This study demonstrates a direct link between overproliferation and retinal dysplasia in the ptc2−/− juvenile retina and establishes ectopic proliferation as the likely cellular underpinning of retinal dysplasia in juvenile ptc2−/− mutants.
Abstract
Purpose.
Patched is a well-studied tumor suppressor and negative regulator of the Hedgehog (Hh) pathway. Earlier work in this laboratory has shown that embryonic zebrafish patched2 (ptc2) mutant retinas possess an expanded ciliary marginal zone (CMZ) and phenotypes similar to those in human patients with basal cell naevus syndrome (BCNS), a congenital disorder linked to mutations in the human PTCH gene. This study extends the analysis of retinal structure and homeostasis in ptc2−/− mutants to juvenile stages, to determine whether Patched 2 function is essential in the postembryonic eye.
Methods.
Histologic, immunohistochemical, and molecular analyses were used to characterize retinal defects in the 6-week-old juvenile ptc2−/− retina.
Results.
Juvenile ptc2−/− mutants exhibited peripheral retinal dysplasias that included the presence of ectopic neuronal clusters in the inner nuclear layer (INL) and regions of disrupted retinal lamination. Retinal dysplasias were locally associated with ectopic proliferation. BrdU/EdU labeling and immunohistochemistry assays demonstrated that a population of ectopically proliferating cells gave rise to the ectopic neuronal clusters in the INL of ptc2−/− mutants and that this contributed to retinal dysplasia in the mutant eye.
Conclusions.
These results demonstrate a direct link between overproliferation and retinal dysplasia in the ptc2−/− juvenile retina and establish ectopic proliferation as the likely cellular underpinning of retinal dysplasia in juvenile ptc2−/− mutants.
The Hedgehog (Hh) pathway is well known to control proliferation, differentiation, and patterning throughout the developing vertebrate central nervous system.1–4 In the vertebrate retina, Hh activity plays an integral role in the coordination of tissue growth and patterning through its influence on cell cycle progression of proliferating neuroblasts, which influences the timing of cell cycle exit and differentiation.5,6 Indeed, the mitogenic effects of the Hh pathway on retinal progenitors during development have been demonstrated in multiple model organisms.7 In zebrafish and Xenopus embryos, Hh pathway activation positively regulates cell cycle progression by influencing the lengths of the G1 and G2 phases of the cell cycle.6 Consistent with these findings, Shh is essential for the maintenance of retinal progenitor proliferation in mice.8,9
The Hh receptor Patched is a tumor suppressor and negative regulator of the Hh pathway.10,11 Mutations in the human PTCH gene have been linked to BCNS (OMIM 109400; Online Mendelian Inheritance in Man http://www.ncbi.nlm.nih.gov/Omim; National Center for Biotechnology Information, Bethesda, MD), a disorder characterized by dental and skeletal defects, as well as a predisposition to early- and late-onset tumorigenesis (reviewed in Ref. 12). Early lethality in Ptch1−/− mice,13 an established model for BCNS,13,14 has limited the investigation of the effects of loss of Patched function to largely developmental contexts. Interestingly, postnatal Ptch1+/− mice possess retinal dysplasias and epiretinal membranes—structural abnormalities that arise at the vitreoretinal interface. Dysplastic regions of the retina in Ptch1+/− mice are characterized by foci of disrupted lamination and the presence of photoreceptor rosettes in the inner nuclear layer (INL).14 Ectopic proliferation of retinal cells is thought to account for retinal dysplasias in Ptch1+/− mice; however, this relationship has not yet been experimentally demonstrated.
In addition to retinal dysplasia and vitreoretinal abnormalities, postnatal Ptch1+/− mice also possess increased proliferation in the retinal margin.15 In lower vertebrates, such as fish, frogs, and chicks, a population of proliferative cells at the retinal margin called the CMZ continues to contribute to the growth of the retina throughout the life of the animal.16,17 The spatial pattern of cells within the CMZ—retinal stem cells in the most peripheral region, followed by proliferative retinal progenitors, and finally differentiating progenitors more centrally—is thought to replicate the temporal sequence of early retinal development.17,18 Loss of a single allele of the mouse Ptch1 gene curiously results in the persistence of a population of retinal progenitors very reminiscent of the CMZ, a structure normally absent in mammals.15
The zebrafish ptc2tj222 (the orthologue of the mammalian Ptch119) mutant was isolated in a genetic screening designed to identify genes involved in the regulation of proliferation during embryonic development.20 The ptc2tj222 mutant possesses a nonsense mutation in the exon encoding the sixth transmembrane domain of Patched 2 that is predicted to abolish its function.20,21 Loss of Patched function results in an overactive Hh pathway, due to derepression of Smoothened, a transmembrane protein responsible for transducing the Hh signal.22,23
Our previous characterization of embryonic retinal development in ptc2 mutants revealed vitreoretinal abnormalities, similar to those observed in Ptch1+/− mice and in human BCNS patients. Our findings suggest that the vitreoretinal abnormalities are developmental in origin.24 Other aspects of retinal development, such as retinal lamination, were normal up to the late embryonic stages (5 days after fertilization [dpf]); however, an increase in the number of proliferative progenitors at the CMZ was observed, suggesting a role during embryonic eye development for Patched2 in negatively regulating the size of this retinal progenitor/stem cell population.24
A small percentage of zebrafish ptc2−/− mutants survive to juvenile stages.20 Given that retinal dysplasias do not appear in Ptch1+/− mice until postnatal stages and the cellular underpinnings of this phenotype is not known, we used juvenile ptc2−/− mutants to gain further insight into the consequences of loss of Patched function on the growth and patterning of the postembryonic retina. Our analyses identified peripheral retinal dysplasias in juvenile ptc2−/− mutants that included disrupted lamination, the presence of ectopic clusters of cells in the INL, and morphologic abnormalities at the retinal margin. Analysis of cell proliferation in the ptc2−/− juvenile retina revealed overproliferation in all retinal regions where proliferative progenitors normally reside (i.e., the CMZ, INL, and outer nuclear layer [ONL]). Continually proliferative progenitors were detected in ptc2−/− mutants adjacent to the CMZ, and proliferative cells were associated locally with dysplastic retinal regions that contained ectopic neurons. Of note, BrdU incorporation assays, combined with immunohistochemistry for retinal neuronal markers, demonstrated that ectopic retinal neurons within these dysplastic regions originated from an ectopic proliferative event. Taken together, our findings support a role for Patched2 in negatively regulating proliferation in the postembryonic retina and demonstrate that ectopic proliferation in the ptc2−/− juvenile retina directly contributes to retinal dysplasias in these mutants.
