Abstract
The functional significance of neuronal death for pathogenesis of epilepsy and the underlying molecular mechanisms thereof remain incompletely understood. The p53 transcription factor has been implicated in seizure damage, but its target genes and the influence of cell death under its control on epilepsy development are unknown. In the present study, we report that status epilepticus (SE) triggered by intra-amygdala kainic acid in mice causes rapid p53 accumulation and subsequent hippocampal damage. Expression of p53-up-regulated mediator of apoptosis (Puma), a proapoptotic Bcl-2 homology domain 3-only protein under p53 control, was increased within a few hours of SE. Induction of Puma was blocked by pharmacologic inhibition of p53, and hippocampal damage was also reduced. Puma induction was also blocked in p53-deficient mice subject to SE. Compared to Puma-expressing mice, Puma-deficient mice had significantly smaller hippocampal lesions after SE. Long-term, continuous telemetric EEG monitoring revealed a ∼60% reduction in the frequency of epileptic seizures in the Puma-deficient mice compared to Puma-expressing mice. These are the first data showing genetic deletion of a proapoptotic protein acting acutely to influence neuronal death subsequently alters the phenotype of epilepsy in the long-term, supporting the concept that apoptotic pathway activation is a trigger of epileptogenesis.—Engel, T., Murphy, B. M., Hatazaki, S., Jimenez-Mateos, E. M., Concannon, C. G., Woods, I., Prehn, J. H. M., Henshall, D. C. Reduced hippocampal damage and epileptic seizures after status epilepticus in mice lacking proapoptotic Puma.
Keywords: apoptosis, Bcl-2, BH3-only protein, epileptogenesis, kainate, p53
Prolonged seizures [status epilepticus (SE)] in adult rodents and humans can cause hippocampal damage and precipitate temporal lobe epilepsy (TLE) (1,2,3,4). Although neuronal death has been implicated as a pathogenic substrate of TLE, this remains largely unproven. However, recent animal studies suggest that hippocampal hilar and CA3 lesions are particularly critical for precipitating post-SE epilepsy (5, 6). Consistent with this, CA3 neuroprotection has been associated with reduced incidence of spontaneous seizures after experimental SE (6,7,8).
The p53 transcription factor is a critical regulator of apoptosis, which has long been implicated as an effector of seizure-induced neuronal death (9). Seizure-like activity in vitro and prolonged seizures in vivo cause p53 accumulation and increased transcriptional activity (9,10,11,12). Genetic ablation or pharmacologic inhibition of p53 reduces neuronal damage following glutamate receptor overactivation in vitro(13, 14) and neuronal loss after seizures in vivo(14,15,16). However, the mechanism by which p53 induces neuronal death after seizures is unknown.
Among the target genes under p53 control is Puma (p53-up-regulated modulator of apoptosis), a member of the Bcl-2 homology domain 3 (BH3)-only subgroup of Bcl-2 family proteins (17,18,19). Puma is one of the most potent proapoptotic proteins, which derives from its avid binding to all prosurvival Bcl-2 family proteins (20) and possibly an ability to directly activate Bax at mitochondria (21). The proapoptotic actions of p53 in neurons have been proposed to derive from transcriptional activation of Puma (22, 23). Presently, we investigated whether Puma is the effector of p53-induced neuronal death after SE. We also tested whether the absence of puma influenced the subsequent development of recurrent spontaneous seizures.
MATERIALS AND METHODS
Mouse model of focal-onset SE
All animal procedures were performed in accordance with the principals of the European Communities Council Directive (86/609/EEC) and were approved by the Research Ethics Committee of the Royal College of Surgeons in Ireland. Adult (20–25 g) male mice were used: C57BL/6 (Harlan, Bicester, UK), p53+/+,−/− on a C57BL/6 background (Jackson Laboratory, Bar Harbor, ME, USA), and puma+/+,+/−,−/− on a C57BL/6 background (Andreas Strasser, Molecular Genetics of Cancer Division, The Walter and Eliza Hall Institute of Medical Research, Parkville, VIC, Australia). Food and water were available ad libitum. Mice underwent seizures induced by unilateral stereotactic microinjection of kainic acid (KA) into the amygdala, as described previously (24, 25). Briefly, mice were anesthetized using isoflurane (3–5% induction, 1–2% maintenance) and kept normothermic in a stereotactic frame. Mice were affixed with skull-mounted electrodes (Bilaney Consultants, Sevenoaks, UK) above dorsal hippocampus and frontal cortex to record surface electroencephalogram (EEG) using a Grass Comet digital EEG (Medivent, Lucan, Ireland). A guide cannula was affixed over the dura (coordinates from bregma: AP=−0.94; L=−2.85 mm), and the entire skull assembly was fixed in place with dental cement. Anesthesia was discontinued, EEG recordings were commenced, and then a 31-gauge internal cannula was inserted into the lumen of the guide to inject KA (Ocean Produce International, Dartmouth, NS, Canada; 0.3 μg in 0.2 μl vehicle; PBS, pH adjusted to 7.4) into the amygdala. Nonseizure control mice received 0.2 μl intra-amygdala vehicle. Lorazepam (6 mg/kg, intraperitoneal or intravenous) was administered 40 min after KA, and the EEG was recorded for up to 1 h thereafter. Mice were euthanized at different time points after anticonvulsant for biochemical analysis and histopathology, or following completion of spontaneous seizure monitoring (see below). Brains were microdissected on ice and processed for mRNA and protein analysis, or flash-frozen whole in 2-methylbutane at −30°C for immunohistochemistry. Brains from additional naive (noninstrumented) wild-type and puma+/−,−/− mice were used to examine hippocampal and amygdala neuroanatomy and gene expression.
Epilepsy monitoring
Long-term EEG recording to define the emergence of epilepsy was performed using implantable EEG telemetry units [Data Sciences International (DSI), St. Paul, MN, USA], as described previously (8, 24). Mice underwent the same initial surgical procedure for affixing the injection cannula but were equipped with EEG transmitters (F20-EET, DSI) to record bilateral EEG from skull overlying the dorsal ipsilateral and contralateral hippocampi. EEG data were acquired using the Dataquest A.R.T. system (DSI). Following surgery, mice received intra-amygdala KA, and then telemetry units were activated. After recordings, mice were euthanized, and brains were processed for histopathology and immunostaining, as described below. Telemetry EEG recordings were manually analyzed for occurrence of epileptic seizures. These were defined as high-frequency (>5 Hz) high-amplitude (>2× baseline) polyspike discharges of ≥5 s duration (8, 24).
