Abstract
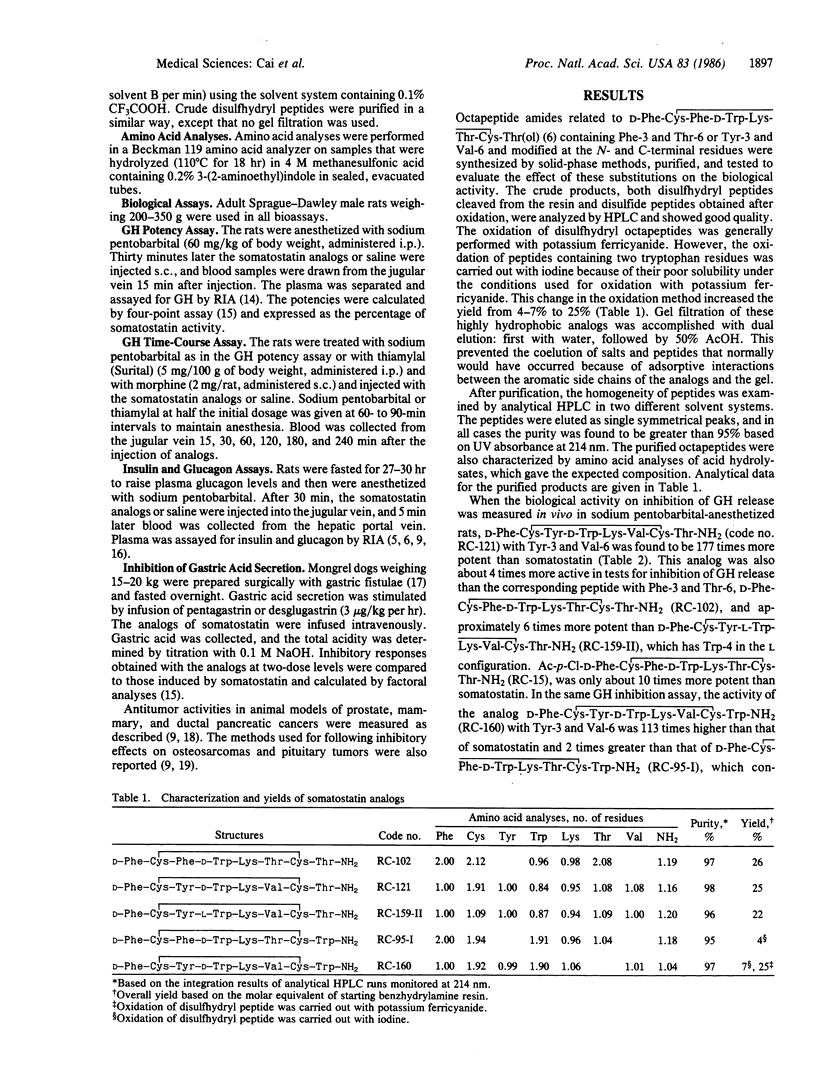
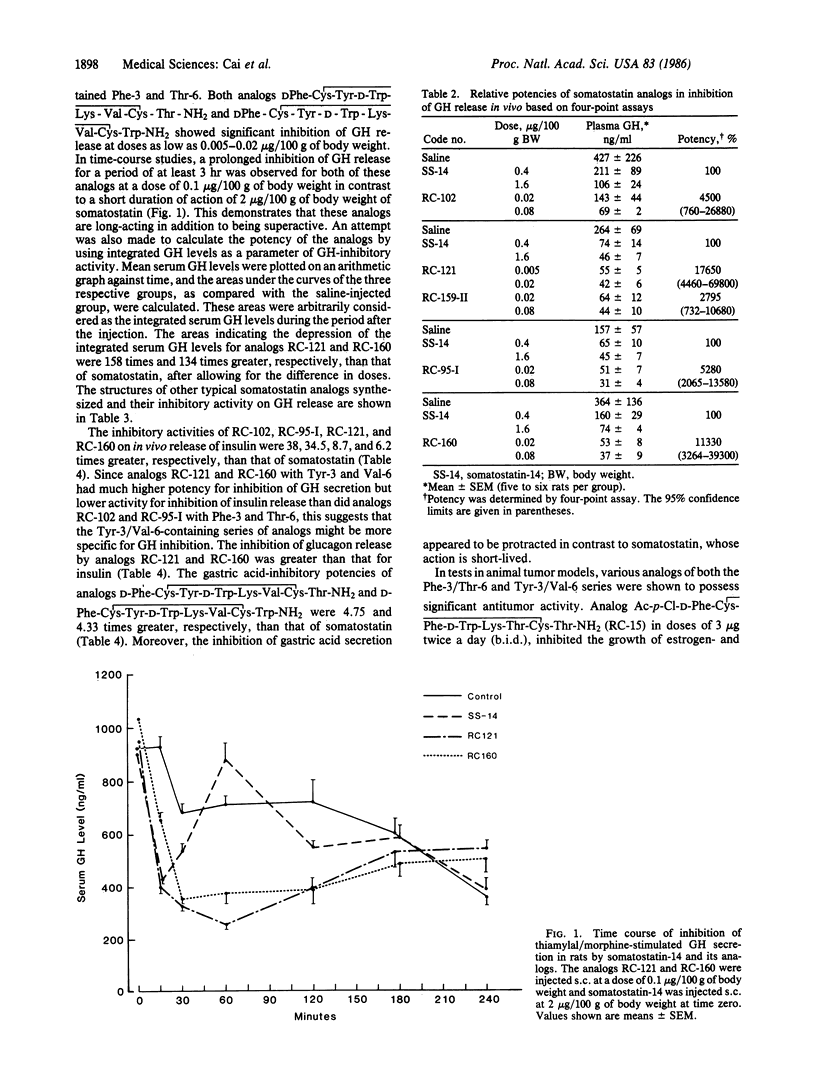
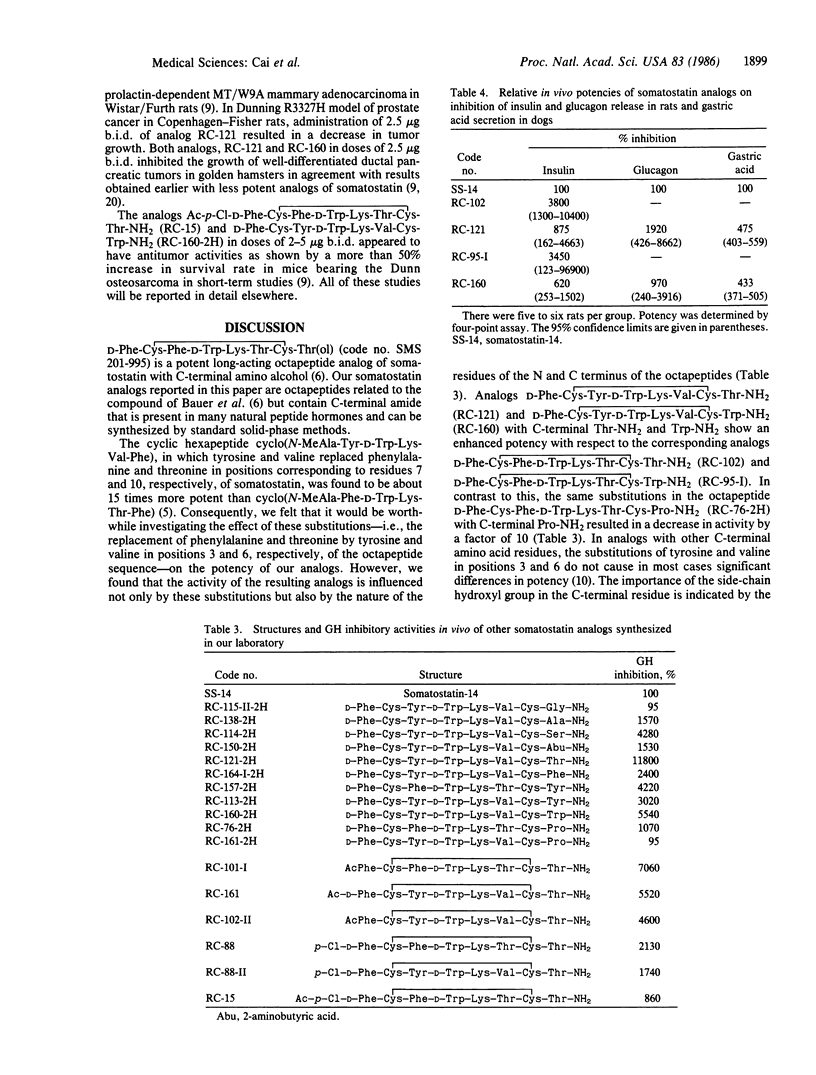
In the search for selective and long-acting analogs of somatostatin, nearly 200 compounds were synthesized by solid-phase methods, purified, and tested biologically. Among these octapeptides, some contained N-terminal (Formula: see text) were 177 times and 113 times more potent, respectively, than somatostatin in tests for inhibition of growth hormone release. These two octapeptides containing tyrosine and valine in positions 3 and 6, respectively, were more active and more selective than their Phe-3 and Thr-6 counterparts, D-Phe-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr-NH2 and D-Phe-Cys-Phe-D-Trp-Lys-Thr-Cys-Trp-NH2. D-Phe-Cys-Tyr-D-Trp-Lys-Val-Cys-Thr-NH2 was also about 6 times more potent than its L-Trp-4 diastereoisomer. The analogs D-Phe-Cys-Tyr-Lys-Val-Cys-Thr-NH2 and D-Phe-Cys-Tyr-D-Trp-Lys-Val-Cys-Trp-NH2 showed a prolonged duration of action and were able to inhibit growth hormone release for at least 3 hr. Analogs of both Phe-3/Thr-6 and Tyr-3/Val-6 classes also suppressed the release of insulin and glucagon in rats and pentagastrin-induced secretion of gastric acid in dogs, but their potencies in these tests were much smaller than the growth-hormone-release inhibitory activity. Some of these analogs possessed antitumor activities as shown by the inhibition of growth of animal models of prostate, mammary, and ductal pancreatic tumors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Briner