Methods
Zebrafish Maintenance and Strains
Zebrafish (Danio rerio) were maintained at 28.5°C on a 14-hour light–10-hour dark cycle. Embryos were obtained from the natural spawning of heterozygous carriers set up in pair-wise crosses. ptc2tj222 and Tg(gfap:GFP)mi2001 outcrosses were obtained from the Zebrafish International Resource Center and were propagated by repeated outcrosses to AB fish. All animals were treated in accordance with provisions established at the University of Texas at Austin governing animal use and care and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Genotyping of ptc2tj222 juvenile fish was performed by isolating genomic DNA, as previously described,25 and amplifying the genomic region containing the mutation by PCR using the following primer set: forward, 5′-ggcagtggtggtggtgtttaac-3′, and reverse, 5′-cgagcctttatttagccagttg-3′. Sequencing was then performed on single fish by using the reverse primer to identify individuals containing the mutation.20
Histology
Histology was performed as described elsewhere.26 Briefly, 6-week-old juvenile zebrafish were euthanized and their corneas punctured with a syringe needle and fixed for 2 days at 4°C in a solution of 1% (wt/vol) paraformaldehyde (PFA), 2.5% glutaraldehyde, and 3% sucrose in PBS/2% OsO4 solution. They were washed three times for 5 minutes each in PBS at room temperature and further dehydrated two times for 10 minutes in propylene oxide and then infiltrated 1 to 2 hours with a 50% propylene oxide/50% Epon-Araldite mixture (Polysciences, Inc., Warrenton, PA). Samples were then incubated overnight at RT in 100% Epon-Araldite resin with caps open to allow for propylene oxide evaporation and resin infiltration, they were embedded and baked at 60°C for 2 to 3 days. Sections (1 μm) were cut, mounted on glass slides, and stained in a 1% methylene blue/1% borax solution. The sections were mounted in DPX (Electron Microscopy Sciences, Hatfield, MA) and photographed with a microscope (DMRB; Leica Microsystems, Bannockburn, IL) equipped with a digital camera (DFC320; Leica).
Immunohistochemistry
Immunohistochemistry was performed as described by Uribe and Gross.27 Briefly, juveniles were euthanized and the corneas were punctured with a syringe needle and fixed for 2 days at 4°C in a 4% PFA solution in PBS. The fish were then washed three times for 10 minutes each in PBS and incubated in a 25% sucrose/PBS solution overnight, followed by 35% sucrose/PBS overnight. Fish were then mounted in tissue-freezing medium (Triangle Biomedical Sciences, Inc., Durham, NC) and immediately transferred to a −80°C freezer for storage. Frozen blocks were sectioned at 10 μm, mounted on gelatin-coated slides, and allowed to dry for 2 hours. The slides were then rehydrated in PBTD (0.1% Tween and 1% DMSO in 1× PBS), blocked with 5% normal goat serum (NGS)/PBTD for 2 hours, and incubated in primary antibody diluted in blocking solution in a humid chamber overnight at 4°C. Nuclei were stained with a green fluorescent nuclear stain (Sytox-green; Invitrogen-Molecular Probes, Carlsbad, CA), diluted 1:10,000, or a nucleic acid stain (TO-PRO-3; Invitrogen) diluted 1:5000 in blocking solution immediately after removal of the primary antibody. The slides were washed three times for 10 minutes each with PBTD and secondary antibody applied in blocking solution for 1 hour. After the slides were washed in PBTD three times for 10 minutes each, they were mounted with antifade medium (Vectashield; Vector Laboratories, Inc.) and coverslipped. The samples were imaged on a Pascal laser scanning confocal microscope (Carl Zeiss Meditec, Dublin, CA). The following primary antibodies were used: rods (zpr3, 1:400, ZIRC), green/red cones (zpr1, 1:200, ZIRC), and mouse anti-Islet1 (39.4D5, 1:10; Developmental Studies Hybridoma Bank, Iowa City, IA).
In Situ Hybridization
Hybridizations on juvenile retinal tissue sections were performed as described28 using digoxigenin-labeled antisense RNA probes. The ptc2 cDNA construct was provided by Brian Perkins (Texas A&M University, College Station, TX).
Fluorescence-Activated Cell Sorting and Quantitative Real-Time PCR (qRT-PCR)
Isolation of Müller glia from 6-week-old juvenile Tg(gfap:GFP)mi2001 retinas was performed, as outlined elsewhere,29 on a cell sorter (FACSAria; BD Biosciences, San Diego, CA). Three retinas, obtained from three different individuals, were pooled to make up each sample. For Hh target gene expression in ptc2−/− and wild-type eyes, whole eyes were used to extract RNA for qRT-PCR experiments and one eye was used for each sample. RNA was extracted (RNAqeous-Micro; Ambion, Austin, TX) according to the manufacturer's protocol. Total RNA was reverse-transcribed with a cDNA synthesis kit (iScript; Bio-Rad, Hercules, CA), and qRT-PCR was performed with a SYBR green kit (Power SYBR Green, performed on the 7900HT system; Applied Biosystems, Foster City, CA). The standard curve method was used to determine expression levels relative to β-actin. The entire experiment was performed three times, and data were analyzed for significance with a Student's t-test. Primer pairs used for qRT-PCR analysis were as follows: ptc2 forward, 5′-ctccattcctgccagcagac-3′, and reverse, 5′-ctcgatggcctccacgaac-3′; gli1 forward, 5′-accactacgacaacaccagcaacc-3′, and reverse, 5′ttcacagactgaccaccagagagc-3′; glutamine synthetase forward, 5′-aggatcgccgcccgt-3′, and reverse, 5′-tgcgaattagggcctcagtg-3′; gfap forward, 5′-gcagacaggtggatggactca-3′, and reverse, 5′-ggccaagttgtctctctcgatc-3′; huc, forward, 5′-tcatcacctcacgcatcctg-3′, and reverse, 5′-cttgatggcctcctctgcttc-3′; rhodopsin forward, 5′-gagcccatacgaatacccacag-3′, and reverse, 5′-cagcttcttgtgctcgatggt-3′; and β-actin forward, 5′-ccgtgacatcaaggagaagct-3′, and reverse, 5′-tcgtggataccgcaagattcc-3′.
BrdU and EdU Incorporation Assays
For 5-bromo-2-deoxyuridine (BrdU) incorporation assays, juvenile fish were incubated in fish water containing 5 μM BrdU (Abcam, Cambridge, MA) for 2 hours, fixed, and prepared for immunohistochemistry as above. Before the blocking step, slides were incubated in 2 N HCl at 37°C for 20 minutes. Rat anti-BrdU (Sigma-Aldrich, St. Louis, MO) was used at a 1:250 dilution. For cell counts, positively stained cells and nuclei were counted from three consecutive sections for each individual. Averages were analyzed and compared by Student's t-test.
For BrdU/EdU double-labeling experiments, the fish were exposed to BrdU for 8 hours and then returned to the tanks for 2 days. EdU incorporation was then performed before the fish were fixed and prepared for immunohistochemistry. For EdU incorporation, a solution of 400 μM 5-ethynyl-2′-deoxyuridine (Invitrogen) in PBS was prepared from a stock of 10 mM EdU in DMSO. EdU solution (100 nL) was injected into each eye through the cornea with a glass injection needle. Fish were allowed to recover for 2 hours in fish water before fixation. EdU detection was performed according to the manufacturer's protocol (Click-iT; Invitrogen), with a 633-nm kit, followed by HCl treatment and subsequent immunohistochemical detection of BrdU.