Clinical seizure scoring
Mice were observed during KA-induced seizures and also during spontaneous epileptic seizures and scored according to a modified Racine scale for mice (24).
p53 inhibitor treatment
Seizure mice received vehicle or pifithrin-α (PFT) [1-(4-methylphenyl)-2-(4,5,6,7-tetrahydro-2-imino-3(2H)-benzothiazolyl)-ethanone hydrobromide] (Sigma-Aldrich, St. Louis, MO, USA). PFT was administered 24 h before and 1 h after the induction of SE. PFT was dissolved in DMSO and PBS. Drug (4 mg/kg) or vehicle was injected via intraperitoneal route, as described previously (14), and animals were euthanized 8 or 24 h after lorazepam.
Genotyping
Genomic DNA was extracted from tail snips. Genotyping of p53 mice was in accordance with supplier recommendations for Trp53tm1Tyj. Puma mice were genotyped according to previously described methods (26).
Subcellular fractionation
Subcellular fractionation was performed to obtain mitochondria- and nuclear-enriched fractions, as described previously (25, 27). Briefly, brain samples were homogenized in a mannitol/sucrose/HEPES buffer, and a crude nuclear fraction was obtained following centrifugation at 1200 g. The supernatant was then subjected to further centrifugation to acquire a mitochondrial fraction (10,000 g). The crude nuclear fraction was further purified by centrifugation in sucrose buffer (10 mM Tris, pH 7.5; 300 mM sucrose; and 1 mM EDTA with 0.1% Nonidet P-40).
Western blot analysis
Western blot analysis was performed as described previously (25). Hippocampi were homogenized in a lysis buffer, boiled in gel-loading buffer, separated on SDS-PAGE gels, and transferred onto nitrocellulose membranes. The following primary antibodies were used: β-actin (1:2000; Sigma-Aldrich), α-tubulin and p53 (Santa Cruz Biotechnology, Heidelberg, Germany), Puma (1:1000; ProSci, Poway, CA, USA), Lamin A/C and phospho-FOXO3a (1:500, Cell Signaling, Beverly, MA, USA), Cytochrome IV oxidase (CoxIV; 1:1000; Molecular Probes, Leiden, The Netherlands), GluR6/7 (1:1000; Chemicon, Hampshire, UK), Grp78 (1:1000; Stressgen Bioscience, Cambridge, UK). A second antibody used to detect Puma protein was a custom-made rabbit polyclonal antibody (Eurogentec S.A., Seraing, Belgium) raised against the C-terminal sequence (RQEEQQRHRPSPWRV) and middle domain sequence (PGGPRSRPRGPRPDG) of the human α Puma isoform. Membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, Plymouth, PA, USA) and bands visualized using Supersignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL, USA). Images were captured using a Fuji-Film LAS-300 (Fuji, Sheffield, UK), and densitometry was performed using AlphaEaseFC4.0 gel-scanning integrated optical density software (Alpha Innotech, San Leandro, CA, USA). Levels were corrected to the intensity reading for the control sample in each independent experiment. For Puma antibody specificity, puma+/+ and puma−/− HCT cells were treated with 3 μM thapsigargin.
Analysis of amygdala KA receptor expression
Bilateral tissue cores (∼1 mm wide by 2 mm deep) of the amygdala (including the basolateral amygdala) or whole hippocampus were extracted from a selection of naive mice of each genotype. Tissue samples were then processed for Western blot analysis as described above.
mRNA analysis
Real-time quantitative PCR analysis of noxa and p21WAF1/CIP1 mRNA levels was undertaken, as described previously (8). Primers were designed using Primer3 software (http://frodo.wi.mit.edu) and verified by BLAST (http://blast.ncbi. nlm.nih.gov/blast.cgi). One microgram of total RNA from control (4 h) and seizure hippocampus (1–24 h) was used to generate cDNA by reverse transcription using Superscript II Reverse Transcriptase enzyme (Invitrogen Corporation, Carlsbad, CA, USA). Quantitative real-time PCR was performed using a LightCycler 1.5 (Roche Diagnostics, Indianapolis, IN, USA) in combination with QuantiTech SYBR Green PCR kit (Qiagen, Crawley, UK). Data were analyzed by LightCycler 1.5 software, and data were normalized to expression of β-actin. For Noxa expression positive control, mouse cortical neurons (5 d in vitro), prepared as described (28) were treated with epoxomicin (50 nM), a selective proteosome inhibitor or vehicle (DMSO, 0.001%) for 24 h.
Histopathology
Histopathology was undertaken as described previously (24, 25). Briefly, fresh-frozen coronal brain sections at the level of the dorsal hippocampus were air-dried, fixed in formalin, and processed for NeuN (Chemicon) immunostaining, which was detected using goat anti-mouse AlexaFluor 568 (Bio Sciences, Dun Laoghaire, Ireland). Neuronal damage was also assessed by Fluoro-Jade B staining (Millipore, Cork, Ireland). Analysis of DNA damage was performed using a fluorescein-based terminal deoxynucleotidyl dUTP nick end labeling (TUNEL) technique, according to manufacturer’s instructions (Promega, Madison, WI, USA) (25). Sections were mounted in medium containing 4,6 diamidino-2-phenylindole (DAPI; Vector Laboratories, Peterborough, UK) to visualize nuclei. Sections were examined and imaged using a Nikon 2000s epifluorescence microscope with a Hamamatsu Orca 285 camera (Micron-Optica, Enniscorthy, Ireland). Semiquantification of damaged and/or surviving cell numbers was performed for the entire CA3 subfield, beginning at the border with CA2 through to the end of CA3c/CA4 within the hilus of the dentate gyrus. Counts were the average of two adjacent sections assessed by an observer masked to experimental group/condition.
Data analysis
Data are presented as means ± se. Data were analyzed using ANOVA with post hoc Fisher’s PLSD test or, for 2-group comparison, Student’s t test (StatView software; SAS Institute, Cary, NC, USA). Significance was accepted at P < 0.05.
RESULTS
Hippocampal damage and p53 up-regulation after focal-onset SE in mice
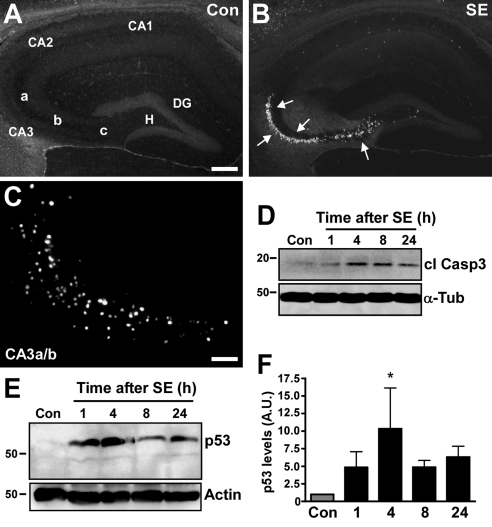
SE was induced in awake, freely moving adult C57BL/6 mice via intra-amygdala microinjection of KA. During seizures, mice displayed manifestations common to limbic SE, including stereotyped masticatory movements, Straub tail, forelimb clonus, and episodes of rearing and falling. Inspection of the ipsilateral hippocampus 72 h after SE in mice revealed extensive CA3 and hilar subfield damage, as assessed by Fluoro-Jade B (FJB) staining (Fig. 1B). Hippocampal cell death was not detected in vehicle-injected mice (Fig. 1A). Dying neurons in the affected hippocampal subfields were pyknotic and positive for DNA fragmentation, as confirmed by TUNEL (Fig. 1C). Complementing these apoptotic features, increased cleaved caspase-3 was detected by Western blot analysis of hippocampal lysates after SE (Fig. 1D).
