Abstract
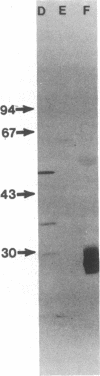
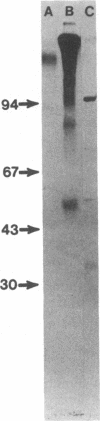
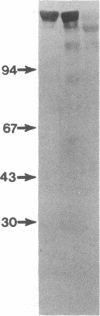
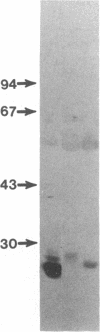
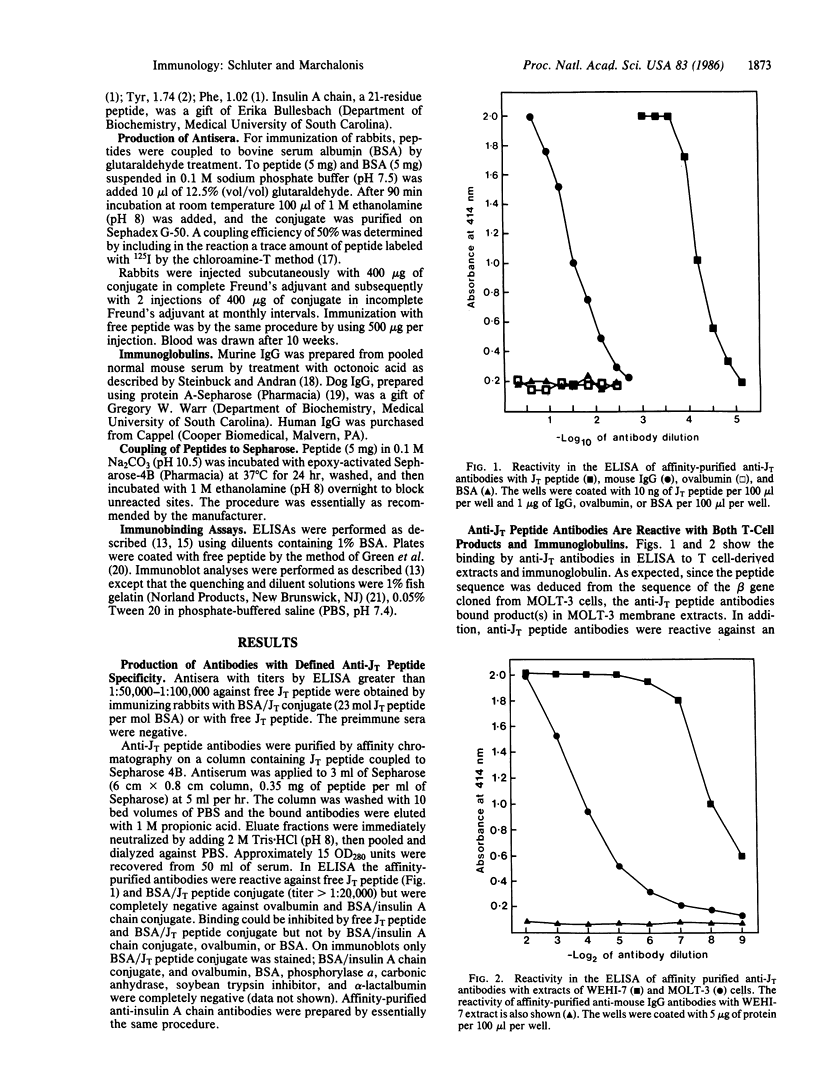
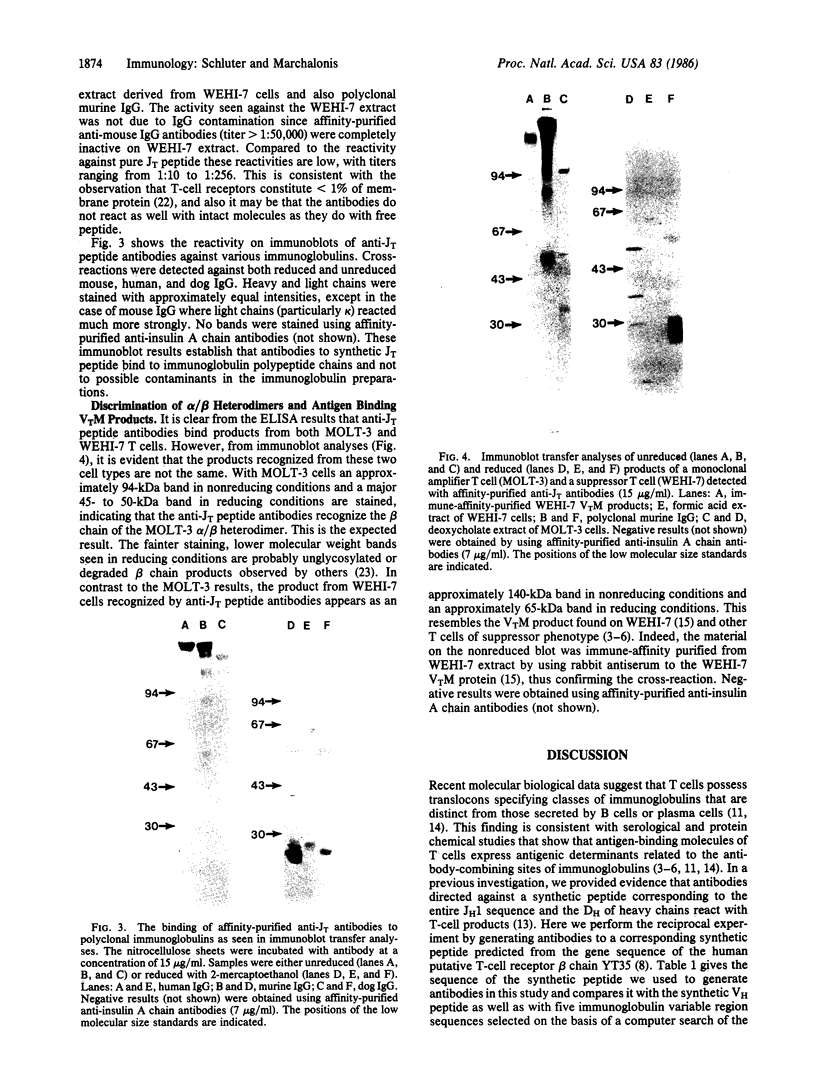
Immunoglobulin light and heavy chains show sequence homology to one another and to the polypeptide chains of putative T-cell receptors in the J (joining) segment of the variable region. Antibodies produced against synthetic peptides corresponding to the entire JH1 region and part of the diversity segment region cross-react serologically with products of normal T cells and monoclonal T-cell lines. In this study we generate immune affinity-purified rabbit antibodies to a synthetic 16-mer peptide consisting of the entire JT sequence and part of the T-cell diversity sequence corresponding to these segments of the human putative T-cell receptor beta gene YT35. Both free peptide and peptide coupled to bovine serum albumin as carrier were found to stimulate the production of antibody. The immune affinity-purified anti-JT peptide antibodies bound to intact immunoglobulin and to light and heavy chain as detected by enzyme-linked immunosorbent assay and by immunoblot transfer. The antibody reacted by these techniques with membrane components of the human monoclonal amplifier T-cell MOLT-3 and the murine suppressor T-cell WEHI-7. The component detected in the MOLT-3 cell corresponded to the beta-chain of the alpha/beta heterodimer putative T-cell receptor; whereas the molecule detected in the WEHI-7 line had properties corresponding to those of antigen-specific T-cell suppressor receptors. The molecular size of this component under reducing conditions was approximately 68 kDa and the intact form had an apparent mass of 140 kDa. These results provide direct proof of serological cross-reaction among products of putative T-cell receptor genes, antigen-binding T-cell receptors, and immunoglobulins, thereby supporting the concept that antigen receptors of T lymphocytes all represent new immunoglobulin translocons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Fabbi M., Bensussan A., Milanese C., Campen T. J., Royer H. D., Reinherz E. L. The human T-cell receptor. J Clin Immunol. 1985 May;5(3):141–157. doi: 10.1007/BF00915505. [DOI] [PubMed] [Google Scholar]