Results
Juvenile ptc2−/− Mutants Possess Peripheral Retinal Dysplasias and Abnormalities at the CMZ and Ciliary Zone
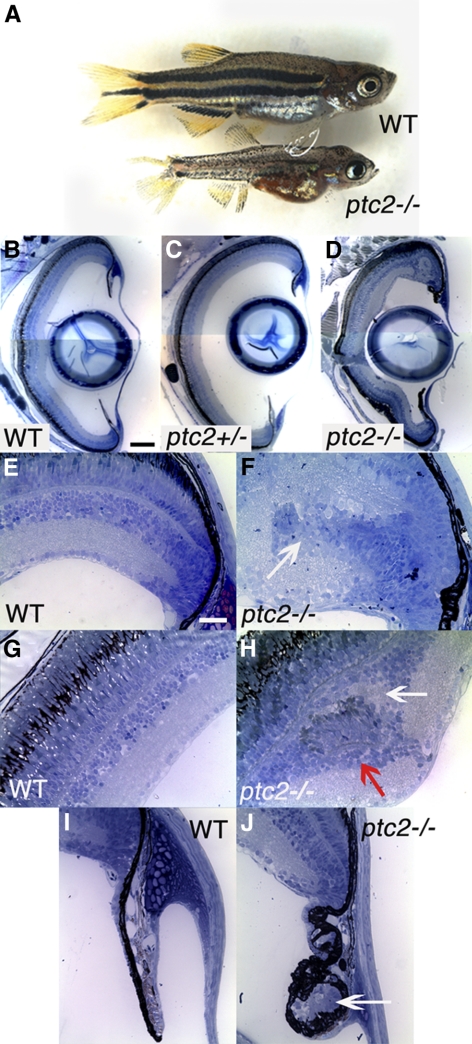
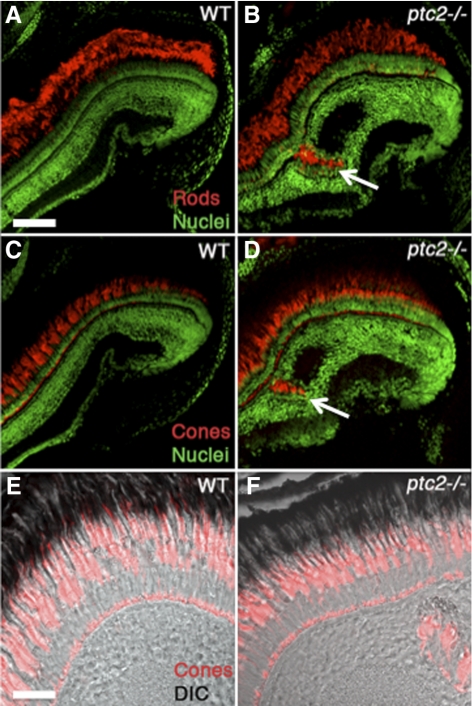
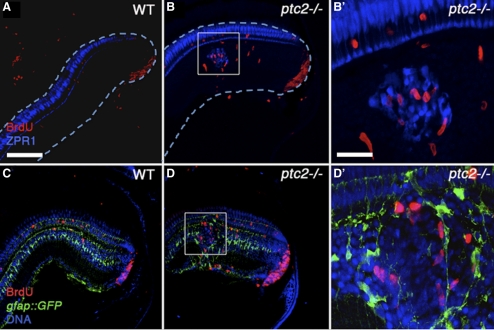
To begin to investigate the effect of the ptc2 mutation on the postembryonic zebrafish retina, histologic analyses were performed on ptc2−/− mutants and their heterozygous and wild-type siblings. Although most ptc2−/− fish do not reach adulthood, ∼2% survive to 6 weeks of age (juveniles). Juvenile ptc2−/− mutants were smaller than their wild-type siblings and had mild pigmentation defects; overall eye structure appeared normal (Fig. 1A). Juvenile ptc2+/− fish were indistinguishable from their wild-type siblings (data not shown) and when examined histologically, retinal morphology, and lamination also appeared to be normal (n = 6; Fig. 1C), when compared with wild-type siblings (Fig. 1B). In ptc2−/− mutants, however, regions of disrupted retinal lamination were detected in the dorsal peripheral retina, as well as morphologic abnormalities in the CMZ and ciliary zone (Fig. 1D). Of the 10 ptc2−/− juveniles that were analyzed by histology, four contained ectopic clusters of cells that appeared to be continuous with the CMZ (n = 4/10; Figs. 1E, 1F). These ectopic cells were incorrectly incorporated into the retina and disrupted normal retinal lamination. Three other ptc2−/− juveniles contained clusters of ectopic cells within the INL; however, lamination in the remainder of the retina in these individuals appeared to be normal (n = 3/10; Figs. 1G, 1H). Examination of sequential histologic sections through the depth of the retina indicated that these clusters did not appear to be continuous with the CMZ (data not shown). These ectopic clusters contained photoreceptors, indicated by the presence of photoreceptor outer segments, and they were associated with an ectopic plexiform layer (Fig. 1H, arrows). Immunohistochemical analyses confirmed the presence of both cone and rod photoreceptors within these dysplastic foci (Figs. 2A–D). In adult syut4+/− mutant fish, which carry a mutant allele of shh, double-cone loss and outer segment abnormalities have been reported.30 Higher magnification images, however, show that in the ptc2−/− juvenile retina, cone morphology was largely normal, and no significant double-cone cell loss was observed (n = 8; Figs. 2E, 2F).
Figure 1.
Juvenile ptc2−/− mutants possess peripheral retinal dysplasias and abnormalities at the ciliary zone. A small percentage of ptc2−/− mutants (∼2%) reach 6 weeks of age. ptc2−/− mutants are smaller than their wild-type siblings and have a disrupted pigmentation pattern (A). Histologic analyses of wild-type (B, E, G, I), ptc2+/− (C), and ptc2−/− (D, F, H, J) 6-week retinas. Retinal organization appeared normal in ptc2+/− mutants (C). In ptc2−/− mutants, retinal disorganization was found in the dorsal peripheral retina, whereas the rest of the retina was laminated normally (D). Within the dorsal peripheral retina of ptc2−/− mutants, ectopic neurons that appeared to be continuous with the CMZ and disrupted lamination were detected (F, arrow; n = 4/10). Ectopic neuronal clusters in the INL were also observed (H, n = 3/10). Neuronal clusters contained photoreceptor outer segments (H, red arrow) and were associated with an ectopic plexiform layer (H, white arrow). Morphologic abnormalities and ectopic cells (J, arrow) were observed in the ciliary zone (n = 3/10). Scale bar: (B–D) 100 μm; (E–J) 25 μm.
Figure 2.
Ectopic neuronal clusters in juvenile ptc2−/− retinas contain rod and cone photoreceptors. Both ZPR3-expressing rods (B, arrow) and ZPR1-expressing red/green double cones (D, arrow) were detected in the ectopic neuronal clusters in ptc2−/− retinas, whereas in wild-type retinas (A, C), photoreceptors were restricted to the ONL. Higher magnification views of cone photoreceptor morphology in wild-type (E) and ptc2−/− (F) retinas. Scale bar: (A–D) 100 μm; (E, F) 50 μm.
