) IFN-γ, (C) IL-17, and (D) TGF-β. The concentration of each cytokine is presented as the mean ± SEM of eight different mice. Statistically significant differences (P ≤ 0.05) were calculated and are labeled with a P value.
Intracellular Cytokine and FoxP3 Expression in IRBPp T Cells from Spleens of EAU-Recovered Mice
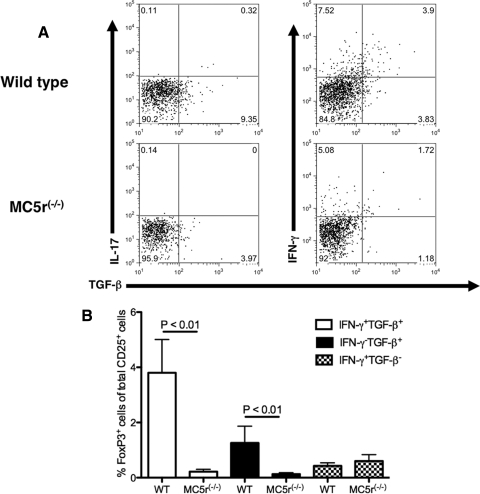
We then asked which cells were producing the secreted cytokines by comparing the CD25+CD4+ IRBPp-stimulated T cells from EAU-recovered wild-type and MC5r−/− mice for intracellular expression of TGF-β, IL-17, IFN-γ, and FoxP3 by flow cytometry (Fig. 2, Supplementary Fig. S1 [http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-8153/-/DCSupplemental]). There was a modest IL-17 expression in both wild-type and MC5r−/− IRBPp-stimulated CD25+CD4+ T cells, corresponding to the cytokines detected in the supernatants assayed in Figure 1. In addition, the levels of TGF-β–expressing CD25+CD4+ T cells were as expected from the results in Figure 1. However, though IFN-γ secretion from EAU-recovered CD25+CD4+ wild-type T cells was greatly suppressed (Fig. 1B), there was enhanced intracellular staining for IFN-γ in the T cells (Fig. 2A). This was not unexpected because a similar finding was made in α-MSH–treated effector T cells in which all intracellular IFN-γ is ubiquitinated.18 The expression of FoxP3 was significantly greater in the CD25+CD4+ T cells from EAU-recovered mice that expressed TGF-β regardless of whether the cells coexpressed IFN-γ (Fig. 2B). There was very little expression of FoxP3 in the CD25+CD4+ T cells from the MC5r−/− mice and in wild-type T cells that did not express TGF-β (Fig. 2B). Therefore, the post-EAU IRBPp-specific CD25+CD4+ Treg cells in wild-type mouse spleens expressed TGF-β and FoxP3.
Figure 2.
Intracellular staining for IFN-γ, IL-17, TGF-β, ανδ FoxP3 in CD25+CD4+ T cells from the spleens of EAU-recovered wild-type and MC5r−/− mice. Spleen cells from EAU-recovered wild-type and MC5r−/− mice were collected and restimulated with IRBPp 1–20 in culture for 24 hours. Cells were collected and stained for CD4, CD25, IFN-γ, IL-17, TGF-β, and FoxP3 for analysis by flow cytometry. (A) Presented are the representative results of gating on CD25+CD4+ cells and assaying for the expression of IFN-γ and IL-17 versus TGF-β. Each quadrant is labeled with the percentage of events within the quadrant relative to all events in the dot plot. (B) The mean ± SEM percentage of CD25+CD4+ cells that are FoxP3+ and TGF-β+IFN-γ+, TGF-β+IFN-γ−, or TGF-β−IFN-γ+ was calculated from independent flow cytometry analysis of three wild-type and nine MC5r−/− EAU-recovered mouse spleen cells. Statistically significant differences (P ≤ 0.05) were calculated and are labeled with a P value.