- Binz H., Wigzell H. T cell receptors with allo-major histocompatibility complex specificity from rat and mouse. Similarity of size, plasmin susceptibility, and localization of antigen-binding region. J Exp Med. 1981 Nov 1;154(5):1261–1278. doi: 10.1084/jem.154.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald L., Ades E. W., Tung E., Marchalonis J. J., Wang A. C. Biochemical characterization of human Thy-1. J Immunogenet. 1984 Oct-Dec;11(5-6):283–296. doi: 10.1111/j.1744-313x.1984.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Capra J. D., Kehoe J. M. Hypervariable regions, idiotypy, and the antibody-combining site. Adv Immunol. 1975;20:1–40. doi: 10.1016/s0065-2776(08)60205-9. [DOI] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Cone R. E., Rosenstein R. W., Janeway C. A., Iverson G. M., Murray J. H., Cantor H., Fresno M., Mattingly J. A., Cramer M., Krawinkel U. Affinity-purified antigen-specific products produced by T cells share epitopes recognized by heterologous antisera raised against several different antigen-specific products from T cells. Cell Immunol. 1983 Dec;82(2):232–245. doi: 10.1016/0008-8749(83)90158-2. [DOI] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Healy C. T., Kapp J. A., Webb D. R. Purification and biochemical analysis of antigen-specific suppressor factors obtained from the supernatant, membrane, or cytosol of a T cell hybridoma. J Immunol. 1983 Dec;131(6):2843–2847. [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Mackel-Vandersteenhoven A., Moseley J. M., Marchalonis J. J. Partial characterization of T cell components related to defined VH (VT) markers. Cell Immunol. 1984 Oct 1;88(1):147–161. doi: 10.1016/0008-8749(84)90060-1. [DOI] [PubMed] [Google Scholar]
- Mage R. G., Bernstein K. E., McCartney-Francis N., Alexander C. B., Young-Cooper G. O., Padlan E. A., Cohen G. H. The structural and genetic basis for expression of normal and latent VHa allotypes of the rabbit. Mol Immunol. 1984 Nov;21(11):1067–1081. doi: 10.1016/0161-5890(84)90117-2. