Abstract
Microglia are critical for amyloid-β peptide (Aβ)-mediated neuronal perturbation relevant to Alzheimer’s disease (AD) pathogenesis. We demonstrate that overexpression of receptor for advanced glycation end products (RAGE) in imbroglio exaggerates neuroinflammation, as evidenced by increased proinflammatory mediator production, Aβ accumulation, impaired learning/memory, and neurotoxicity in an Aβ-rich environment. Transgenic (Tg) mice expressing human mutant APP (mAPP) in neurons and RAGE in microglia displayed enhanced IL-1β and TNF-α production, increased infiltration of microglia and astrocytes, accumulation of Aβ, reduced acetylcholine esterase (AChE) activity, and accelerated deterioration of spatial learning/memory. Notably, introduction of a signal transduction-defective mutant RAGE (DN-RAGE) to microglia attenuates deterioration induced by Aβ. These findings indicate that RAGE signaling in microglia contributes to the pathogenesis of an inflammatory response that ultimately impairs neuronal function and directly affects amyloid accumulation. We conclude that blockade of microglial RAGE may have a beneficial effect on Aβ-mediated neuronal perturbation relevant to AD pathogenesis.—Fang, F., Lue, L.-F., Yan, S., Xu, H., Luddy, J. S., Chen, D., Walker, D. G., Stern, D. M., Yan, S., Schmidt, A. M., Chen, J. X., Yan, S. S. RAGE-dependent signaling in microglia contributes to neuroinflammation, Aβ accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease.
Keywords: Aβ-binding protein, microglia/neuron interaction, cytokine, animal model
Microglia are active participants in the events that lead to neurotoxicity of amyloid-β peptide (Aβ) in Alzheimer’s disease (AD) (1,2,3,4). Increased microglial activation, microglial association with senile plaques, and elevated levels of proinflammatory mediators, such as cytokines, chemokines, and free radicals, have been observed in AD brain and AD mouse models. Several lines of evidence indicate that activated microglia and their by-products contribute to neuronal damage (5,6,7,8,9,10,11). Although Aβ-induced cellular stress has been observed in vitro by direct application of Aβ to a range of cells, neuronal toxicity of Aβ is likely amplified in the presence of activated microglia (12,13,14,15). Such effects are probably due to the presence of a range of cytotoxic (nitric oxide, superoxide, hydrogen peroxide, and proteases) and inflammatory mediators released following cellular activation by Aβ, as well as other factors in the AD milieu. Precise delineation of mechanisms of Aβ-mediated microglial inflammatory responses remains to be elucidated. Here, we demonstrate that a receptor for advanced glycation end products (RAGE)-dependent signaling in microglia stimulates inflammatory responses and processes that exacerbate neuronal damage in a transgenic (Tg) mouse model of AD.
RAGE, a multiligand receptor in the immunoglobulin superfamily, binds a broad repertoire of ligands, including products of nonenzymatic glycoxidation (AGEs), Aβ, the S100/calgranulin family of proinflammatory cytokine-like mediators, and high mobility group box 1 nonhistone DNA binding protein (HMGB1 or amphoterin) (16,17,18). RAGE consists of an extracellular domain (V-type followed by two C-type regions) and a single transmembrane domain followed by a short cytosolic tail, the latter mediating signal transduction. The biology of RAGE is largely dictated by expression and/or accumulation of its ligands. Thus, in mature healthy animals, RAGE expression is relatively low in most tissues, including the central nervous system (CNS), whereas deposition of ligands during disease states increases expression levels of RAGE. When pathogenic Aβ species accumulate in AD brain, or Tg models of β-amyloidosis, RAGE expression increases in affected cerebral vessels, neurons, and microglia (16, 19,20,21). This mechanism has the potential to exacerbate cellular dysfunction due to RAGE-ligand interaction in multiple cell types, as increased expression of the receptor allows for more profound RAGE-induced cellular perturbation (16, 20, 22,23,24,25). Furthermore, anti-RAGE IgG correlates strongly with global scores of dementia (26,27,28,29,30,31).
Studies have demonstrated that RAGE plays an important role in Aβ-mediated cellular perturbation (16, 17, 20, 22,23,24, 27, 32,33,34,35). Transgenic (Tg) mice overexpressing mutant human amyloid precursor protein (mAPP)/Aβ and RAGE in neurons displayed early-stage deficits of spatial learning/memory and neuropathologic changes (22). In view of increased expression of RAGE in microglia and the significance of microglia in an Aβ-rich environment, we hypothesized that interaction of microglial RAGE with Aβ enhances microglial activation and migration and leads to induction of proinflammatory mediators. Such events, we reasoned, would result in sustained generation of toxic mediators and, ultimately, exaggerated neuroinflammation leading to neuronal stress and injury. Although there may be multiple mechanisms through which Aβ activates microglia and enhances inflammation to cause neuronal damage, the results reported herein support the hypothesis that microglial RAGE expression may be an important contributor to neuroinflammation and accelerator of neuronal stress relevant to the pathogenesis of AD.