In addition, 5 of the 10 ptc2−/− juveniles examined possessed abnormalities in the ciliary zone, a region located at the base of the iris, which contains a nonpigmented epithelium continuous with the CMZ (n = 5/10).31 These abnormalities varied in severity between affected individuals and were characterized by the presence of ectopic cells surrounded by pigmented tissue (Figs. 1I, 1J). Immunohistochemical analyses revealed that this abnormal ciliary zone contained differentiated neurons characteristic of the neural retina (Supplementary Fig. S1, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-8033/-/DCSupplemental).
The Juvenile ptc2 Retina Is Overproliferative
The Hh pathway promotes retinal progenitor proliferation in multiple model organisms,15,32,33 and in Ptch1+/− mice, ectopic proliferation has been observed, both within the mature retina, where proliferation is normally minimal at post-embryonic stages, and in the retinal periphery.14,15 Indeed, ectopic proliferation is thought to underlie the formation of retinal dysplasia in Ptch1+/− mice14; however, a direct relationship between proliferation and retinal dysplasia has not been experimentally established. To begin to address this question, it was first necessary to determine whether proliferation was indeed perturbed in juvenile zebrafish ptc2−/− mutants.
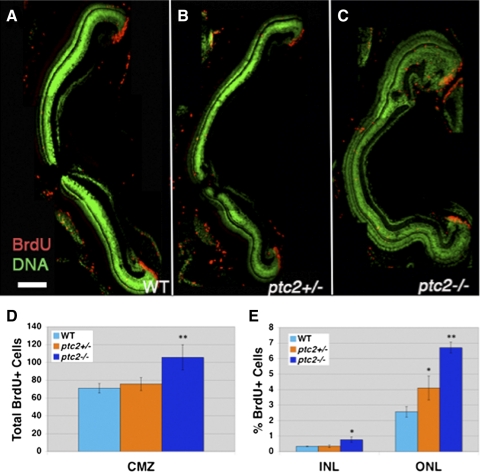
To quantify proliferation, a BrdU incorporation assay was performed on ptc2−/−, ptc2+/−, and wild-type siblings. Six-week-old fish were exposed to BrdU for 2 hours and euthanized and the percentage of BrdU+ cells of the total cells was determined for the INL and ONL, and the total number of BrdU+ cells was determined for the CMZ. Statistically significant increases in the percentage of BrdU+ cells were observed in ptc2−/− mutants in the INL (n = 4, 2.30-fold increase; P < 0.01) and ONL (n = 4, 2.62-fold increase; P < 0.001; Figs. 3A, 3C, 3E), when compared with wild-type fish. In addition, the total number of BrdU+ cells in the CMZ increased by 54% in ptc2−/− mutants when compared with wild-type siblings (n = 4, Figs. 3A, 3C, 3D). Interestingly, overproliferation within the INL was mostly confined to the retinal periphery (Supplementary Fig. S2, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-8033/-/DCSupplemental), where proliferation is normally restricted to a small population of Müller glia. In the INL, Müller glia proliferation is thought to give rise to rod progenitor cells that then migrate to the ONL and differentiate into rod photoreceptors.34,35 Overproliferation in the INL could therefore be due to Müller glia overproliferation, whereas the increase in BrdU+ cells in the ONL suggests an overproduction of rod progenitors. Finally, although no differences in the INL or CMZ were observed in ptc2+/− fish, a 1.58-fold increase in the percentage of BrdU+ cells in the ONL was observed when compared with those in the siblings (n = 4, P < 0.01; Figs. 3B, 3D, 3E).
Figure 3.
The ptc2−/− retina is overproliferative. BrdU incorporation (2 hours) at 6 weeks revealed an increase in the number of proliferative cells in the ptc2−/− retina (C) when compared to wild-type (A) (n = 4). The total number of BrdU+ cells in the CMZ was increased by 54% in ptc2−/− mutants when compared with wild-type siblings (**P < 0.001, n = 4) (D). The percentage of BrdU+ cells in the INL (2.30-fold, *P < 0.01, n = 4) and ONL (2.62-fold, **P < 0.001, n = 4) were significantly increased in ptc2−/− mutants when compared to wild-type siblings (E). A statistically significant increase in the percentage of BrdU+ cells was also observed in the ONL of ptc2+/− mutants (B, E) (1.54-fold, *P < 0.01, n = 4) when compared to wild-type (A, E). Scale bar: 100 μm.
ptc2 Is Expressed in the Progenitor/Stem Cell Populations of the Juvenile Retina
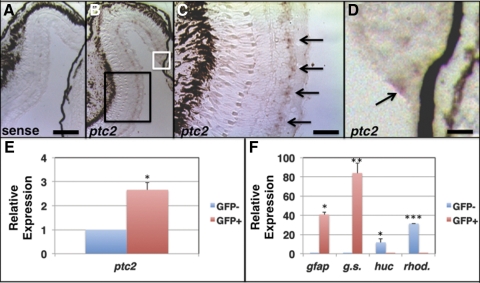
Given the overproliferation phenotypes in ptc2−/− mutants, we next wanted to define the cell types in which ptc2 was expressed in the juvenile eye. In situ hybridization for ptc2 transcripts was performed on retinal cryosections from 6-week-old wild-type embryos. ptc2 expression was observed in a discrete population of cells located within the INL (Figs. 4B, 4C). In the mouse, Ptch1 (the orthologue of the zebrafish ptc219) is expressed in Müller glia,36 and the location of ptc2-expressing cells in the zebrafish INL suggested that they were Müller glia. To determine whether zebrafish Müller glia express ptc2, a gfap::GFP transgenic line was used that expresses GFP in Müller glia.29,37 GFP+ and GFP− cells were isolated from dissociated retinas by FACS, and quantification of ptc2 levels by qRT-PCR in GFP+ and GFP− cell populations revealed a 2.66-fold enrichment in GFP+ cells (n = 3, P < 0.01; Fig. 4E). In addition, transcripts of the Müller glial markers gfap and glutamine synthetase were significantly enriched in the isolated GFP+ cell population and those of neuronal markers huc and rhodopsin were significantly enriched in GFP− cells, verifying the efficacy of cell sorting (Fig. 4F).
Figure 4.
ptc2 is expressed in progenitor/stem cell populations of the juvenile zebrafish retina. In situ hybridization for the ptc2 transcript in 6-week-old juvenile wild-type retinas. Staining was absent throughout the retina when hybridization was performed using a ptc2 sense control probe (A). (B) ptc2 expression was detected in a population of cells in the INL (C; High magnification of the black box) and in the retinal margin (D; high magnification of white box), where CMZ progenitors reside. qRT-PCR analysis of ptc2 expression from FACS-sorted GFP+ Müller glia cells isolated from a gfap:GFP transgenic line revealed enrichment of the ptc2 transcript in GFP+ Müller glia when compared to GFP−, non-Müller glial cells (E, *P < 0.01, n = 3). GFP+ cells were enriched for the Müller glial markers gfap (***P < 0.01, n = 3) and glutamine synthetase (**P < 0.001, n = 3), and GFP− cells were enriched for the neuronal markers huc (*P < 0.01, n = 3) and rhodopsin (***P < 0.0001, n = 3) (F). Scale bars: (A, B) 100 μm; (C) 50 μm; (D) 25 μm.
ptc2 expression was previously reported in the most peripheral region of the CMZ in embryonic zebrafish,24,38 and in Ptch1lacZ+/− mice, lacZ staining is detected in a small population of cells at the retinal margin.15 In juvenile zebrafish, ptc2 was also detected at low levels in a few cells at the CMZ (Figs. 4B, 4D). Thus, ptc2 is expressed in the two progenitor–stem cell populations of the postembryonic zebrafish retina, the CMZ, and Müller glia, suggesting that it could have a role within these cell populations to regulate proliferation.