U., Doepfner W., Haller R., Huguenin R., Marbach P., Petcher T. J., Pless SMS 201-995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982 Sep 13;31(11):1133–1140. doi: 10.1016/0024-3205(82)90087-x. [DOI] [PubMed] [Google Scholar]
- HOPE D. B., MURTI V. V., DU VIGNEAUD V. A highly potent analogue of oxytocin, desamino-oxytocin. J Biol Chem. 1962 May;237:1563–1566. [PubMed] [Google Scholar]
- Konturek S. J., Tasler J., Krol R., Dembinski A., Coy D. H., Schally A. V. Effect of somatostatin analogs on gastric and pancreatic secretion. Proc Soc Exp Biol Med. 1977 Sep;155(4):519–522. doi: 10.3181/00379727-155-39842. [DOI] [PubMed] [Google Scholar]
- Meyers C. A., Coy D. H., Huang W. Y., Schally A. V., Redding T. W. Highly active position eight analogues of somatostatin and separation of peptide diastereomers by partition chromatography. Biochemistry. 1978 Jun 13;17(12):2326–2331. doi: 10.1021/bi00605a011. [DOI] [PubMed] [Google Scholar]
- Meyers C. A., Murphy W. A., Redding T. W., Coy D. H., Schally A. V. Synthesis and biological actions of prosomatostatin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6171–6174. doi: 10.1073/pnas.77.10.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. A., Meyers C. A., Coy D. H. Potent, highly selective inhibition of growth hormone secretion by position 4 somatostatin analogs. Endocrinology. 1981 Aug;109(2):491–495. doi: 10.1210/endo-109-2-491. [DOI] [PubMed] [Google Scholar]