MATERIALS AND METHODS
Generation of Tg mice and characterization of transgene expression
Selective overexpression of human wild-type RAGE in microglia was achieved using the macrophage scavenger receptor type A (MSR) promoter (Tg RAGE), in view of its previous success in driving overexpression of the dominant-negative (DN) RAGE in microglia (36). The human RAGE cDNA was subcloned into the MSR vector using the appropriate cloning sites. For production of Tg mice, transgenic cassettes were created by releasing the XhoI fragment, and these were injected into mouse B6CBAF1/J oocytes. Oocytes were then implanted into pseudopregnant females, which were subsequently mated with B6CBAF1/J males resulting in the generation of founders. These founders were bred into the C57BL/6 background 10 generations. Founders were verified by PCR analysis of tail DNA using a probe encoding the full-length RAGE cDNA (22). Transgene expression was analyzed by RT-PCR and quantitative real-time PCR, by Western blotting with rabbit antibody produced against human soluble RAGE (which is also immunoreactive with mouse soluble RAGE), and by immunostaining with the same antibody (22, 36). Tg mice overexpressing DN-RAGE in microglia driven by the MSR promoter (Tg DN-RAGE) have been previously described and used in our studies (36). Human RAGE or DN-RAGE transgene was identified by PCR using primers (146F-5′-AGCGGCTGGAATGGAAACTGAACA and 847B-5′-GAAGGGGCAAGGGCACACCATC for human RAGE; 4A-5′AGGATCAGGGCTGGGAACTCTA, and 4B-5′-TCCCCCTGAACCTGAAACATAAAA for human DN-RAGE). Tg mice overexpressing an alternatively spliced hAPP minigene that encodes hAPP695, hAPP751, and hAPP770 bearing mutations linked to familial AD (V717F, K670M/N671L) have been described previously (line J20) (22). These mice were in the C57BL/6 background and were crossed with either Tg RAGE or Tg DN-RAGE mice. NonTg littermates matched for sex were used as controls in our studies.
Microglia were isolated from d 1 and 2 in neonatal mice following the protocol described previously (37). The cultured microglia were >95% homogeneous. Microglia derived from RAGE, DN-RAGE mice, and nonTg littermate controls were stained for CD68.
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and flush-perfused transcardially with 0.9% saline. Brains were removed and divided at midline. One hemibrain was postfixed in phosphate-buffered 4% paraformaldehyde (pH 7.4) at 4°C for 26 h for vibratome sectioning. Hippocampus and cortex were dissected from the other hemibrain, snap-frozen, and stored at −80°C for RNA or protein analysis. All animal studies were carried out according to protocols approved by the Institutional Animal Care and Use Committee of Columbia University.
RNA analyses
Total RNA was extracted from cerebral cortices of Tg mice using TRIzol reagents (Invitrogen, Carlsbad, CA, USA) and was processed directly to produce cDNA using TaqMan reverse transcription reagents kit (Applied Biosystems, Foster City, CA, USA). RT-PCR was performed to detect RAGE transcripts in Tg RAGE and Tg DN-RAGE mice. Real-time PCR was utilized for quantification of inflammatory mediator (TNF-α and IL-1β) gene expression. TNF-α, IL-1β, human RAGE, and ribosomal RNA (18S) probes and primers were purchased from Applied Biosystems. Mouse RAGE primer and probe consist of forward primer, 5′-GGACCCTTAGCTGGCACTTAGA-3′; reverse primer, 5′-GAGTCCCGTCTCA-GGGTGTCT-3′; and probe (6FAM-ATTCCCGATGGCAA-AGAAACACTCGTG-TAMRA). Quantitative real-time PCR was performed using the ABI Prism 7900 Sequence Detection System (Applied Biosystems). Data are calculated using the 2−ΔΔCt method as described by the manufacturer and are expressed as fold-increase over the indicated controls (1.0) in each figure.
Immunoblotting was performed as described previously (22). Expression of human APP was studied by immunoblotting with 6E10 (mouse monoclonal antibody to human Aβ [1–16], Signet Laboratories, Dedham, MA, USA), and human and mouse APP was analyzed with 369W (provided by Dr. S. Gandy, Mount Sinai School of Medicine, New York, NY, USA).