Requirement of MC5r for Post-EAU Regulatory Immunity in the Spleen Is with the APCs
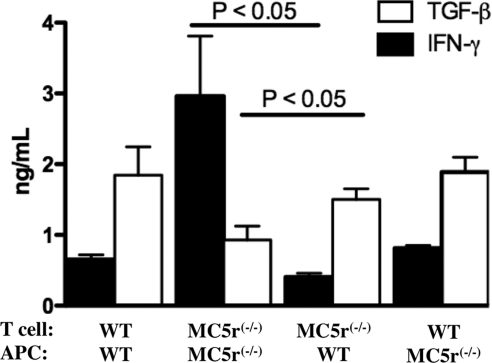
APCs from the spleens of wild-type mice were assayed to determine whether they were the mediators of the IRBP-specific regulatory immunity in the spleens of EAU-recovered wild-type mice. APCs and T cells were isolated from the spleens of wild-type and MC5r−/− mice that recovered from EAU. The spleen T cells were added to cultures of APCs that were first pulsed with IRBPp for 24 hours. Conditioned media were assayed for IFN-γ and TGF-β 48 hours later (Fig. 3). Cultures with wild-type T cells stimulated with wild-type APCs and cultures of MC5r−/− T cells stimulated with MC5r−/− APCs had cytokine levels such as those shown in Figure 1, with the wild-type cultures expressing TGF-β and the MC5r−/− cultures expressing IFN-γ. When the wild-type T cells were stimulated with the MC5r−/− APCs, the cytokine levels were no different from the wild-type T cells stimulated with wild-type APCs. There was a significant change in cytokine production by the MC5r−/− T cells when stimulated by the wild-type APCs compared with the cytokine production of MC5r−/− T cells stimulated by MC5r−/− APCs. Therefore, though the spleen APCs from EAU-recovered MC5r−/− were capable of stimulating regulatory immunity from an already established population of Treg cells, unlike the spleen APCs from EAU-recovered wild-type mice, the EAU-recovered MC5r−/− spleen APCs appeared to be unable to promote regulatory immunity from a mixed population of effector T cells.
Figure 3.
MC5r effects on cytokine production from EAU-recovered T cells and APCs. APCs from the spleens of EAU-recovered wild-type mice were isolated and incubated in culture with T cells from the spleens of EAU-recovered wild-type and MC5r−/− mice. In addition, APCs from the spleens of EAU-recovered MC5r−/− mice were isolated and incubated in culture with T cells from the spleens of EAU-recovered wild-type and MC5r−/− mice. After 48 hours of incubation, the culture supernatants were assayed for IFN-γ and TGF-β. Presented are the mean ± SEM of TGF-β and IFN-γ production from five independent cultures of cell combinations from five EAU-recovered mice.
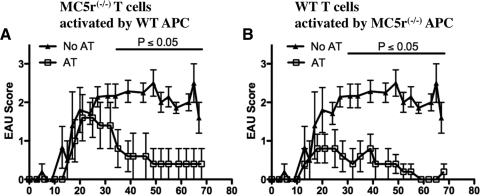
To demonstrate that the wild-type EAU-recovered APCs were inducing functional MC5r−/− Treg cells, the in vitro–stimulated cells were adoptively transferred into wild-type mice immunized for EAU. The adoptive transfer of MC5r−/− T cells stimulated with wild-type APCs accelerated the resolution of EAU (Fig. 4B). Therefore, the APCs from wild-type EAU-recovered mouse spleens promoted tolerogenic activity in the MC5r−/− T cells. In comparison, the adoptive transfer of wild-type T cells stimulated with MC5r−/− APCs were, as expected, effective in suppressing EAU (Fig. 4B) because the wild-type T cells were already Treg cells. Therefore, the induction of regulatory immunity in the spleens of EAU-recovered mice is mediated by APCs also associated with EAU recovery and dependent on MC5r expression. Interestingly, these results indicate that the EAU-recovered MC5r−/− APCs are capable of stimulating the Treg cells already present in the EAU-recovered spleen.
Figure 4.
Effects of MC5r expression on EAU-recovered T cells and APCs to mediate regulatory immunity. Clinical EAU scores ± SEM of each group on a given day of wild-type mice immunized for EAU and receiving an adoptive transfer (AT) of cultured stimulated T cells. (A, B) EAU scores of mice that received IRBP incubated spleen cells from naive wild-type mice (▴, n = 7). EAU scores of mice (□, n = 5) injected with T cells from (A) the cultures of EAU-recovered MC5r−/− T cells activated by APCs from EAU-recovered wild-type mice or (B) from the cultures of EAU-recovered wild-type T cells activated by APCs from EAU-recovered MC5r−/− mice. Statistically significant differences (P ≤ 0.05) were calculated and are labeled with a P value.