Figure 1.
Up-regulation of p53 in hippocampus during seizure-induced neuronal death. A, B) Photomicrographs show representative FJB staining at 72 h in hippocampus of vehicle-injected control mice (A) and mice that underwent SE (B). Arrows denote cells (white) undergoing degeneration. DG, dentate gyrus; H, hilus; CA1–3, cornu ammonis; CA3a-c, subfields of CA3. C) Photomicrograph (×20 lens) showing representative TUNEL staining in the ipsilateral CA3a/b subfield of a mouse 72 h after SE. D) Representative Western blot (n=4/lane) showing presence of cleaved caspase 3 (cl Casp3) after SE but not in control (Con). Tubulin (α-Tub) is shown as a guide to protein loading. E) Western blot (n=1/lane) showing increased levels of p53 after SE compared to control. Same blot was reprobed for actin as a guide to protein loading. F) Graph showing semiquantification of p53 protein levels. Data are from 10 independent experiments. A.U., arbitrary units. *P < 0.05 vs. control. Scale bars = 250 μm (A, B); 50 μm (C).
Hippocampal levels of p53 protein were very low in control mice (Fig. 1E). In contrast, hippocampal lysates from mice subject to SE contained higher levels of p53 protein, beginning as early as 1 h and peaking at 4 h (Fig. 1E, F).
Puma is induced after SE
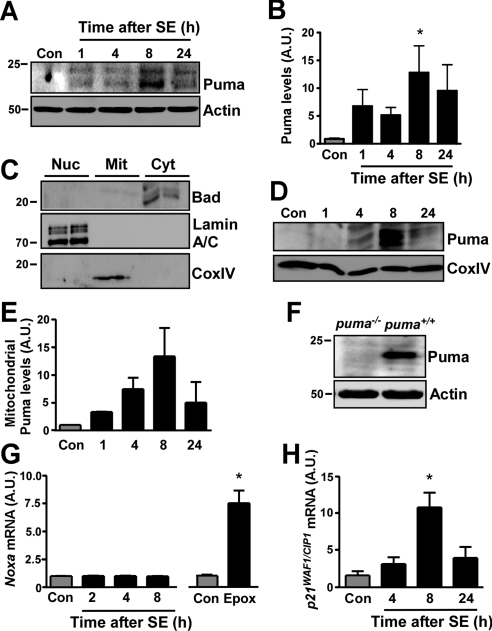
We next performed Western blot analysis of the p53 target gene Puma. Hippocampal extracts were probed with both commercially available and a custom-made antibody against Puma, and results were similar with either antibody. Immunoblot analysis of hippocampal whole-cell lysates determined Puma was essentially undetectable in control mice (Fig. 2A, B). SE resulted in a significant increase of Puma protein, beginning at 1 h and peaking at 8 h (Fig. 2A, B). Since Puma has been shown to target mitochondria (17, 29, 30), we also analyzed this fraction in control and seizure-damaged hippocampus. Western blot analysis confirmed that mitochondrial fractions were enriched for mitochondrial protein and absent of contamination by cytosolic or nuclear proteins (Fig. 2C). We observed an increase in Puma levels within the mitochondrial fraction beginning 4 h after seizures, which also peaked at 8 h (Fig. 2D, E). The specificity of the Puma antibody was confirmed by Western blot analysis of cell lysates expressing Puma protein and lysates from puma−/− cells (Fig. 2F).
Figure 2.
Puma is induced shortly after SE. A) Representative Western blot (n=1/lane) of hippocampal whole-cell lysates showing increased expression of Puma after SE. B) Graph showing semiquantitative analysis of Puma levels in hippocampus after SE (n=6/group). C) Marker analysis of hippocampal fractions. Cytoplasm (Cyt) contained only Bad, the nuclear fraction (Nuc) only lamin A/C, and the mitochondrial fraction (Mit) CoxIV. D) Representative Western blot (n=4/lane) showing increasing Puma levels in the mitochondrial fraction of seizure-damaged mouse hippocampus. CoxIV is shown as a loading control. E) Graph showing semiquantitative analysis of Puma levels in pooled mitochondrial fractions of hippocampus after SE (data from 2 independent experiments). F) Lysates from puma-expressing and puma−/− HCT cells treated with 3 μM thapsigargin were blotted to confirm specificity of the Puma antibody. G) Graph showing noxa mRNA levels, corrected to β-actin, within hippocampus after SE in mice. Graph at right shows noxa expression in mouse cortical neurons treated with the proteasome inhibitor epoximicin (Epox; 50 nM) as a positive control (n=4/group). H) Graph showing a significant increase of the p53 target gene p21WAF1/CIP1 mRNA, corrected to β-actin (n=4/group). A.U., arbitrary units. *P < 0.05 vs. control.
We next looked at the expression of Noxa, another BH3-only protein under p53 control (31). Because tests of two different Noxa-specific antibodies failed to detect any band in control or seizure hippocampus, we measured levels of noxa mRNA. Expression of noxa was unchanged up to 8 h after SE (Fig. 2G). To confirm that noxa is inducible in neurons, we treated primary cultures of cortical neurons with the proteasome inhibitor epoxomicin (32). Epoxomicin induced a large and significant increase in noxa expression (Fig. 2G). Finally, to support evidence that p53 is transcriptionally active after SE in this model, we measured levels of the cyclin-dependent kinase inhibitor p21WAF1/CIP1, which is coinduced in a p53-dependent manner with Puma (17). SE caused a time-dependent increase in hippocampal expression of p21WAF1/CIP1 beginning at 4 h, which became significant at 8 h (Fig. 2H).
Coactivation of p53-independent pathways of Puma induction after SE
Studies have demonstrated that Puma can be induced independently of p53 via endoplasmic reticulum (ER) stress (33) and the FOXO3a transcription factor (34). To assess ER stress, we immunoblotted hippocampal extracts with antibodies against glucose-regulated protein 78 (Grp78, also known as BiP). A rapid and sustained increase in hippocampal Grp78 levels was evident after SE (see Supplemental Fig. S1). Next, we examined FOXO3a, which is transcriptionally active when dephosphorylated. Western blot analysis revealed FOXO3a dephosphorylation also occurred following seizures (see Supplemental Fig. S1).