Quantification of glial fibrillary acid protein (GFAP)
A custom ELISA was used to measure concentration of GFAP in brain tissues. Tissue samples were extracted in 1% SDS, diluted to 0.1 μg protein/100 μl cortical brain extract, and applied to ELISA plate wells in triplicate. A cocktail of GFAP antibodies (BD Pharmingen, San Jose, CA, USA) was used as capture antibody; a rabbit polyclonal antibody to GFAP (Dako, Carpinteria, CA, USA) was used as a detection antibody. Bound antibody was detected by subsequent reaction with horseradish peroxidase-conjugated anti-rabbit IgG, and then tetramethybenzidine peroxidase substrate. Plates were read on a microplate reader at a wavelength of 450 nm. A standard curve generated from dilutions of purified human GFAP (Calbiochem, San Diego, CA, USA) included on each plate was used for quantification.
Immunocytochemical and histochemical analyses
Serial vibratome sections (20 μm) were cut from 4% paraformaldehyde-fixed brains. Immunocytochemical analyses were performed using rabbit anti-RAGE IgG (no. 6.5, immunoreactive with human and mouse forms of RAGE, generated from soluble human RAGE protein in our laboratory; 50 μg/ml). To detect endogenous mouse RAGE in nonTg and mAPP mice, an antibody to mouse RAGE (2.5, generated from the soluble mouse RAGE protein in our laboratory) was used for immunofluorescent staining. To detect plaque-associated microglia and astrocytes, sections were processed utilizing free-floating dual-color peroxidase or fluorescence immunohistochemistry following our previously published procedures (22). Microglia were identified by staining with rat anti-MHCII IgG or CD45 (dilution 1: 25 for MHC II and CD45; BD Pharmingen). Reactive astrocytes were visualized with rabbit anti-GFAP antibody (dilution 1:3000; Dako). Sections were subsequently reacted with anti-Aβ antibody 3D6 (dilution 1:3000; provided by Eli Lilly, Indianapolis, IN, USA) to identify Aβ deposits. Numbers of plaque-associated microglial or astrocytic clusters were counted in the entire hippocampus and cortex from multiple sections at the same level in each experimental group. The area of each region counted for each animal at each age group was determined by image analysis, using Universal Images software (Universal Imaging Corp., Downingtown, PA, USA). Acetylcholine esterase (AChE) activity was determined histochemically, as described previously (22) and quantitatively using a commercial kit (Molecular Probes, Hercules, CA, USA). For on-slide immunohistochemistry, sites of primary antibody binding were visualized using peroxidase-conjugated goat anti-rat or -mouse IgG and 3-amino-9-ethylcarbazole or diaminobenzidine (Sigma) as the detection system. To ensure objective assessments and reliability of results, brain sections from mice in any given experiment were blind coded and processed in parallel. Codes were revealed only after the analysis was completed (Invitrogen).
Measurement of p38 and extracellular signal-regulated kinases (ERK1/2)
The p38 and ERK1/2 in cortical tissues from each genotype of mice (RAGE, DN-RAGE, mAPP, mAPP/RAGE, mAPP/DN-RAGE, and nonTg littermates) were determined by utilization of an ELISA kit.
Behavioral study
Behavioral studies were performed to assess spatial learning and memory in the radial arm water maze, as described previously (22, 38). The investigators were masked to mouse genotypes until behavioral testing was completed.
Statistical analyses
Statistical analyses using StatView 5.0 for Windows (SAS Institute, Cary, NC, USA) were performed using 2-way ANOVA for repeated measures followed by Fisher’s protected least significant difference test for post hoc comparisons. Results are expressed as means ± se.
RESULTS
Generation and characterization of Tg RAGE and Tg DN-RAGE mice
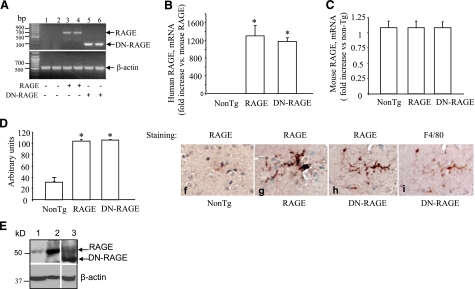
In view of increased numbers of RAGE-immunoreactive microglia in AD-affected regions (e.g., hippocampus) of the brain (19, 20), we sought to determine whether RAGE signaling in microglia contributes to Aβ-mediated neuroinflammation, potentially exacerbating neurodegeneration in AD pathogenesis. For this purpose, we developed Tg mice expressing human full-length RAGE. To determine whether RAGE functions solely as a cell-surface binding site for Aβ, possibly increasing local levels of Aβ to interact with other cell surface molecules, or alternatively whether it has a direct role in transducing a signal important to the process of Aβ-induced cellular perturbation, we also prepared Tg mice expressing DN-RAGE in microglia lineage. DN-RAGE, a construct comprising the extracellular and membrane-spanning domains of RAGE, but devoid of the cytosolic tail, has previously been shown to block RAGE-dependent intracellular signaling mechanisms (22, 24, 39). RAGE and DN-RAGE mice were made under the control of the scavenger receptor type A promoter (MSR) to drive expression of the RAGE or DN-RAGE transgene in microglia lineage as described previously (36). RAGE and DN-RAGE mice display normal gross development and normal reproductive fitness and life span. RT-PCR, quantitative real-time-PCR, and immunostaining