Activation of the Hedgehog pathway results in the expression of Hedgehog target genes, such as Gli1 and Ptch,39,40 as do mutations in Ptch.13 To determine whether Hh-dependent transcriptional activity is perturbed in the juvenile ptc2−/− retina, we performed qRT-PCR analysis to quantify transcript levels of gli1 and ptc2 in eyes isolated from juvenile ptc2−/− fish and their wild-type siblings. A statistically significant increase in the relative amount of both gli1 (3.63-fold increase, n = 3, P < 0.001) and ptc2 (3.03-fold increase, n = 3, P < 0.01; both in Supplementary Fig. S3, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-8033/-/DCSupplemental) was observed, suggesting that the Hh pathway is indeed overactive in the ptc2−/− mutant eye.
Peripheral Dysplasias in the ptc2−/− Juvenile Retina Contain Continually Proliferative Cells That Do Not Express Müller Glia Markers
BrdU incorporation assays revealed proliferative cells in the peripheral retina, adjacent to the CMZ (Fig. 2; Supplementary Fig. S2, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-8033/-/DCSupplemental), and therefore, overproliferation at the ptc2−/− retinal periphery may be due to CMZ-derived proliferative progenitors that abnormally remain in the cell cycle. Alternatively, Müller glia, which function as endogenous progenitors in the postembryonic retina,35 could be overproliferative. Indeed, in zebrafish, immature Müller glia express higher levels of stem cell markers and are more proliferative than their mature, centrally located counterparts.41,42 Moreover, Shh promotes the proliferation of rat Müller glia in vitro,43 suggesting that Müller glia proliferation is sensitive to Hh pathway activity.
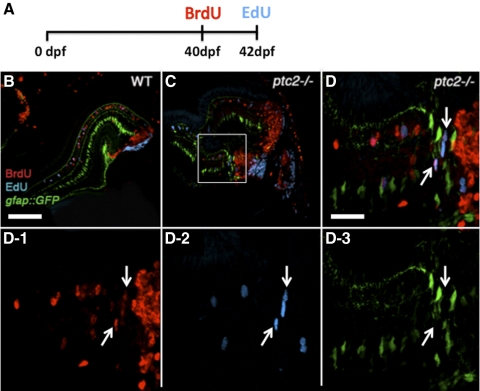
To address these possibilities, a ptc2;gfap::GFP line was generated and a BrdU/EdU double-labeling experiment was performed in this line (Fig. 5A). Briefly, 6-week-old fish were exposed to BrdU for 8 hours, returned to their tanks for 2 days, and then injected with EdU. After a 4-hour recovery, the fish were euthanized, fixed, and processed for immunohistochemistry. In this assay, continually proliferative cells would be BrdU+/EdU+, whereas those that ceased proliferation after the first exposure would only be BrdU+. The presence of GFP suggests a Müller glial identity, whereas an absence suggests a non-Müller glial origin. In the wild-type retina, BrdU+ cells were properly incorporated into the differentiated retina, and a few BrdU+/GFP+ or EdU+/GFP+ cells were observed, indicating proliferative Müller glia (Fig. 5B and data not shown). EdU+ cells were largely restricted to the CMZ and no BrdU+/EdU+ cells were detected outside of the CMZ. Of the 10 ptc2−/−;gfap::GFP fish analyzed, three possessed retinal dysplasia. In all three of these fish, beyond the normal populations of BrdU+ or EdU+ cells, several BrdU+/EdU+ cells were detected in the retinal periphery, physically separated from the EdU+ cells of the CMZ (n = 3/3, Figs. 5C, 5D1–3). Importantly, these BrdU+/EdU+ cells did not co-localize with GFP, indicating that they were not likely to be Müller glia (Figs. 5D1–3).
Figure 5.
Continually proliferative cells in regions of retinal dysplasia in ptc2−/− mutants. A BrdU/EdU double-labeling experiment was performed on wild-type gfap:GFP (B) and ptc2−/−;gfap:GFP mutant retinas (C, D, D1–3). The fish were exposed to BrdU for 8 hours at 40 dpf, returned to their tanks for 2 days, injected with EdU at 42 dpf (6 weeks), and fixed for BrdU and EdU immunohistochemistry and detection (A). In wild-type retinas, cells that were proliferative at the time of fixation (EdU+) were mostly confined to the CMZ, whereas cells that were proliferative 2 days before fixation (BrdU+) had incorporated into the retina. In addition, a few GFP+ Müller glia were also either BrdU+ or EdU+ (B). In ptc2−/−;gfap:GFP retinas, in addition to BrdU+/GFP+ or EdU+/GFP+ Müller glia, BrdU+/EdU+ double-labeled cells were detected in the peripheral retina, and these did not express GFP (C, and white arrows in high magnification of boxed region in D and in D1–3, n = 3/3). Scale bars: (B, C) 100 μm; (D, D1–3) 20 μm.
Ectopic Neuronal Clusters in Regions of Retinal Dysplasia Are Associated with Ectopic Proliferation
We next wanted to investigate the relationship between proliferation and the formation of ectopic photoreceptor-containing neuronal clusters observed in the ptc2−/− retina. Examination of multiple neuronal clusters in the INL revealed ectopic proliferation that was locally associated with these cells (n = 4/5; Figs. 6A, 6B, 6B′). In the Ptch1+/− mouse retina, rosettes are associated with Müller glial reactivity that is thought to occur in response to the presence of the rosettes.31 Reactivity is often characterized by the upregulation of GFAP, and sometimes by increased proliferation.44 To determine whether proliferation around the neuronal clusters in ptc2−/− juveniles resulted from Müller glial reactivity, we examined the spatial relationship between proliferative cells, Müller glia, and regions of retinal dysplasia within the INL of ptc2−/−;gfap::GFP juveniles (Figs. 6C, 6D, 6D′). Although some BrdU+ cells were located adjacent to GFP+ Müller glia, co-localization of BrdU and GFP was not observed in any of these clusters, suggesting that proliferating cells associated with the ectopic neuronal clusters were not Müller glia (n = 0/5; Figs. 6D, 6D′). This finding was also corroborated by the lack of colocalization of GFAP and BrdU, using an anti-GFAP antibody in immunohistochemical assays (Supplementary Fig. S4, http://www.iovs.org/lookup/suppl/doi:10.1167/iovs.11-8033/-/DCSupplemental).
Figure 6.