Pharmacologic inhibition of p53 prevents Puma induction after SE
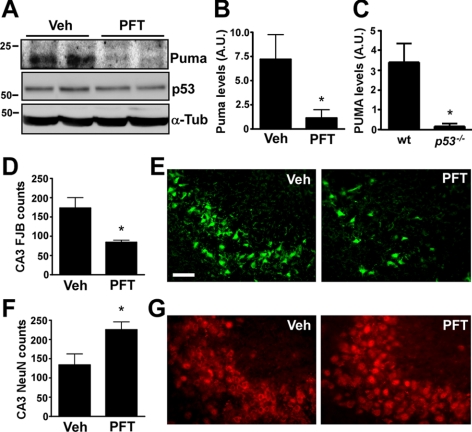
To determine which of the three potential pathways were responsible for inducing Puma, we focused on p53, for which specific pharmacological and genetic disruption was possible. We first examined the effects of pifithrin-α (PFT), which inhibits the transcriptional activity of p53 (14, 35). Vehicle- and PFT-treated mice were subjected to SE and then hippocampal extracts immunoblotted for gene expression at 8 h, corresponding to the time of maximal Puma expression (see Fig. 2A, D). Western blot analysis showed near-complete inhibition of Puma expression in PFT-treated mice after SE when compared to vehicle-treated seizure mice (Fig. 3A, B). Levels of p53 were not significantly affected by PFT treatment (Fig. 3A).
Figure 3.
Puma induction after SE in mice is p53-dependent. A) Representative Western blots (n=1/lane) showing hippocampal Puma and p53 expression 8 h after SE in vehicle (Veh)- and pifithrin-α (PFT)-treated mice. Note that Puma levels are reduced in PFT-treated mice, while p53 levels were similar between groups. B) Graph showing significantly lower Puma levels in PFT-treated mice when compared to vehicle-treated mice after SE (n=3/group). C) Graph showing lower Puma levels in p53−/− mice when compared to wild-type (wt) mice 8 h after SE (n=3/group). D–G) Graphs (D, F) and representative photomicrographs (×40 lens; E, G) showing hippocampal damage 24 h after SE as assessed by counts of FJB-positive CA3 cells (D, E), and CA3 cells with normal-appearing NeuN immunoreactivity (F, G), in PFT-treated mice compared to vehicle-treated mice (n=4–7/group). Note significantly fewer FJB-positive cells and significantly more surviving neurons in PFT-treated mice. A.U., arbitrary units. *P < 0.05 vs. control. Scale bar = 100 μm.
Puma is not induced after SE in p53−/− mice
To complement the PFT data, we analyzed Puma expression after SE in p53+/+ and p53−/− mice. Puma expression following SE was nearly absent in hippocampal extracts from p53−/− mice compared to p53+/+ mice (Fig. 3C).
Hippocampal damage after SE is reduced by p53 inhibition or p53 deficiency
To determine whether p53 activity is required for neuronal death in the model, we examined hippocampal FJB staining in vehicle- and PFT-treated mice. PFT-treated mice had significantly fewer degenerating neurons in the CA3 subfield (Fig. 3D, E). Analysis of hippocampal sections stained with antibodies against NeuN demonstrated that mice given PFT had significantly more surviving neurons after SE compared to vehicle-treated seizure mice (Fig. 3F, G). No differences were observed in seizure duration during SE between PFT- and vehicle-treated mice (data not shown). Similar data were obtained when hippocampal damage after SE was compared between p53+/+ and p53−/− mice. Mice lacking p53 displayed 45% less FJB staining and 35% more surviving NeuN counts at 72 h compared to p53-expressing controls (n=10–20/group, data not shown).
Normal brain architecture and KA response in Puma-deficient mice
To investigate the functional significance of Puma during seizure-induced neuronal death, we undertook experiments using mice lacking Puma (26). Previous studies have established that puma−/− mice have normal hippocampal development (28), and this was confirmed by our observations (Supplemental Fig. S2). Expression of KA receptors (GluR6/7) was also normal in the amygdala and the hippocampus of these mice (Supplemental Fig. S2). Hippocampal p53 levels in naive Puma-deficient mice were also equivalent to those in wild-type mice (Supplemental Fig. S2). EEG analysis determined there were no differences between wild-type, puma+/−, and puma−/− mice for delay to seizure onset or seizure duration during SE (Supplemental Fig. S2).
Reduced hippocampal damage after SE in Puma-deficient mice
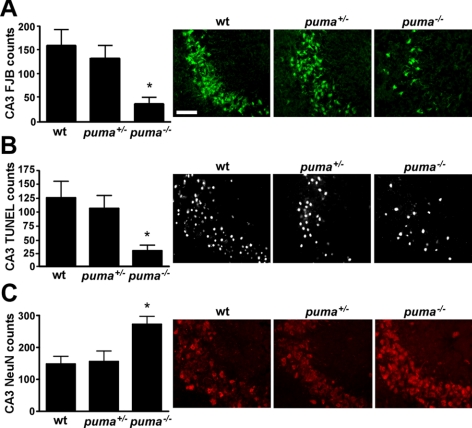
To determine whether Puma contributes to hippocampal lesions after SE, we compared damage in wild-type mice to puma+/− and puma−/− mice. Counts of FJB-positive cells within the CA3 subfield of the hippocampus revealed ∼70–80% less in puma−/− mice compared to wild-type and puma+/− mice (Fig. 4A). A similar finding was obtained when hippocampal sections were stained for DNA fragmentation using TUNEL (Fig. 4B). Last, we compared neuronal survival between the different genotypes by assessing numbers of NeuN-stained CA3 cells displaying normal neuronal morphology. Counts of surviving CA3 neurons were significantly higher in puma−/− mice when compared to wild-type and puma+/− mice (Fig. 4C). Induction of p53 after SE assayed by Western blot analysis was equivalent between wild-type and puma−/− mice (see Supplemental Fig. S2).
Figure 4.
Reduced hippocampal damage after SE in puma−/− mice. A) Graph and representative photomicrographs (×40 lens) showing significantly lower FJB counts in the ipsilateral CA3 in puma−/− mice when compared to puma+/− and wild-type (wt) mice. B) Graph and representative photomicrographs showing significantly lower TUNEL counts in ipsilateral CA3 from puma−/− mice when compared to puma+/− and wild-type mice. C) Graph and representative photomicrographs showing significantly more surviving NeuN-positive cells in ipsilateral CA3 from puma−/− mice when compared to puma+/− and wild-type mice. *P < 0.05 vs. wild-type and puma+/− mice (n=5–7/group). Scale bar = 50 μm.