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. Antigen-binding molecules of T cells: distinction from MHC-restricted molecules and segmental homology to immunoglobulin VH and T-cell receptor genes. Scand J Immunol. 1985 Feb;21(2):99–107. doi: 10.1111/j.1365-3083.1985.tb01408.x. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. Lymphocyte surface immunoglobulins. Science. 1975 Oct 3;190(4209):20–29. doi: 10.1126/science.1101378. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Warr G. W., Rodwell J. D., Karush F. Surface component of primate thymus-derived lymphocytes related to a heavy chain variable region. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3625–3629. doi: 10.1073/pnas.77.6.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. 1984 Jun 28-Jul 4Nature. 309(5971):757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- Seiden M. V., Clevinger B., McMillan S., Srouji A., Lerner R., Davie J. M. Chemical synthesis of idiotopes. Evidence that antisera to the same JH1 peptide detect multiple binding site-associated idiotopes. J Exp Med. 1984 May 1;159(5):1338–1350. doi: 10.1084/jem.159.5.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda S., Schlamowitz M. Studies of 125I trace labeling of immunoglobulin G by chloramine-T. Immunochemistry. 1970 Nov;7(11):885–898. doi: 10.1016/0019-2791(70)90051-0. [DOI] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Suzuki G., Cramer M., Hayakawa K., Okumura K., Tada T. Idiotypic and fine specificity analysis of a (4-hydroxy-3-nitrophenyl)acetyl (NP)-specific suppressor T cell hybridoma at the level of cell surface structures, isolated receptor material and functional suppressor factor. Eur J Immunol. 1983 Sep;13(9):711–719. doi: 10.1002/eji.1830130905. [DOI] [PubMed] [Google Scholar]
- Vasta G. R., Marchalonis J. J., Kohler H. Invertebrate recognition protein cross-reacts with an immunoglobulin idiotype. J Exp Med. 1984 Apr 1;159(4):1270–1276. doi: 10.1084/jem.159.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr G. W., Hart I. R. Binding of canine IgM and IgG to protein A of Staphylococcus aureus: a simple method for the isolation of canine immunoglobulins from serum and the lymphocyte surface. Am J Vet Res. 1979 Jul;40(7):922–926. [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]