Enhancement of TGF-β Expression in Effector T Cells Is Mediated by APCs from EAU-Recovered Wild-Type Mice
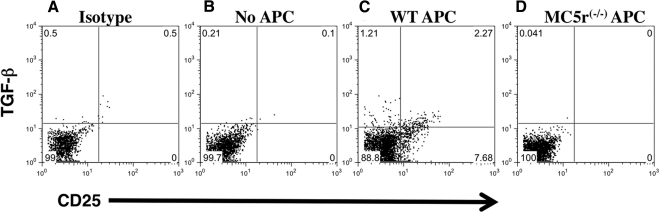
If the signal to express regulatory activity as promotion of TGF-β production in T cells comes from APCs that emerge in the spleens of EAU-recovered mice, when the APCs are used to activate a mixed population of effector T cells they should be enhanced in TGF-β production. The expression of intracellular TGF-β was assayed in wild-type effector IRBPp-specific T cells that were stimulated by spleen APCs from wild-type and MC5r−/− mice that have recovered from EAU. The expression of intracellular TGF-β in the CD4+ T cells was assayed by flow cytometry relative to CD25 expression (Fig. 5). Spleen APCs from EAU-recovered wild-type mice enhanced TGF-β expression in CD25+ and CD25−CD4+ T cells (Fig. 5C). In contrast, the spleen APCs from MC5r−/− EAU-recovered mice suppressed TGF-β expression in the effector T cells (Fig. 5D) compared with unstimulated T cells and with background control (Figs. 5A, 5B). The results demonstrate that the post-EAU wild-type APCs must have selectively promoted the activation of Treg cells out of a mixed population of antigen-specific effector T cells, thereby presumably establishing regulatory immunity. The results also demonstrate that MC5r is associated with an APC-mediated type of immune deviation through selective activation of Treg cells, a functionality not seen in spleen APCs from EAU-recovered MC5r−/− mice.
Figure 5.
Induction of TGF-β expression in IRBP-specific effector T cells by APCs from EAU-recovered mice. (A) IRBPp-specific Th1 cells were stimulated with APCs from EAU-recovered mice and stained with isotype controls. IRBPp-specific Th1 cells were (B) resting, (C) stimulated by APCs from EAU-recovered wild-type mice, or (D) stimulated by APCs from EAU-recovered MC5r−/− mice. After 24 hours of incubation, the cells were stained for CD4, CD5, and intracellular TGF-β. Stained cells were analyzed by flow cytometry. Presented are representative dot plots of TGF-β versus CD25 expression gated on the CD4+ cells of three experiments. The percentage of events is labeled in each quadrant.
Discussion
The natural process of EAU recovery in the mouse model of autoimmune uveitis provides an opportunity to evaluate self-regulated mechanisms that effectively suppress autoimmune disease. Although it has been assumed that the resolution of EAU is due to a recovery in the mechanisms of immune privilege,10,12,19,20 what has been observed is an increase in regulatory T-cell activity during and after the resolution of EAU.12,19,20 It has been observed that the emergence of Treg cells is dependent on the mice being immunized to IRBP, and the number of IRBP-specific Treg cells increases with the progression and resolution of uveitis. The inability to find these IRBP-specific Treg cells before immunization is an indication that the expanding population of Treg cells is IRBP-specific inducible Treg cells.12,19,20 In addition, the need to have an intact eye after immunization and a uveitic response to find the IRBP-specific Treg cells in the spleen suggests that the intraocular mechanisms resolving uveitis are associated with the expansion of IRBP-specific Treg cells. A consequence is the emergence in the spleen of IRBP-specific Treg cells that have the capacity to suppress the reactivation of EAU.12,20 Although Treg cells may be induced that act locally in the EAU-recovered MC5r−/− mice, these mice do not express IRBP-specific Treg cell responses in their spleens.13 In this study we found that though MC5r−/− mice had the potential to induce such protective regulatory immunity after EAU recovery, they did not have APCs in their spleens that could promote the activation of TGF-β–producing CD25+CD4+ FoxP3+ Treg cells, in contrast to EAU-recovered wild-type mice.
The IRBP-stimulated T-cell response in spleens of EAU-recovered mice was very different between the wild-type and the MC5r−/− mice. That agreed with previous findings describing the functionality of IRBP-stimulated splenic T cells between the two types of EAU-recovered mice.12,13 Although recovery from EAU is not dependent on MC5r expression, the emergence of an IRBP-specific Treg cell response in the spleen was dependent on MC5r expression. The EAU-recovered wild-type mice had an expanded population of TGF-β–producing CD25+CD4+ FoxP3+ Treg cells. The CD25+CD4+ T cells in the EAU-recovered MC5r−/− mice, when restimulated, expressed a cytokine pattern of effector T cells and not of Treg cells; however, when these MC5r−/− spleen T cells were stimulated by APCs from the spleens of EAU-recovered wild-type mice, they produced TGF-β, were suppressed in IFN-γ production, and accelerated recovery when adoptively transferred into mice with EAU. The effects of the APCs from the EAU-recovered wild-type mice on the EAU-recovered spleen MC5r−/− T cells and on the immunized lymph node T-cell populations were to promote the activation of Treg cells and potentially to select the Treg cells out of a mixed population of effector T cells for expansion.