for RAGE were performed on the cerebral cortical tissues and microglia isolated from these mice to analyze expression of RAGE in Tg mice. Notably, RT-PCR analysis revealed increases in RAGE transcripts in the cerebral cortex of Tg RAGE and DN-RAGE mice, as compared to nonTg littermates (Fig. 1A). By quantitative real-time PCR, levels of human RAGE mRNA significantly increased in the cerebral cortex of Tg RAGE and DN-RAGE mice, whereas no significant changes in mouse RAGE mRNA were found between Tg mice and nonTg littermates (Fig. 1B, C), suggesting that the introduction of human RAGE transgene does not interfere with endogenous mouse RAGE expression. Consistent with these results, levels of human RAGE protein were elevated in Tg RAGE and DN-RAGE mice compared to the nonTg littermates (Fig. 1D, E). Further, immunostaining using specific antibody to human RAGE revealed an increase in RAGE antigen levels in the cerebral cortex of both Tg RAGE and DN-RAGE mice (Fig. 1F–H). RAGE was present in brain microglia of Tg RAGE or Tg DN-RAGE mice as compared with nonTg mice (Fig. 1F–H). Sequential staining of a microglial section with anti-RAGE IgG (generated against extracellular domain epitopes) (Fig. 1H) and anti-F4/80 IgG (a microglia marker, Fig. 1I) showed immunoreactivity for both antigens.
Figure 1.
Identification and characterization of RAGE and DN-RAGE mice. A) RT-PCR analysis of total RNA harvested from cerebral cortex of Tg RAGE mice (+, lanes 3–4), Tg DN-RAGE (+, lanes 5–6), and nonTg littermates (−, lanes 1–2) using primers for human RAGE. B, C) Quantitative real-time PCR for human (B) and mouse (C) RAGE mRNA in the brain of Tg mice. D, E) Immunoblotting of brain homogenates for RAGE. D) Quantification of RAGE immunoreactive bands in the brain of the indicated Tg mice. E) Top panel: representative immunoblots of brain homogenates from nonTg (lane 1), Tg RAGE (lane 2), and Tg DN-RAGE mice (lane 3). Bottom panel: immunoblots for β-actin used as protein-loading control. F–H) Immunostaining of brain sections from nonTg (F), Tg RAGE (G), and Tg DN-RAGE mice (H) for RAGE. I) Sequential staining of the section in panel H with F4/80 IgG (a microglial marker) shows an overlapping distribution of cells stained with antibodies to RAGE and F4/80. Sections were counterstained with hematoxylin. Above studies were performed on mice 3–4 mo of age.
To further verify the expression of RAGE in microglia, we measured levels of RAGE mRNA and protein in neonatal microglia cultured from Tg mice. Indeed, both human RAGE mRNA and protein were significantly increased in microglia derived from Tg RAGE and DN-RAGE mice compared to nonTg microglia (Supplemental Fig. S1A–C). Expression levels of RAGE and DN-RAGE were comparable between RAGE and DN-RAGE mice. Confocal analysis of double immunostaining for RAGE with CD68 (a microglial marker) demonstrated that RAGE was homogenously present in microglia positive for CD68 (Supplemental Fig. S1D). Quantification of RAGE staining revealed that RAGE expression levels were increased by 3- to 4-fold in RAGE or DN-RAGE microglia compared to nonTg microglia (Supplemental Fig. S1E, F; this comparison reflects increased expression of human transgenic RAGE vs. levels of endogenous murine RAGE mRNA). These data clearly demonstrate that RAGE or DN-RAGE mice express increased levels of microglial RAGE, as compared to nonTg mice. Therefore, microglial RAGE or DN-RAGE mice are appropriate models to investigate the effect of RAGE signaling in microglia on Aβ-mediated cellular perturbation.
We then further analyzed the effect of RAGE signaling in microglia using an in vivo AD mouse model. For these studies, Tg mice modeling amyloid plaque development by expressing a mutant form of human APP and Aβ (J-20 line, mAPP) were used as described in our previous studies (24, 38, 40,41,42,43). The mAPP mouse model is well suited to studies for determination of whether increased RAGE expression or blockade of RAGE signaling in microglia may enhance/accelerate or diminish neuroinflammation and neuropathological changes, since these animals have been previously characterized with respect to neuropathological, biochemical, and behavioral endpoints (22, 38, 43). Furthermore, it has been previously established that there is increased RAGE expression in neurons and microglia in mAPP mice as these animals age and accumulate Aβ (22). To examine the effects of Aβ overproduction on endogenous mouse RAGE expression in mAPP mice, as compared to those in nonTg littermates, we perfomed the double immunofluorescent staining using an antibody specific for mouse RAGE. As shown in Supplemental Fig. S2, mAPP mice showed increases in neuronal and microglial RAGE expression (Supplemental Fig. S2A3, C2, 3) compared to those in nonTg littermates (Supplemental Fig. S2A2, B2, 3). RAGE-deficient mice did not display a specific staining pattern with anti-RAGE antibody (Supplemental Fig. S2A1). In addition, no specific staining patterns were observed when anti-RAGE antibody was omitted, replaced by nonimmune IgG, or preabsorbed with the corresponding antigen (sRAGE protein) (Supplemental Fig. S2D).