Ectopic neuronal clusters in regions of retinal dysplasia are locally associated with ectopic proliferation. Immunohistochemical analysis of wild-type retinas after a 2-hour exposure to BrdU revealed few proliferative cells in the INL (A, C), whereas in dysplastic regions of the ptc2−/− retina, BrdU+ cells were locally associated with ectopic ZPR1-expressing red/green double cones in the INL (B). (B′: high magnification of white box.) Analysis of adjacent sections from the same individuals showed that these proliferative cells were not Müller glia, as they did not label with GFP in the gfap:GFP transgenic background (D). (D′: high magnification of white box). Scale bar: 100 μm.
Ectopically Proliferating Cells Generate New Neurons in the Dysplastic Regions of the ptc2−/− Retina
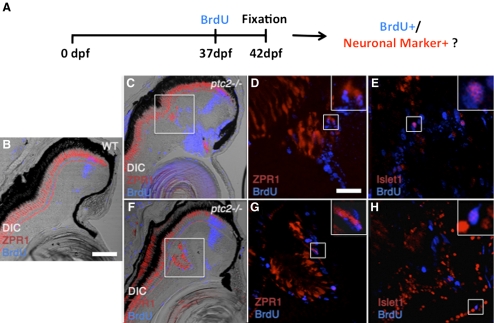
Results thus far indicate that ectopic proliferation is locally associated and correlates with dysplastic regions of the ptc2−/− retina. However, whether these ectopically proliferative cells actually generate the ectopic neurons and thereby contribute to the formation of retinal dysplasias in ptc2−/− mutants is still not known. To directly analyze this possibility, we performed a BrdU incorporation assay combined with immunohistochemistry for the detection of differentiated retinal neurons, to determine whether proliferative cells differentiated into new neurons in ptc2−/− retinas. Experimentally, ptc2−/− juveniles and their wild-type siblings, were exposed to BrdU for 8 hours at the age of 37 days, returned to their tanks for an additional 5 days, and fixed for immunohistochemical analysis at 42 days of age (Fig. 7A). In wild-type siblings, a population of BrdU+ cells was evident as a tight band of cells within the differentiated retina. These BrdU+ cells corresponded to CMZ-derived progenitors that were proliferative at day 37, but by 42 dpf had differentiated and become incorporated into the retina (Fig. 7B). Analysis of ptc2−/− juveniles in this assay revealed dysplasias in 6 of the 22 individuals analyzed. In five of the six ptc2-/– mutants with dysplasias, zpr1-positive red/green double cone photoreceptor outer segments were directly associated with BrdU+ nuclei (n = 5/6, Figs. 7C, 7D, 7F, 7G). Unlike rod photoreceptors that are produced from rod progenitors within the differentiated retina, cone photoreceptors are produced only in the CMZ. Thus, our findings indicate that ectopically proliferative cells can generate cone photoreceptors in juvenile ptc2−/− mutants.
Figure 7.
New neurons are generated by ectopic proliferation in the dysplastic ptc2−/− juvenile retina. A combined BrdU incorporation and immunohistochemical assay to determine whether ectopically proliferative cells generate new neurons in dysplastic regions of the ptc2−/− retina (A). Fish were exposed to BrdU for 8 hours at 37 dpf, returned to their tanks for 5 days, and fixed for immunohistochemistry at 42 dpf (6 weeks). In wild-type siblings (B), BrdU+ cells were detected in a narrow band within the differentiated retina, corresponding to CMZ-derived cells that were proliferative 5 days before fixation, as well as in scattered rod progenitors located in the ONL. Red/green double cones, labeled by ZPR1, were restricted to the ONL. In two different ptc2−/− retinas, BrdU+ cells colocalized with ZPR1 (C, F, high magnification of boxed areas in D, G). In adjacent sections from the same individuals, BrdU+ cells were also positive for Islet1, a marker of retinal ganglion cells, and subsets of differentiated amacrine, bipolar, and horizontal cells (E, H). (D, E, G, H, insets) Higher magnification of the respective boxed regions in each panel, showing co-localization of BrdU and the neuronal marker. Scale bars: (B, C, F) 100 μm; (D, E, G, H) 20 μm.
To further determine whether neurons within dysplastic regions arise from ectopically proliferating cells, sections adjacent to those used for zpr1 immunohistochemistry were stained for Islet1, a marker of retinal ganglion cells and subsets of differentiated amacrine, bipolar, and horizontal cells.45,46 Indeed, in these sections, Islet1 co-localized with BrdU (n = 3/4, Figs. 7E, 7H), indicating that ectopically proliferating cells in the ptc2−/− mutant retina also generated Islet1-expressing neurons. Taken together, these results point to ectopic proliferation as the source of ectopic neurons in the ptc2−/− retina and as the likely cellular underpinning of the retinal dysplasias observed in juvenile ptc2−/− mutants.
Discussion
Our analyses have revealed abnormalities in the juvenile ptc2−/− mutant zebrafish retina, confined to the peripheral retina, that included ectopic proliferation and retinal dysplasias, composed of ectopic neuronal clusters, within the INL. These phenotypes are similar to those observed in the dysplastic Ptch1+/− mouse retina.14,15 From studies of the Ptch1+/− mouse, it has been hypothesized that retinal dysplasias could result from retinal progenitor overproliferation. Our results demonstrate that retinal neurons within the dysplastic regions of the ptc2−/− juvenile retina do indeed arise from an ectopic proliferative event, supporting a model in which loss of Patched 2 function leads to overproliferation of cells within the retina, ultimately contributing to retinal dysplasias in ptc2−/− mutants.
It is of interest, considering the known role of Patched as a tumor suppressor,47,48 that retinal phenotypes in the ptc2 juvenile retina occur in regions that are either known to, or thought to, contain retinal stem cells. Although the lineage in the ectopically proliferating cells in the ptc2−/− juvenile retina is still unknown, expression of ptc2 in both the CMZ and in Müller glia suggests that one of these progenitor/stem cell populations is the source of these proliferative cells. The presence of ectopically proliferating cells adjacent to the CMZ (Fig. 5), as well as locally associated with neuronal clusters (Fig. 6) in the ptc2−/− juvenile retina, may account for the increased number of proliferative cells in the INL. Unlike the CMZ which gives rise to multiple retinal cell types, Müller glia give rise to lineage restricted rod progenitors that then migrate to the ONL and differentiate into rod photoreceptors.34,35 However, on exposure to growth factors or in response to physical injury to the retina, Müller glia are thought to dedifferentiate, proliferate, and give rise to all neuronal cells types.35,49,50 Analysis of proliferation and retinal dysplasia in ptc2−/−;gfap::GFP transgenic fish revealed that ectopically proliferating cells were not likely to have been derived from Müller glia. Moreover, preliminary lineage tracing studies using ptc2−/−;tuba1a::CreERT2;β-actin2::LCLG transgenic fish51 have not detected Müller glia–derived cells in ptc2−/− mutants (JB, unpublished observations, 2011). Although we cannot exclude the possibility that the ectopically proliferating cells in juvenile ptc2−/− mutant retinas are of Müller glia origin, our findings to date do not support this possibility. Identification of the cellular origin of the these ectopic neurons can only be unambiguously completed using targeted transgenic lineage tracing tools, and multiple cellular origins for the ectopically proliferating cells are possible; however, our results suggest that misregulation of progenitor cell proliferation in the CMZ is very likely to contribute to the process and lead to the formation of ectopic neurons and retinal dysplasias in ptc2−/− mutants.