Treg cells found in the spleens of EAU-recovered wild-type mice were activated by spleen APCs from EAU-recovered MC5r−/− mice and retained Treg cell activity. This demonstrates that once EAU-recovered spleen Treg cells are induced, they do not require MC5r to be expressed on the APCs to be restimulated. The results also indicate that these Treg cells are afferent suppressors. This means that in previous studies, EAU-recovered regulatory immunity in the spleen suppressed a recall response to induce EAU because the Treg cells suppressed the afferent response to the IRBP reimmunization, not necessarily to the responding IRBP-specific memory T effector cells. Therefore, the results demonstrate that the dependence on MC5r is with the APCs, not the T cells, to promote protective regulatory immunity in the spleens of EAU-recovered mice.
It has been known for some time that there is a link between the ocular microenvironment and the spleen associated with an APC that presents ocular antigens to induce tolerance.3 The results of this study present an unexpected and new finding that the expression of regulatory immunity to ocular autoantigen in the spleens of EAU-recovered mice is dependent on APC-expressing MC5r. Although it has yet to be definitively demonstrated that spleen APCs are from the EAU-recovering eye, the induction of IRBP-specific regulatory immunity requires intact eyes and EAU.12 Therefore, the possibility exists for the EAU-resolving ocular microenvironment to induce tolerogenic APCs that migrate to the spleen and promote protective regulatory immunity. These tolerogenic APCs that emerge as a result of recovery from EAU could be the same tolerogenic APCs that are required for the anterior chamber-associated immune deviation phenomenon. If this is dependent on the expression of MC5r, then it suggests that activation of the MC5r melanocortin pathway in APCs is by local ocular production of α-MSH.
Most of the understanding about the effects of α-MSH on macrophages and dendritic cells is to suppress their inflammatory activity; however, there is evidence that macrophages and dendritic cells are suppressed by α-MSH in their ability to present antigen and activate Th1 cells.21,22 The MC5r is 1 of 4 G-protein–coupled melanocortin receptors in which α-MSH, a constitutively expressed neuropeptide in the ocular microenvironment, is the ligand.16,23 The neuropeptide α-MSH is a potent suppressor of inflammation and inhibits proinflammatory signaling in macrophages and dendritic cells through MC1r and MC3r.23,24 The results of this study strongly suggest that α-MSH regulates APC functionality through MC5r, another new finding that has not been previously reported. The ligation of MC5r by α-MSH activates the JAK2/STAT1 pathway in B cells, but the ERK1/2 pathway through PI3-kinase not linked to cAMP in MC5r-transfected HEK293 cells.25,26 Therefore, α-MSH has the potential though MC5r to mediate cellular differentiation and cytokine production.
Our results suggest that part of the mechanism of EAU recovery is the induction of a tolerogenic APC dependent on MC5r expression that, in the spleen, promotes and possibly maintains IRBP-specific protective immunity. The implications of the results are that reestablishing the mechanisms of immunomodulation in the uveitic ocular microenvironment should generate a novel MC5r-dependent APC that induces and stands ready to reactivate ocular autoantigen-specific Treg cells.
Supplementary Material
Acknowledgments
The authors thank David Yee and Randy Huang for their technical assistance and for the use of the Schepens Eye Research Institute flow cytometry and animal core facilities.
Footnotes
Supported in part by the Massachusetts Lions Eye Research Foundation and by National Institutes of Health/National Eye Institute Public Health Service Grant EY010752.