Mice expressing human RAGE or DN-RAGE were crossed with mAPP mice. Six genotypes were generated in the expected Mendelian ratio; single Tgs (RAGE, DN-RAGE, and mAPP), double Tgs (mAPP/RAGE, mAPP/DN-RAGE), and nonTg littermate controls (Supplemental Fig. S3A). RAGE expression in each of the genotypes was analyzed by quantitative PCR of the cerebral cortex. Levels of RAGE transcripts were greatly increased in the cerebral cortex of RAGE, mAPP/RAGE, DN-RAGE, and mAPP/DN-RAGE mice at the age of 3–4 mo, as compared to mAPP and nonTg littermates of the same age under the conditions used for PCR amplification (Supplemental Fig. S3B). Consistent with previous results, RAGE levels were higher in the cerebral cortex of mAPP mice than in that of nonTg littermates at the age of 3–4 mo (22). Similar differences in RAGE expression were observed at 9–10 mo of age. There were no significant differences in mAPP transgene expression (including human and endogenous mouse forms of APP) between mAPP, mAPP/RAGE, and mAPP/DN-RAGE mice (Supplemental Fig. S3C–E).
Effects of microglial RAGE on proinflammatory mediators and neuroinflammation in mAPP/RAGE and mAPP/DN-RAGE mice
We previously demonstrated in human microglia obtained post mortem that RAGE serves as an important cell-surface receptor-mediating chemotactic and inflammatory responses to Aβ and other proinflammatory ligands, specifically S100/calgranulins (18, 19, 44). Increased RAGE expression was found in microglia from AD brains compared to those from age-matched, nondemented control brains.
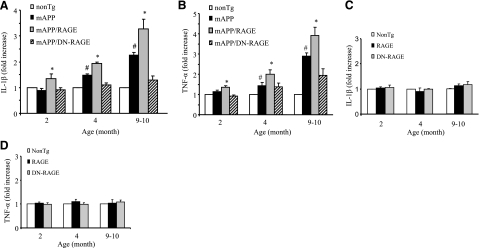
On the basis of these findings, we hypothesized that overexpression of RAGE in microglia may exacerbate neuroinflammation in an Aβ-rich environment. To test this concept, we first assessed expression of proinflammatory cytokines in cortical tissue. Tg mAPP/RAGE mice showed increasing levels of IL1-β and TNF-α in the cerebral cortex in an age-dependent manner. This increased cytokine expression in Tg mAPP/RAGE mice occurred as early as 2 mo prior to the induction of the same cytokines in mAPP mice (Fig. 2A, B). Notably, introduction of the DN-RAGE transgene into mAPP mice (mAPP/DN-RAGE mice) delayed and attenuated the increases in brain cytokine levels in mAPP mice from 2 to 10 mo of age. (Fig. 2A, B). Levels of IL-1β and TNF-α were comparable among single Tg RAGE and DN-RAGE mice and nonTg littermate controls, suggesting no effect due to the RAGE or DN-RAGE transgene on cytokine production in the absence of RAGE ligands (Fig. 2C, D).
Figure 2.
Effect of microglial RAGE on induction of proinflammatory cytokines in brains of mAPP/RAGE and mAPP/DN-RAGE mice. Quantitative real-time PCR analysis of cytokines (IL-1β; A, C; and TNF-α; B, D) of total RNA extracted from cerebral cortex of the indicated Tg mice at 2, 4–5, and 9–10 mo of age (4–6 mice/group). There is no significant difference in IL-1β or TNF-α levels between nonTg and RAGE or DN-RAGE mice (P>0.01). #P < 0.01 vs. nonTg group; *P < 0.01 vs. other groups.