Interestingly, the retinal lamination defects and the production of newly born neurons within the differentiated retina that are observed in the ptc2−/− retina resemble the histologic disruptions previously observed in retinas that have regenerated after injury.52,53 Like Müller glia, the CMZ has been shown to overproliferate in response to retinal damage or on exposure to growth factors.54,55 Increased retinal proliferation in the ptc2−/− retina can therefore also be due to a secondary effect in response to retinal damage, which may explain the observation that retinal dysplasias are local and do not occur throughout the retina. Why these dysplastic regions are restricted to the juvenile dorsal retinal periphery is unclear. Expression of a BMP reporter transgene in the dorsal, but not ventral, zebrafish CMZ suggests differences in transcriptional activities between the dorsal and ventral growth zones of the adult retina.56 Such differences may contribute to the different phenotypes observed in the dorsal and ventral retinal periphery.
Although the mitogenic role of Patched during embryonic neural development is well established,13,57 less is known regarding its function in the postembryonic central nervous system (CNS). In zebrafish embryos, a loss-of-function mutation in ptc1 (the orthologue of the mammalian Ptch219) results in morphologic abnormalities during early eye development, whereas retinal proliferation appears to be unaffected.58 ptc2 loss-of-function, however, is associated with increased retinal proliferation at the CMZ,24 a function that appears to be retained in the later, juvenile stages examined in our study. Histologic analysis of adult ptc1−/− mutant retinas revealed no obvious defects (JB, unpublished observations, 2010), suggesting that ptc1 does not play an important role in homeostasis of the adult retina.
In the postnatal mouse cerebellum, the Hh pathway promotes precursor cell proliferation,59,60 and medulloblastomas, tumors of the cerebellum, have been described in both Ptch1+/− mice and in BCNS patients.13,61 In the mouse, loss of Ptch1 function results in increased retinal proliferation, suggesting that Ptch1 acts to negatively regulate progenitor/stem cell proliferation.14,15 The overproliferation in the progenitor/stem cell populations of the juvenile ptc2−/− retina certainly supports this role. Although, to our knowledge, no histologic characterizations of human BCNS retinas have been published to date, the results presented herein could provide valuable insight into the contributing causes of visual impairment in BCNS patients. Whether ptc2−/− juvenile fish possess BCNS-related phenotypes in tissues other than the retina remains to be investigated; however, the utility of the ptc2−/− mutant line for such studies provides an additional, and valuable, in vivo model for the study of Patched function during development and in diseases like BCNS.
Supplementary Material
Acknowledgments
The authors thank Daniel Goldman (University of Michigan, Ann Arbor, MI) for providing us with tuba1a:CreERT2;β-actin2:LCLG transgenic fish; Ann Morris, Peter Hitchcock, Ryan Thummel, Eric Bachmann, and Rima Aimas for technical advice; and members of the Gross laboratory for helpful suggestions.
Footnotes
Supported by National Institutes of Health (NIH) Grant R01-EY18005 (JMG). Antisera and zebrafish lines were obtained from the Zebrafish International Resource Center, supported by National Institutes of Health, National Center for Research Resources (NIH-NCRR) Grant P40 RR012546.
Disclosure: J. Bibliowicz, None; J.M. Gross, None
References
- 1. Ekker SC, Ungar AR, Greenstein P, et al. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol. 1995;5:944–955 [DOI] [PubMed] [Google Scholar]
- 2. Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756 [DOI] [PubMed] [Google Scholar]
- 3. Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100 [DOI] [PubMed] [Google Scholar]
- 4. Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ohnuma S, Hopper S, Wang KC, Philpott A, Harris WA. Co-ordinating retinal histogenesis: early cell cycle exit enhances early cell fate determination in the Xenopus retina. Development. 2002;129:2435–2446 [DOI] [PubMed] [Google Scholar]
- 6. Locker M, Agathocleous M, Amato MA, Parain K, Harris WA, Perron M. Hedgehog signaling and the retina: insights into the mechanisms controlling the proliferative properties of neural precursors. Genes Dev. 2006;20:3036–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wallace VA. Proliferative and cell fate effects of Hedgehog signaling in the vertebrate retina. Brain Res. 2008;1192:61–75 [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Dakubo GD, Thurig S, Mazerolle CJ, Wallace VA. Retinal ganglion cell-derived sonic hedgehog locally controls proliferation and the timing of RGC development in the embryonic mouse retina. Development. 2005;132:5103–5113 [DOI] [PubMed] [Google Scholar]
- 9. Sakagami K, Gan L, Yang XJ. Distinct effects of Hedgehog signaling on neuronal fate specification and cell cycle progression in the embryonic mouse retina. J Neurosci. 2009;29:6932–6944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the Hedgehog receptor. Nature. 1996;384:176–179 [DOI] [PubMed] [Google Scholar]
- 11. Stone DM, Hynes M, Armanini M, et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–134 [DOI] [PubMed] [Google Scholar]
- 12. High A, Zedan W. Basal cell nevus syndrome. Curr Opin Oncol. 2005;17:160–166 [DOI] [PubMed] [Google Scholar]
- 13. Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113 [DOI] [PubMed] [Google Scholar]
- 14. Black GC, Mazerolle CJ, Wang Y, et al. Abnormalities of the vitreoretinal interface caused by dysregulated Hedgehog signaling during retinal development. Hum Mol Genet. 2003;12:3269–3276 [DOI] [PubMed] [Google Scholar]
- 15. Moshiri A, Reh TA. Persistent progenitors at the retinal margin of ptc+/− mice. J Neurosci. 2004;24:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Negishi K, Teranishi T, Kato S. Growth zone of the juvenile goldfish retina revealed by fluorescent flat mounts. J Neurosci Res. 1982;7:321–330 [DOI] [PubMed] [Google Scholar]
- 17. Wetts R, Serbedzija GN, Fraser SE. Cell lineage analysis reveals multipotent precursors in the ciliary margin of the frog retina. Dev Biol. 1989;136:254–263 [DOI] [PubMed] [Google Scholar]
- 18. Perron M, Kanekar S, Vetter ML, Harris WA. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev Biol. 1998;199:185–200 [DOI] [PubMed] [Google Scholar]
- 19. Lewis KE, Concordet JP, Ingham PW. Characterisation of a second patched gene in the zebrafish Danio rerio and the differential response of patched genes to Hedgehog signalling. Dev Biol. 1999;208:14–29 [DOI] [PubMed] [Google Scholar]