- Plewe G., Beyer J., Krause U., Neufeld M., del Pozo E. Long-acting and selective suppression of growth hormone secretion by somatostatin analogue SMS 201-995 in acromegaly. Lancet. 1984 Oct 6;2(8406):782–784. doi: 10.1016/s0140-6736(84)90706-2. [DOI] [PubMed] [Google Scholar]
- Redding T. W., Schally A. V. Inhibition of growth of pancreatic carcinomas in animal models by analogs of hypothalamic hormones. Proc Natl Acad Sci U S A. 1984 Jan;81(1):248–252. doi: 10.1073/pnas.81.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier J., Brown M., Vale W. D-Trp8-somatostatin: an analog of somatostatin more potent than the native molecule. Biochem Biophys Res Commun. 1975 Jul 22;65(2):746–751. doi: 10.1016/s0006-291x(75)80208-7. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Comaru-Schally A. M., Redding T. W. Antitumor effects of analogs of hypothalamic hormones in endocrine-dependent cancers. Proc Soc Exp Biol Med. 1984 Mar;175(3):259–281. doi: 10.3181/00379727-175-41797. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Coy D. H., Meyers C. A. Hypothalamic regulatory hormones. Annu Rev Biochem. 1978;47:89–128. doi: 10.1146/annurev.bi.47.070178.000513. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Redding T. W., Comaru-Schally A. M. Potential use of analogs of luteinizing hormone-releasing hormones in the treatment of hormone-sensitive neoplasms. Cancer Treat Rep. 1984 Jan;68(1):281–289. [PubMed] [Google Scholar]