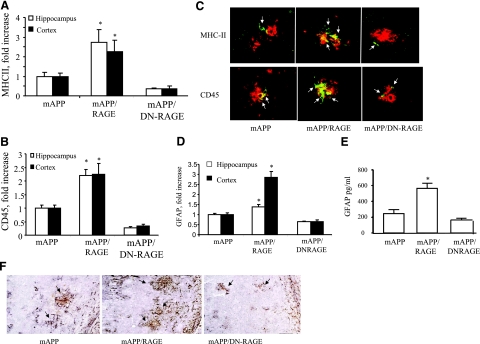
Next, we compared microgliosis and astrocytosis in mAPP/RAGE, mAPP/DN-RAGE, and mAPP mice. Microglia were visualized immunohistochemically with antibodies to MHCII and CD45, markers for activated microglia. Astrocytes were visualized with antibody to GFAP. mAPP/RAGE mice displayed significantly increased plaque-associated microglial clusters (Fig. 3A–C) and astrocyte infiltration (Fig. 3D, F), compared to mAPP mice at 9–10 mo of age. In contrast, mAPP/DN-RAGE mice do not show significant microglial and astrocyte infiltration in the cerebral cortex and hippocampus (Fig. 3). ELISA results further confirmed an increase in GFAP levels in the cerebral cortex of mAPP/RAGE mice, which was attenuated in the mAPP/DN-RAGE animals (Fig. 3E).
Figure 3.
Effect of microglial RAGE on microgliosis and astrocytosis in brains of mAPP/RAGE and mAPP/DN-RAGE mice. A, B) Quantification of plaque-associated microglia positive for MHC II (A) or CD45 (B) in the cerebral cortex and hippocampus (5–6 mice/genotype). C) Representative sections from mAPP, mAPP/RAGE, and mAPP/DN-RAGE mice at 9–10 mo of age to demonstrate plaque-associated microglia. Arrows indicate cells reactive with microglial activation marker (MHC II or CD45; green). Amyloid plaques are red. D) Image analysis of plaque-associated astrocytosis in cortex and hippocampus of the indicated Tg mice at 9–10 mo of age (5–6 mice/genotype). E) Quantification of levels of GFAP in the cerebral cortex from the indicated Tg mice at 9–10 mo of age; n = 5–6 mice/group. *P < 0.05 vs. other groups; ELISA. F) Representative images from mAPP, mAPP/RAGE, and mAPP/DN-RAGE mice at 9–10 mo of age to demonstrate plaque-associated astrocytes. GFAP immunoreactive astrocyte clusters are black; amyloid plaques are brown.
Effect of microglial RAGE on Aβ levels and amyloid plaque formation
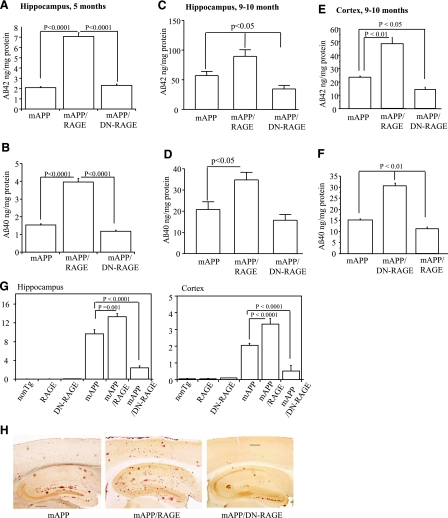
Microglia are believed to have an active role in regulating Aβ levels and amyloid burden in the brain. These considerations led us to assess levels of neocortical and hippocampal Aβ by ELISA in brain extracts prepared from animals of each of the genotypes at 5 and 9–10 mo of age. At 5 mo of age, though levels of Aβ(1-40) and Aβ(1-42) were relatively low in all the groups, significantly higher concentrations were observed in Tg mAPP/RAGE animals (Fig. 4A, B). As animals aged to 9–10 mo, there was a prominent increase in levels of Aβ in brains of Tg mAPP mice. However, Tg mAPP/RAGE mice displayed significantly more Aβ(1-40)and Aβ(1-42) in the hippocampus and cortex than Tg mAPP mice (Fig. 4C–F). Introduction of the DN-RAGE transgene into Tg mAPP mice resulted in lower Aβ levels, as shown by a downward trend in Aβ in Tg mAPP/DN-RAGE mice vs. Tg mAPP [this trend achieved statistical significance for Aβ(1-42) in hippocampus and cortex; Fig. 4C, E]. Thus, these data reveal for the first time that overexpression of RAGE in microglia provoked striking increases in levels of Aβ(1-40) and Aβ(1-42) in the hippocampus and cortex.
Figure 4.
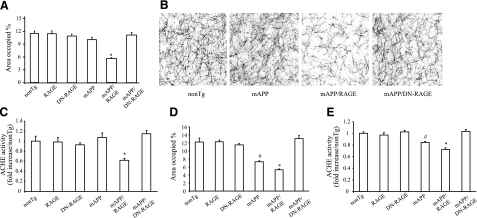
Aβ levels and Aβ plaque load in brains of Tg mice. A–F) Aβ(1-40) and Aβ(1-42) levels were determined by ELISA in the hippocampus and cortex from mice of each of the genotypes at 5 mo (A, B) and 9–10 mo of age (C–F) (n=7–10 mice/group). G) Aβ deposits in hippocampus and neocortex were quantified by histological image analysis in the same group of mice after staining of brain sections with 3D6 antibody as indicated. H) Representative sections stained with 3D6 from Tg mAPP, Tg mAPP/MSR-RAGE, and Tg mAPP/MSR-DN-RAGE mice at 9–10 mo of age. Results are means ± se. Scale bar = 30 μm.