Reduced occurrence of spontaneous seizures in Puma-deficient mice
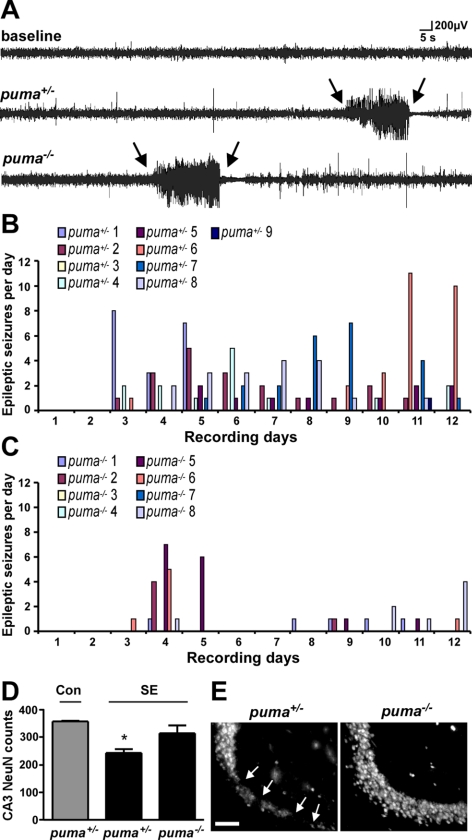
Last, we asked whether the neuroprotection in Puma-deficient mice would influence the development of epilepsy after SE. For the purposes of these experiments, puma−/− mice were compared to littermate puma+/− mice. Continuous EEG recording was used for epileptic seizure monitoring, beginning during the latent period (1 d after SE) and lasting for 12 d, similar to before (8). The durations of KA-induced SE between the groups were not different (data not shown).
The daily epileptic event data for individual mice from each genotype are shown in Fig. 5B, C. Of the puma+/− mice subjected to SE, 8 of 9 (89%) developed epilepsy during the 12-d recordings. Spontaneous seizure rates in puma+/− mice were typically 1–2/d (range 0–11). Only 5 of 8 (63%), puma−/− mice developed epilepsy during the 12-d recordings. Average delay to first spontaneous seizure was 5.1 ± 1.5 d in puma+/− mice and 7 ± 2.3 d in puma−/− mice. The average duration of a spontaneous seizure was the same between genotypes (19±6 for puma+/− mice vs. 19±5 s in puma−/− mice), and no differences were noted in the clinical features of epileptic seizures. However, spontaneous seizure occurrence was significantly less frequent in puma−/− mice compared to puma+/− mice over the 12-d monitoring period. Compared to puma+/− mice, puma−/− mice had reduced epileptic seizure counts (14±3 vs. 5±2, P<0.05), fewer daily events (1.2±3 vs. 0.4±0.2, P<0.05) and lower sum epileptic seizure time (279±76 vs. 96±32, P<0.05).
Figure 5.
Milder epileptic phenotype and long-term neuroprotection in Puma-deficient mice. A) Representative EEG traces showing baseline EEG (top lane) and examples of typical epileptic seizures captured by EEG telemetry (between arrows) in puma+/− and puma−/− mice. B, C) Graphs depict daily epileptic seizure occurrence for puma+/− (B) and puma−/− (C) mice over 12 d of continuous seizure monitoring. D) Graph showing counts of NeuN-positive cells in CA3 72 h after epilepsy monitoring in puma−/− mice when compared to puma+/− (n=4–7/group). Note that NeuN counts in puma−/− mice remained higher. E) Representative photomicrographs (×40 lens) of NeuN staining within the ipsilateral hippocampal CA3 region 15 d after SE in puma+/− and puma−/− mice. Arrows mark sites of neuronal loss. *P < 0.05 vs. control and puma−/− mice. Scale bar = 100 μm.
Investigation of hippocampal damage at the end of the long-term EEG recordings in mice subject to SE determined there were significantly more surviving ipsilateral CA3 neurons in puma−/− mice when compared to puma+/− mice (Fig. 5D, E).
DISCUSSION
Despite animal and human data showing seizures cause apoptosis-associated signaling in the hippocampus, little direct evidence has supported a causal role in seizure-induced neuronal death and epileptogenesis (36). We demonstrate here that genetic deletion of a proapoptotic gene acting acutely to influence neuronal death reduced the occurrence of spontaneous seizures in the long term, supporting the concept that apoptotic pathway activation is a trigger of epileptogenesis.
The present studies began by exploring whether neuronal death after SE involved p53 but expanded on previous work by attempting to identify the effectors. There was strong p53 induction in the mouse hippocampus after SE triggered by intra-amygdala KA, a model previously demonstrated to display an apoptotic component (25, 37). We also found that p53 contributed significantly to hippocampal damage, because both a p53 inhibitor (PFT) and p53 deficiency were protective against SE. This is in agreement with reports in other seizure models (14,15,16). While transcription-independent mechanisms have been proposed (38), most evidence supports p53-induced neuronal death as requiring p53 transactivation (22, 23). Protection in our model was evident in p53-deficient mice and in mice given PFT, an inhibitor of p53 transcriptional activity (14, 35). Thus, our data argue both that p53 has a causal role in the hippocampal cell death, and its transcriptional activity is required. However, PFT can have actions independent of p53 transcriptional activity (39), and such an effect was not excluded presently.
While p53 can regulate several proapoptotic genes (22, 40, 41), the main p53 transcriptional target effecting neuronal death in response to stimuli, including DNA damage and oxidative stress, appears to be Puma (22, 23, 41). Our studies establish Puma as the likely effector of p53-induced neuronal death after SE, based on several findings. Foremost, Puma-deficient mice were potently protected against seizure-induced neuronal death and Puma induction was blocked by either PFT or p53 deficiency. Second, Puma but not Noxa, was induced after SE, in agreement with other reports showing Puma rather than Noxa is required for p53-dependent cell killing (17, 29). Third, the temporal profile of Puma induction and maximal expression lagged slightly behind p53, and p21WAF1/CIP1 was induced over the same time frame as Puma, which was also noted in the original discovery of Puma (17).
The cause of p53 accumulation following seizures, and thus indirectly the trigger for Puma, was not a focus of our study. DNA damage, oxidative stress, NMDA receptor activation and calpain activation are capable of causing p53 and/or Puma induction, including in neurons (17, 29, 42, 43). Our data appear somewhat at odds with the finding that NMDA-induced CA1 lesions are not associated with Puma induction, and CA1 neurons are not protected against NMDA toxicity in Puma-deficient mice (28). Thus, non-NMDA class glutamate receptor activation or other mechanisms appear important for Puma induction. Both kainate and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate) receptors are important in delayed glutamate-induced neuronal death (44). Also, experiments in kainate receptor-deficient mice showed a requirement for kainate receptors in synaptic transmission in the mossy fiber pathway, central to neuronal death in the present model, and for CA3 injury after kainate seizures in vivo(45). Studies have also suggested that kainate receptors may be more important than NMDA receptors for p53 induction (46), and both p53 and Puma can be induced by cell depolarization independent of glutamate receptor activation (47).