- Torres-Aleman I., Redding T. W., Schally A. V. Inhibition of growth of a prolactin and growth hormone-secreting pituitary tumor in rats by D-tryptophan-6 analog of luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1252–1256. doi: 10.1073/pnas.82.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veber D. F., Freidlinger R. M., Perlow D. S., Paleveda W. J., Jr, Holly F. W., Strachan R. G., Nutt R. F., Arison B. H., Homnick C., Randall W. C. A potent cyclic hexapeptide analogue of somatostatin. Nature. 1981 Jul 2;292(5818):55–58. doi: 10.1038/292055a0. [DOI] [PubMed] [Google Scholar]
- Veber D. F., Holly F. W., Nutt R. F., Bergstrand S. J., Brady S. F., Hirschmann R., Glitzer M. S., Saperstein R. Highly active cyclic and bicyclic somatostatin analogues of reduced ring size. Nature. 1979 Aug 9;280(5722):512–514. doi: 10.1038/280512a0. [DOI] [PubMed] [Google Scholar]
- Veber D. F., Holly F. W., Paleveda W. J., Nutt R. F., Bergstrand S. J., Torchiana M., Glitzer M. S., Saperstein R., Hirschmann R. Conformationally restricted bicyclic analogs of somatostatin. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2636–2640. doi: 10.1073/pnas.75.6.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veber D. F., Saperstein R., Nutt R. F., Freidinger R. M., Brady S. F., Curley P., Perlow D. S., Paleveda W. J., Colton C. D., Zacchei A. G. A super active cyclic hexapeptide analog of somatostatin. Life Sci. 1984 Apr 2;34(14):1371–1378. doi: 10.1016/0024-3205(84)90009-2. [DOI] [PubMed] [Google Scholar]
- Wynants C., Van Binst G., Loosli H. R. Hexapeptide analogue of somatostatin, Sandoz 201-456. Conformational study in dimethylsulfoxide by 1D and 2D n.m.r. methods. Int J Pept Protein Res. 1985 Jun;25(6):622–627. [PubMed] [Google Scholar]