We next determined amyloid burden (Aβ plaque load) in the hippocampus and cerebral cortex in groups of Tg mice using the monoclonal antibody 3D6. The area occupied by immunoreactive Aβ deposits in Tg mAPP/RAGE mice was significantly greater vs. that observed in Tg mAPP mice at 9–10 mo of age. Deposits of Aβ were more extensively distributed in the double Tgs (Tg mAPP/RAGE mice) compared with Tg mAPP (Fig. 4G, H). Remarkably, plaque load in the hippocampus and cerebral cortex of Tg mAPP/DN-RAGE mice was strikingly reduced compared to that observed in Tg mAPP or double Tg mice (Fig. 4G, H). Taken together, these data indicate that overexpression of microglial RAGE enhanced plaque load in Tg mAPP mice and highlight critical roles for RAGE signal transduction in microglia in these processes, as evidenced by a striking reduction of plaques in Tg mAPP/DN-RAGE mice (vs. Tg mAPP).
Effects of microglial RAGE on neuropathological findings in mAPP/RAGE and mAPP/DN-RAGE mice
To determine whether elevated levels of microglial RAGE and neuroinflammation in mAPP/RAGE mice would also be reflected in signs of increased neuronal stress, we next assessed the density of cholinergic fibers and synapses, which has been shown to be diminished in AD-like pathology. Thus, we histochemically analyzed the density of neurites containing AChE activity (which predominantly labels cholinergic neurites) (22, 45) in Tg mice. AChE-positive neurites were determined as the area occupied by positively staining neurites in the subiculum (Sb). At age 4–5 mo, the area occupied by AChE-positive neurites in the Sb was decreased only in mAPP/RAGE mice (P<0.01), as compared to nonTg littermate controls (Fig. 5A, B). Representative images of AChE histochemically stained Sb sections from each mouse genotype at 4–5 mo of age are shown in Fig. 5B. We also performed biochemical AChE activity analysis. Consistent with histochemical results, AChE activity was significantly reduced in the Sb regions of the double Tg mAPP/RAGE mice compared to other groups of mice (Fig. 5C). The decrease in AChE-positive neuritic processes and AChE activity appeared to be progressive by 9–10 mo of age in mAPP mice (Fig. 5D, E; compared to same mice at 4–5 mo), though not in nonTg littermates (RAGE or DN-RAGE alone), indicating that loss of cholinergic fibers was not simply a function of age. Of note, levels of AChE-positive neurites and AChE activity were significantly lower in mAPP/RAGE mice than in mAPP mice. Tg mAPP/DN-RAGE mice were protected from Aβ-mediated reduction of AChE-positive neurites (Fig. 5A, B, D) and AChE activity (Fig. 5C, E) at ages 4–5 and 9–10 mo, as compared to nonTg littermate controls. These data indicate that neuropathological changes are accelerated in mAPP/RAGE mice, as early as 4–5 mo of age, whereas nonTg and Tg mAPP mice do not display such abnormalities at that age. Introduction of DN-RAGE attenuates Aβ-mediated regional neuropathological changes, suggesting the critical role of microglial RAGE signaling in neuronal dysfunction.
Figure 5.
Effect of microglial RAGE on AChE activity of mAPP/RAGE and mAPP/DN-RAGE mice. A) AChE-positive neurites were visualized histochemically in the subiculum (Sb) of indicated genotypes at 4–5 mo of age. B) Representative images of AChE staining for indicated groups of mice at 4–5 mo of age. Scale bar = 5 μm. C) AChE activity in subiculum at 4–5 mo of age. D) AChE-positive neurites in subiculum at 9–10 mo of age. E) AChE activity in subiculum at 9–10 mo of age. n = 7–9 mice/group (A, B, D); 9–10 mice/group (C, E). *P < 0.01 vs. nonTg, RAGE, DN-RAGE, and mAPP/DN-RAGE groups; #P < 0.05 vs. nonTg, RAGE, DN-RAGE, and mAPP/RAGE groups. No significant differences were found among RAGE, DN-RAGE, nonTg, and mAPP/DN-RAGE groups.