Disclosure: D.J. Lee, None; A.W. Taylor, None
References
- 1. Taylor AW. Ocular immune privilege. Eye. 2009;23:1885–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003;74:179–185 [DOI] [PubMed] [Google Scholar]
- 3. Stein-Streilein J. Immune regulation and the eye. Trends Immunol. 2008;29:548–554 [DOI] [PubMed] [Google Scholar]
- 4. Niederkorn JY. Immune privilege in the anterior chamber of the eye. Crit Rev Immunol. 2002;22:13–46 [PubMed] [Google Scholar]
- 5. Darrell RW, Wagener HP, Kurland LT. Epidemiology of uveitis: incidence and prevalence in a small urban community. Arch Ophthalmol. 1962;68:502–514 [DOI] [PubMed] [Google Scholar]
- 6. Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California: the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500; discussion 500 [DOI] [PubMed] [Google Scholar]
- 7. Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–308 [DOI] [PubMed] [Google Scholar]
- 8. Knox EG, Mayho A, Cant W, Truelove P. Iridocyclitis: incidence, distribution and health service usage. Community Med. 1980;2:97–101 [PubMed] [Google Scholar]
- 9. Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120:3073–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohta K, Wiggert B, Taylor AW, Streilein JW. Effects of experimental ocular inflammation on ocular immune privilege. Invest Ophthalmol Vis Sci. 1999;40:2010–2018 [PubMed] [Google Scholar]
- 11. Ohta K, Yamagami S, Taylor AW, Streilein JW. IL-6 antagonizes TGF-beta and abolishes immune privilege in eyes with endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2000;41:2591–2599 [PubMed] [Google Scholar]
- 12. Kitaichi N, Namba K, Taylor AW. Inducible immune regulation following autoimmune disease in the immune-privileged eye. J Leukoc Biol. 2005;77:496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor AW, Kitaichi N, Biros D. Melanocortin 5 receptor and ocular immunity. Cell Mol Biol. 2006;52:53–59 [PubMed] [Google Scholar]
- 14. Taylor AW, Lee D. Applications of the role of alpha-MSH in ocular immune privilege. Adv Exp Med Biol. 2010;681:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Namba K, Kitaichi N, Nishida T, Taylor AW. Induction of regulatory T cells by the immunomodulating cytokines alpha-melanocyte-stimulating hormone and transforming growth factor-beta2. J Leukoc Biol. 2002;72:946–952 [PubMed] [Google Scholar]
- 16. Taylor AW, Streilein JW, Cousins SW. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr Eye Res. 1992;11:1199–1206 [DOI] [PubMed] [Google Scholar]
- 17. Taylor AW, Alard P, Yee DG, Streilein JW. Aqueous humor induces transforming growth factor-beta (TGF-beta)-producing regulatory T-cells. Curr Eye Res. 1997;16:900–908 [DOI] [PubMed] [Google Scholar]
- 18. Lee DJ, Biros DJ, Taylor AW. Injection of an alpha-melanocyte stimulating hormone expression plasmid is effective in suppressing experimental autoimmune uveitis. Int Immunopharmacol. 2009;9:1079–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun M, Yang P, Du L, Zhou H, Ren X, Kijlstra A. Contribution of CD4+CD25+ T cells to the regression phase of experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2010;51:383–389 [DOI] [PubMed] [Google Scholar]
- 20. Sun M, Yang P, Du L, et al. Increased regulatory T cells in spleen during experimental autoimmune uveoretinitis. Ocul Immunol Inflamm. 2010;18:38–43 [DOI] [PubMed] [Google Scholar]
- 21. Luger TA, Kalden D, Scholzen TE, Brzoska T. alpha-Melanocyte-stimulating hormone as a mediator of tolerance induction. Pathobiology. 1999;67:318–321 [DOI] [PubMed] [Google Scholar]
- 22. Taylor AW, Streilein JW, Cousins SW. Alpha-melanocyte-stimulating hormone suppresses antigen-stimulated T cell production of gamma-interferon. Neuroimmunomodulation. 1994;1:188–194 [DOI] [PubMed] [Google Scholar]
- 23. Lam CW, Getting SJ. Melanocortin receptor type 3 as a potential target for anti-inflammatory therapy. Curr Drug Targets Inflamm Allergy. 2004;3:311–315 [DOI] [PubMed] [Google Scholar]
- 24. Li D, Taylor AW. Diminishment of alpha-MSH anti-inflammatory activity in MC1r siRNA-transfected RAW264.7 macrophages. J Leukoc Biol. 2008;84:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buggy JJ. Binding of alpha-melanocyte-stimulating hormone to its G-protein-coupled receptor on B-lymphocytes activates the Jak/STAT pathway. Biochem J. 1998;331(pt 1):211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodrigues AR, Pignatelli D, Almeida H, Gouveia AM. Melanocortin 5 receptor activates ERK1/2 through a PI3K-regulated signaling mechanism. Mol Cell Endocrinol. 2009;303:74–81 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.