Puma joins an expanding group of BH3-only proteins that includes Bad, Bid, and Bim, shown to be activated in models of SE (36). Whether Puma has a more important role than other BH3-only proteins in this model is unknown but plausible, on the basis of the amount of protection in Puma-deficient mice. This may derive from molecular properties of Puma and an apical site of function. Indeed, Puma inhibits all antiapoptotic Bcl-2 family proteins and can directly activate Bax (20, 21, 29, 30), and Puma levels increased shortly after SE. Indeed, the onset of Puma induction may precede cytochrome c release and caspase cleavage, which emerge after 4 h in the present model (present data and ref. 25). Nevertheless, some neuronal loss was evident in Puma-deficient mice, indicating involvement of other BH3-only proteins or nonapoptotic cell death mechanisms in seizure-induced neuronal death in vivo in this model.
Two potential caveats could be considered in the present study. First, we did not undertake full stereological counting in our assessments of hippocampal damage in the p53- or Puma-deficient mice. Second, Schauwecker and Steward (48) reported that p53 deficiency did not protect mice that had a 129/SvEMS instead of C57BL/6 background. It is therefore possible that neuroprotection could differ in p53- or Puma-deficient mice of another strain. However, interstrain damage variability is strongly model-dependent and does not reflect intrinsic differences in susceptibility to seizure-induced neuronal death (49). The model used in the present studies is less influenced by strain differences than the model used by Schauwecker and Steward (24, 25, 37).
The present study also characterized epilepsy development in Puma-deficient mice after SE. The relationship between neuronal death and epileptogenesis is complex. Indeed, neuronal death is not required for epilepsy to develop following early-life seizures (50, 51), and neuroprotection in adult SE models has failed to influence epileptogenesis in some studies (52,53,54,55). However, an influence of hippocampal damage on the frequency of seizures in epilepsy patients has been reported (56, 57). Also, recent animal studies show that hippocampal CA3 lesion size may be a critical determinant of epileptic seizure frequency (6,7,8). A major finding in the present study was that mice lacking Puma had fewer epileptic seizures after SE than Puma-expressing controls. These are the first data to show that targeting a single apoptosis-associated gene results in acute neuroprotection and a milder form of epilepsy. This supports a direct relation between neuronal death and epileptogenesis. Indeed, the reduction in epileptic seizure frequency is of a similar degree to that reported by us using a nongenetic seizure-preconditioning paradigm, in which mice display ∼50% less CA3 damage (8). We speculate that the significant sparing of hippocampal neurons in Puma-deficient mice contributes to reduced ictogenesis per se, a failure of initiated seizures to secondarily generalize/propagate, or a combination of these effects. Why was an effect seen presently but not in certain previous studies in which neuroprotection was achieved? It is possible the amount of protection or the continuous EEG we began soon after the precipitating insult, rather than intermittent EEG or behavior-only monitoring (52,53,54,55), explains the detected disease-modifying effect. It is noteworthy that only the frequency of epileptic seizures in our study, and not the duration of events or the proportion of animals developing epilepsy, were reduced in puma−/− mice. Thus, the specific molecular pathways disrupted in puma−/− mice, attendant lasting neuroprotection and model employed may be important influences on epileptic phenotype.
In summary, the present study identifies Puma as the p53 transcriptional target contributing to seizure-induced neuronal death in vivo. Moreover, we find the neuroprotection afforded by loss of Puma has an antiepileptic effect. Targeting the pathway may have therapeutic applications for preventing neuronal death and epileptogenesis.
Supplementary Material
Acknowledgments
The authors thank Andreas Strasser (Walter and Eliza Hall Institute of Medical Research, Parkville, Australia) and Andreas Villunger (University of Innsbruck, Innsbruck, Austria) for providing Puma-deficient mice, and Genshin Mouri, Liam Tuffy and Clara Schindler for technical support. This work was supported by grants from Health Research Board Ireland (RP/2005/24, RP/2007/37, RP/2008/69, PD/2009/31), the Wellcome Trust (GR076576), Science Foundation Ireland (08/IN1/B1875), Marie Curie ToK FP-14499, and a fellowship (to T.E.) from the Irish Research Council for Science Engineering and Technology.
References
- DeGiorgio C. M., Tomiyasu U., Gott P. S., Treiman D. M. Hippocampal pyramidal cell loss in human status epilepticus. Epilepsia. 1992;33:23–27. doi: 10.1111/j.1528-1157.1992.tb02278.x. [DOI] [PubMed] [Google Scholar]
- Fujikawa D. G., Itabashi H. H., Wu A., Shinmei S. S. Status epilepticus-induced neuronal loss in humans without systemic complications or epilepsy. Epilepsia. 2000;41:981–991. doi: 10.1111/j.1528-1157.2000.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Mikaeloff Y., Jambaque I., Hertz-Pannier L., Zamfirescu A., Adamsbaum C., Plouin P., Dulac O., Chiron C. Devastating epileptic encephalopathy in school-aged children (DESC): a pseudo encephalitis. Epilepsy Res. 2006;69:67–79. doi: 10.1016/j.eplepsyres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Pitkanen A., Kharatishvili I., Karhunen H., Lukasiuk K., Immonen R., Nairismagi J., Grohn O., Nissinen J. Epileptogenesis in experimental models. Epilepsia. 2007;48(Suppl. 2):13–20. doi: 10.1111/j.1528-1167.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- Bumanglag A. V., Sloviter R. S. Minimal latency to hippocampal epileptogenesis and clinical epilepsy after perforant pathway stimulation-induced status epilepticus in awake rats. J Comp Neurol. 2008;510:561–580. doi: 10.1002/cne.21801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Ren G., Lusardi T., Wilz A., Lan J. Q., Iwasato T., Itohara S., Simon R. P., Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest. 2008;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini G., Baldelli E., Longo D., Contri M. B., Guerrini U., Sironi L., Gelosa P., Zini I., Ragsdale D. S., Avoli M. Proepileptic influence of a focal vascular lesion affecting entorhinal cortex-CA3 connections after status epilepticus. J Neuropathol Exp Neurol. 