- 20. Koudijs MJ, den Broeder MJ, Keijser A, et al. The zebrafish mutants dre, uki, and lep encode negative regulators of the hedgehog signaling pathway. PLoS Genet. 2005;1:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson RL, Milenkovic L, Scott MP. In vivo functions of the patched protein: requirement of the C terminus for target gene inactivation but not Hedgehog sequestration. Mol Cell. 2000;6:467–478 [DOI] [PubMed] [Google Scholar]
- 22. van den Heuvel M, Ingham PW. Smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature. 1996;382:547–551 [DOI] [PubMed] [Google Scholar]
- 23. Murone M, Rosenthal A, de Sauvage FJ. Sonic hedgehog signaling by the patched-smoothened receptor complex. Curr Biol. 1999;9:76–84 [DOI] [PubMed] [Google Scholar]
- 24. Bibliowicz J, Gross JM. Expanded progenitor populations, vitreo-retinal abnormalities, and Muller glial reactivity in the zebrafish leprechaun/patched2 retina. BMC Dev Biol. 2009;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio). 4th ed Eugene, OR: The University of Oregon Press; 2000 [Google Scholar]
- 26. Nuckels RJ, Gross JM. Histological preparation of embryonic and adult zebrafish eyes. CSH Protocols. 2007;2007:pdb prot4846 [DOI] [PubMed] [Google Scholar]
- 27. Uribe RA, Gross JM. Immunohistochemistry on cryosections from embryonic and adult zebrafish eyes. CSH Protocols. 2007;2007:pdb prot4779 [DOI] [PubMed] [Google Scholar]
- 28. Barthel LK, Raymond PA. In situ hybridization studies of retinal neurons. Methods Enzymol. 2000;316:579–590 [DOI] [PubMed] [Google Scholar]
- 29. Qin Z, Barthel LK, Raymond PA. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc Natl Acad Sci USA. 2009;106:9310–9315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stenkamp DL, Satterfield R, Muhunthan K, Sherpa T, Vihtelic TS, Cameron DA. Age-related cone abnormalities in zebrafish with genetic lesions in sonic hedgehog. Invest Ophthalmol Vis Sci. 2008;49:4631–4640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soules KA, Link BA. Morphogenesis of the anterior segment in the zebrafish eye. BMC Dev Biol. 2005;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jensen AM, Wallace VA. Expression of Sonic hedgehog and its putative role as a precursor cell mitogen in the developing mouse retina. Development. 1997;124:363–371 [DOI] [PubMed] [Google Scholar]
- 33. Moshiri A, McGuire CR, Reh TA. Sonic hedgehog regulates proliferation of the retinal ciliary marginal zone in posthatch chicks. Dev Dyn. 2005;233:66–75 [DOI] [PubMed] [Google Scholar]
- 34. Otteson DC, D'Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232:62–76 [DOI] [PubMed] [Google Scholar]
- 35. Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang YP, Dakubo G, Howley P, et al. Development of normal retinal organization depends on Sonic hedgehog signaling from ganglion cells. Nat Neurosci. 2002;5:831–832 [DOI] [PubMed] [Google Scholar]
- 37. Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene Expr Patterns. 2006;6:1007–1013 [DOI] [PubMed] [Google Scholar]
- 38. Stenkamp DL, Frey RA, Prabhudesai SN, Raymond PA. Function for Hedgehog genes in zebrafish retinal development. Dev Biol. 2000;220:238–252 [DOI] [PubMed] [Google Scholar]
- 39. Lee J, Platt KA, Censullo P, Ruiz i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–2552 [DOI] [PubMed] [Google Scholar]
- 40. Hidalgo A, Ingham P. Cell patterning in the Drosophila segment: spatial regulation of the segment polarity gene patched. Development. 1990;110:291–301 [DOI] [PubMed] [Google Scholar]
- 41. Julian D, Ennis K, Korenbrot JI. Birth and fate of proliferative cells in the inner nuclear layer of the mature fish retina. J Comp Neurol. 1998;394:271–282 [PubMed] [Google Scholar]
- 42. Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wan J, Zheng H, Xiao HL, She ZJ, Zhou GM. Sonic hedgehog promotes stem-cell potential of Muller glia in the mammalian retina. Biochem Biophys Res Commun. 2007;363:347–354 [DOI] [PubMed] [Google Scholar]
- 44. Sahel JA, Albert DM, Lessell S. Proliferation of retinal glia and excitatory amino acids (in French). Ophtalmologie. 1990;4:13–16 [PubMed] [Google Scholar]
- 45. Link BA, Fadool JM, Malicki J, Dowling JE. The zebrafish young mutation acts non-cell-autonomously to uncouple differentiation from specification for all retinal cells. Development. 2000;127:2177–2188 [DOI] [PubMed] [Google Scholar]
- 46. Shkumatava A, Fischer S, Muller F, Strahle U, Neumann CJ. Sonic hedgehog, secreted by amacrine cells, acts as a short-range signal to direct differentiation and lamination in the zebrafish retina. Development. 2004;131:3849–3858 [DOI] [PubMed] [Google Scholar]
- 47. Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851 [DOI] [PubMed] [Google Scholar]
- 48. Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671 [DOI] [PubMed] [Google Scholar]
- 49. Fischer AJ, Reh TA. Potential of Muller glia to become neurogenic retinal progenitor cells. Glia. 2003;43:70–76 [DOI] [PubMed] [Google Scholar]
- 50. Das AV, Mallya KB, Zhao X, et al. Neural stem cell properties of Muller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302 [DOI] [PubMed] [Google Scholar]
- 51. Ramachandran R, Reifler A, Parent JM, Goldman D. Conditional gene expression and lineage tracing of tuba1a expressing cells during zebrafish development and retina regeneration. J Comp Neurol. 2010;518:4196–4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hitchcock PF, Lindsey Myhr KJ, Easter SS J., Mangione-Smith R, Jones DD. Local regeneration in the retina of the goldfish. J Neurobiol. 1992;23:187–203 [DOI] [PubMed] [Google Scholar]
- 53. Stenkamp DL, Powers MK, Carney LH, Cameron DA. Evidence for two distinct mechanisms of neurogenesis and cellular pattern formation in regenerated goldfish retinas. J Comp Neurol. 2001;431:363–381 [DOI] [PubMed] [Google Scholar]
- 54. Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220:197–210 [DOI] [PubMed] [Google Scholar]
- 55. Fischer AJ, Reh TA. Growth factors induce neurogenesis in the ciliary body. Dev Biol. 2003;259:225–240 [DOI] [PubMed] [Google Scholar]
- 56. Collery RF, Link BA. Dynamic smad-mediated BMP signaling revealed through transgenic zebrafish. Dev Dyn. 2011;240:712–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hahn H, Christiansen J, Wicking C, et al. A mammalian patched homolog is expressed in target tissues of sonic hedgehog and maps to a region associated with developmental abnormalities. J Biol Chem. 1996;271:12125–12128 [DOI] [PubMed] [Google Scholar]
- 58. Lee J, Willer JR, Willer GB, Smith K, Gregg RG, Gross JM. Zebrafish blowout provides genetic evidence for Patched1-mediated negative regulation of Hedgehog signaling within the proximal optic vesicle of the vertebrate eye. Dev Biol. 2008;319:10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114 [DOI] [PubMed] [Google Scholar]
- 60. Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448 [DOI] [PubMed] [Google Scholar]
- 61. Peringa J, Fung KM, Muragaki Y, Trojanowski JQ. The cellular and molecular biology of medulloblastoma. Curr Opin Neurol. 1995;8:437–440 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.