2008;67:687–701. doi: 10.1097/NEN.0b013e318181b8ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Mateos E. M., Hatazaki S., Johnson M. B., Bellver-Estelles C., Mouri G., Bonner C., Prehn J. H., Meller R., Simon R. P., Henshall D. C. Hippocampal transcriptome after status epilepticus in mice rendered seizure damage-tolerant by epileptic preconditioning features suppressed calcium and neuronal excitability pathways. Neurobiol Dis. 2008;32:442–453. doi: 10.1016/j.nbd.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Sakhi S., Bruce A., Sun N., Tocco G., Baudry M., Schreiber S. S. p53 induction is associated with neuronal damage in the central nervous system. Proc Natl Acad Sci U S A. 1994;91:7525–7529. doi: 10.1073/pnas.91.16.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Rong Y., Baudry M., Schreiber S. S. Status epilepticus induces p53 sequence-specific DNA binding in mature rat brain. Brain Res Mol Brain Res. 1999;63:248–253. doi: 10.1016/s0169-328x(98)00285-x. [DOI] [PubMed] [Google Scholar]
- Tan Z., Sankar R., Tu W., Shin D., Liu H., Wasterlain C. G., Schreiber S. S. Immunohistochemical study of p53-associated proteins in rat brain following lithium-pilocarpine status epilepticus. Brain Res. 2002;929:129–138. doi: 10.1016/s0006-8993(01)03360-1. [DOI] [PubMed] [Google Scholar]
- Araki T., Shinoda S., Schindler C. K., Quan-Lan J., Meller R., Taki W., Simon R. P., Henshall D. C. Expression, interaction, and proteolysis of death-associated protein kinase and p53 within vulnerable and resistant hippocampal subfields following seizures. Hippocampus. 2004;14:326–336. doi: 10.1002/hipo.10184. [DOI] [PubMed] [Google Scholar]
- Xiang H., Hochman D. W., Saya H., Fujiwara T., Schwartzkroin P. A., Morrison R. S. Evidence for p53-mediated modulation of neuronal viability. J Neurosci. 1996;16:6753–6765. doi: 10.1523/JNEUROSCI.16-21-06753.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C., Zhu X., Yu Q. S., Chan S. L., Camandola S., Guo Z., Greig N. H., Mattson M. P. A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid beta-peptide. J Neurochem. 2001;77:220–228. doi: 10.1046/j.1471-4159.2001.t01-1-00220.x. [DOI] [PubMed] [Google Scholar]
- Morrison R. S., Wenzel H. J., Kinoshita Y., Robbins C. A., Donehower L. A., Schwartzkroin P. A. Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J Neurosci. 1996;16:1337–1345. doi: 10.1523/JNEUROSCI.16-04-01337.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebaili M., Lerner-Natoli M., Montpied P., Baille V., Bockaert J., Rondouin G. Molecular events involved in neuronal death induced in the mouse hippocampus by in-vivo injection of kainic acid. Brain Res Mol Brain Res. 2001;93:190–198. doi: 10.1016/s0169-328x(01)00197-8. [DOI] [PubMed] [Google Scholar]
- Yu J., Zhang L., Hwang P. M., Kinzler K. W., Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- Nakano K., Vousden K. H. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Han J., Flemington C., Houghton A. B., Gu Z., Zambetti G. P., Lutz R. J., Zhu L., Chittenden T. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci U S A. 2001;98:11318–11323. doi: 10.1073/pnas.201208798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis S. N., Adams J. M. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron P. F., Gallenne T., Bougras G., Gautier F., Manero F., Vusio P., Meflah K., Vallette F. M., Juin P. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Cregan S. P., Arbour N. A., Maclaurin J. G., Callaghan S. M., Fortin A., Cheung E. C., Guberman D. S., Park D. S., Slack R. S. p53 activation domain 1 is essential for PUMA upregulation and p53-mediated neuronal cell death. J Neurosci. 2004;24:10003–10012. doi: 10.1523/JNEUROSCI.2114-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uo T., Kinoshita Y., Morrison R. S. Apoptotic actions of p53 require transcriptional activation of PUMA and do not involve a direct mitochondrial/cytoplasmic site of action in postnatal cortical neurons. J Neurosci. 2007;27:12198–12210. doi: 10.1523/JNEUROSCI.3222-05.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouri G., Jimenez-Mateos E., Engel T., Dunleavy M., Hatazaki S., Paucard A., Matsushima S., Taki W., Henshall D. C. Unilateral hippocampal CA3-predominant damage and short latency epileptogenesis after intra-amygdala microinjection of kainic acid in mice. Brain Res. 2008;1213:140–151. doi: 10.1016/j.brainres.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Murphy B., Dunleavy M., Shinoda S., Schindler C., Meller R., Bellver-Estelles C., Hatazaki S., Dicker P., Yamamoto A., Koegel I., Chu X., Wang W., Xiong Z., Prehn J., Simon R., Henshall D. Bcl-w protects hippocampus during experimental status epilepticus. Am J Pathol. 2007;171:1258–1268. doi: 10.2353/ajpath.2007.070269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villunger A., Michalak E. M., Coultas L., Mullauer F., Bock G., Ausserlechner M. J., Adams J. M., Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Schindler C. K., Pearson E. G., Bonner H. P., So N. K., Simon R. P., Prehn J. H., Henshall D. C. Caspase-3 cleavage and nuclear localization of caspase-activated DNase in human temporal lobe epilepsy. J Cereb Blood Flow Metab. 2006;26:583–589. doi: 10.1038/sj.jcbfm.9600219. [DOI] [PubMed] [Google Scholar]
- Concannon C. G., Ward M. W., Bonner H. P., Kuroki K., Tuffy L. P., Bonner C. T., Woods I., Engel T., Henshall D. C., Prehn J. H. NMDA receptor-mediated excitotoxic neuronal apoptosis in vitro and in vivo occurs in an ER stress and PUMA-independent manner. J Neurochem. 2008;105:891–903. doi: 10.1111/j.1471-4159.2007.05187.x. [DOI] [PubMed] [Google Scholar]
- Steckley D., Karajgikar M., Dale L. B., Fuerth B., Swan P., Drummond-Main C., Poulter M. O., Ferguson S. S., Strasser A., Cregan S. P. Puma is a dominant regulator of oxidative stress induced Bax activation and neuronal apoptosis. J Neurosci. 2007;27:12989–12999. doi: 10.1523/JNEUROSCI.3400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallenne T., Gautier F., Oliver L., Hervouet E., Noel B., Hickman J. A., Geneste O., Cartron P. F., Vallette F. M., Manon S., Juin P. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol. 2009;185:279–290. doi: 10.1083/jcb.200809153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda E., Ohki R., Murasawa H., Nemoto J., Shibue T., Yamashita T., Tokino T., Taniguchi T., Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- Qin J. Z., Ziffra J., Stennett L., Bodner B., Bonish B. K., Chaturvedi V., Bennett F., Pollock P. M., Trent J. M., Hendrix M. J., Rizzo P., Miele L., Nickoloff B. J. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 2005;65:6282–6293. doi: 10.1158/0008-5472.CAN-05-0676. [DOI] [PubMed] [Google Scholar]
- Reimertz C., Kogel D., Rami A., Chittenden T., Prehn J. H. Gene expression during ER stress-induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J Cell Biol. 2003;162:587–597. doi: 10.1083/jcb.200305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H., Pellegrini M., Tsuchihara K., Yamamoto K., Hacker G., Erlacher M., Villunger A., Mak T. W. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarov P. G., Komarova E. A., Kondratov R. V., Christov-Tselkov K., Coon J. S., Chernov M. V., Gudkov A. V. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- Engel T., Henshall D. Apoptosis, Bcl-2 family proteins and caspases: the ABCs of seizure-damage and epileptogenesis? Int J Physiol Pathophysiol Pharmacol. 2009;1:97–115. [PMC free article] [PubMed] [Google Scholar]
- Shinoda S., Araki T., Lan J. Q., Schindler C. K., Simon R. P., Taki W., Henshall D. C. Development of a model of seizure-induced hippocampal injury with features of programmed cell death in the BALB/c mouse. J Neurosci Res. 2004;76:121–128. doi: 10.1002/jnr.20064. [DOI] [PubMed] [Google Scholar]
- Endo H., Kamada H., Nito C., Nishi T., Chan P. H. Mitochondrial translocation of p53 mediates release of cytochrome c and hippocampal CA1 neuronal death after transient global cerebral ischemia in rats. J Neurosci. 2006;26:7974–7983. doi: 10.1523/JNEUROSCI.0897-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn D., Graupner V., Neise D., Essmann F., Schulze-Osthoff K., Janicke R. U. Pifithrin-alpha protects against DNA damage-induced apoptosis downstream of mitochondria independent of p53. Cell Death Differ. 2009;16:869–878. doi: 10.1038/cdd.2009.17. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Akhtar R. S., Geng Y., Klocke B. J., Latham C. B., Villunger A., Michalak E. M., Strasser A., Carroll S. L., Roth K. A. BH3-only proapoptotic Bcl-2 family members Noxa and Puma mediate neural precursor cell death. J Neurosci. 2006;26:7257–7264. doi: 10.1523/JNEUROSCI.0196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhi S., Bruce A., Sun N., Tocco G., Baudry M., Schreiber S. S. Induction of tumor suppressor p53 and DNA fragmentation in organotypic hippocampal cultures following excitotoxin treatment. Exp Neurol. 1997;145:81–88. doi: 10.1006/exnr.1997.6451. [DOI] [PubMed] [Google Scholar]
- Sedarous M., Keramaris E., O'Hare M., Melloni E., Slack R. S., Elce J. S., Greer P. A., Park D. S. Calpains mediate p53 activation and neuronal death evoked by DNA damage. J Biol Chem. 2003;278:26031–26038. doi: 10.1074/jbc.M302833200. [DOI] [PubMed] [Google Scholar]
- Tokita Y., Bessho Y., Masu M., Nakamura K., Nakao K., Katsuki M., Nakanishi S. Characterization of excitatory amino acid neurotoxicity in N-methyl-d-aspartate receptor-deficient mouse cortical neuronal cells. Eur J Neurosci. 1996;8:69–78. doi: 10.1111/j.1460-9568.1996.tb01168.x. [DOI] [PubMed] [Google Scholar]
- Mulle C., Sailer A., Perez-Otano I., Dickinson-Anson H., Castillo P. E., Bureau I., Maron C., Gage F. H., Mann J. R., Bettler B., Heinemann S. F. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Djebaili M., De Bock F., Baille V., Bockaert J., Rondouin G. Implication of p53 and caspase-3 in kainic acid but not in N-methyl-d-aspartic acid-induced apoptosis in organotypic hippocampal mouse cultures. Neurosci Lett. 2002;327:1–4. doi: 10.1016/s0304-3940(02)00137-4. [DOI] [PubMed] [Google Scholar]
- Gomez-Lazaro M., Galindo M. F., Fernandez-Gomez F. J., Prehn J. H., Jordan J. Activation of p53 and the pro-apoptotic p53 target gene PUMA during depolarization-induced apoptosis of chromaffin cells. Exp Neurol. 2005;196:96–103. doi: 10.1016/j.expneurol.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Schauwecker P. E., Steward O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci U S A. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienzler F., Norwood B. A., Sloviter R. S. Hippocampal injury, atrophy, synaptic reorganization, and epileptogenesis after perforant pathway stimulation-induced status epilepticus in the mouse. J Comp Neurol. 2009;515:181–196. doi: 10.1002/cne.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raol Y. S., Budreck E. C., Brooks-Kayal A. R. Epilepsy after early-life seizures can be independent of hippocampal injury. Ann Neurol. 2003;53:503–511. doi: 10.1002/ana.10490. [DOI] [PubMed] [Google Scholar]
- Dube C., Richichi C., Bender R. A., Chung G., Litt B., Baram T. Z. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129:911–922. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre V., Ferrandon A., Marescaux C., Nehlig A. Electroshocks delay seizures and subsequent epileptogenesis but do not prevent neuronal damage in the lithium-pilocarpine model of epilepsy. Epilepsy Res. 2000;42:7–22. doi: 10.1016/s0920-1211(00)00153-4. [DOI] [PubMed] [Google Scholar]
- Andre V., Ferrandon A., Marescaux C., Nehlig A. The lesional and epileptogenic consequences of lithium-pilocarpine-induced status epilepticus are affected by previous exposure to isolated seizures: effects of amygdala kindling and maximal electroshocks. Neuroscience. 2000;99:469–481. doi: 10.1016/s0306-4522(00)00209-8. [DOI] [PubMed] [Google Scholar]
- Brandt C., Potschka H., Loscher W., Ebert U. N-methyl-d-aspartate receptor blockade after status epilepticus protects against limbic brain damage but not against epilepsy in the kainate model of temporal lobe epilepsy. Neuroscience. 2003;118:727–740. doi: 10.1016/s0306-4522(03)00027-7. [DOI] [PubMed] [Google Scholar]
- Narkilahti S., Nissinen J., Pitkanen A. Administration of caspase 3 inhibitor during and after status epilepticus in rat: effect on neuronal damage and epileptogenesis. Neuropharmacology. 2003;44:1068–1088. doi: 10.1016/s0028-3908(03)00115-1. [DOI] [PubMed] [Google Scholar]
- Tasch E., Cendes F., Li L. M., Dubeau F., Andermann F., Arnold D. L. Neuroimaging evidence of progressive neuronal loss and dysfunction in temporal lobe epilepsy. Ann Neurol. 1999;45:568–576. doi: 10.1002/1531-8249(199905)45:5<568::aid-ana4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Bernasconi A., Tasch E., Cendes F., Li L. M., Arnold D. L. Proton magnetic resonance spectroscopic imaging suggests progressive neuronal damage in human temporal lobe epilepsy. Prog Brain Res. 2002;135:297–304. doi: 10.1016/S0079-6123(02)35027-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.