Effect of microglial RAGE on spatial learning/memory in mAPP/RAGE and mAPP/DN-RAGE mice
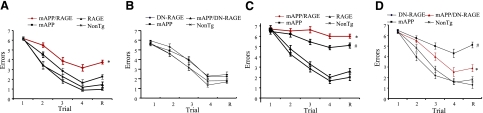
Finally, to analyze whether neuropathological changes are reflected in neuronal dysfunction, we performed behavioral analysis using the radial arm water maze to assess spatial learning/memory in Tg mice. At 4–5 mo of age, RAGE, DN-RAGE, nonTg, and mAPP littermates showed strong learning and memory capacity (Fig. 6A, B). In contrast, mAPP/RAGE mice averaged ∼3–4 errors by trial 4 and retention test, indicating impaired spatial memory for platform location between trials, as well as reference memory (during the 30-min delay before retention test). At 9–10 mo of age, although mAPP mice displayed deficits in learning and memory compared to nonTg mice (∼5–5.5 errors in mAPP mice vs. ∼2 errors in nonTg mice between trials 3 and 4 and retention test), mAPP/RAGE mice showed more errors (6.5–7 errors) than single mAPP mice (Fig. 6C). mAPP/DN-RAGE mice were similarly assessed to test the effect of the DN-RAGE transgene. At 4–5 mo of age, mAPP/DN-RAGE mice had normal behavioral function as compared to nonTg littermate controls (Fig. 6B). Notably, at 9–10 mo of age, mAPP/DN-RAGE mice showed significantly improved learning and memory (∼3 errors), whereas ∼5–5.5 errors occurred by trials 3 and 4 and retention test in mAPP mice (Fig. 6D). Tg RAGE or DN-RAGE mice did not exhibit abnormal behavior as compared to nonTg mice. Control studies showed the six genotypes to be indistinguishable in their speed of swimming or time required to reach a visible platform (data not shown). Taken together, these data suggest an early acceleration of learning and memory impairment consequent to overexpression of microglial RAGE in an Aβ-enriched environment, such as the AD mouse model. In contrast, introduction of DN-RAGE produces an apparently protective effect.
Figure 6.
Behavioral studies in mAPP/RAGE and mAPP/DN-RAGE mice. Spatial learning and memory were tested in the radial arm water maze at age 4–5 mo (A, B) and 9–10 mo (C, D) for the indicated mice; n = 7–9 male mice/genotype. Trials: 1–4, acquisition trials; R, retention trial. mAPP/RAGE mice demonstrated worsened spatial learning memory compared to nonTg mice and mAPP mice (A, C). Single mAPP mice at 9–10 mo of age showed impaired learning memory compared to nonTg littermate controls (C, D). mAPP/DN-RAGE mice significantly improved learning memory (D). *P < 0.01 (A, D), *P < 0.05 (C) vs. mAPP; #P < 0.01 vs. nonTg.
RAGE-dependent activation of mitogen-activated protein kinase (MAPK) p38 and ERK1/2 in microglia
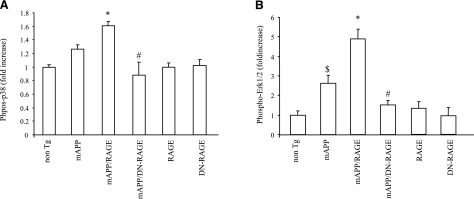
Activation of MAPK p38 and ERK1/2 has previously been shown to regulate gene expression and lead to increased production of proinflammatory cytokines (11, 46) and further has been reported to occur in neuronal RAGE overexpressing mAPP mice (22, 24). Using ELISA, we examined phosphorylated forms of p38 and ERK1/2 in the cerebral cortex of Tg mice at 4–5 mo of age. This time point was chosen because of increased cytokine production and impaired spatial learning memory in mice at this age. Levels of phosphorylated p38 and ERK1/2 were significantly increased in brain extracts of mAPP and mAPP/RAGE mice, as compared to nonTg mice, while mAPP/RAGE brain exhibited an even higher level of phosphorylated p38 and ERK1/2 than mAPP brain. It is noted that mAPP/DN-RAGE mice revealed significantly less phosphorylation of p38 and ERK1/2, as compared with mAPP/RAGE mice (Fig. 7). Total p38 and ERK1/2 in the brains were comparable among all of the mice groups. There were no changes in the levels of phosphorylated p38 and ERK1/2 in the single Tg RAGE or DN-RAGE mice.
Figure 7.
Effect of RAGE on activation of phosphorylation of p38 and ERK1/2 for in vivo mAPP mice. Phosphorylation of p38 (A) and ERK1/2 (B) in brain homogenates of indicated Tg mice was measured by ELISA; n = 3–8 mice/group. Vertical axis indicates fold-increased phosphorylated p38 (A) and phosphorylated ERK1/2 (B) (normalized to total p38 or ERK1/2, respectively) in the indicated Tg mice relative to nonTg mice. *P < 0.05 vs. nonTg, mAPP, RAGE, DN-RAGE, and mAPP/DN-RAGE groups; #P < 0.05 vs. mAPP group; $P < 0.05 vs. nonTg group.
DISCUSSION
A close association between neuroinflammatory mechanisms and Aβ-related pathological events has been recognized for some time (6, 11, 47). Pathological analyses of brains from AD patients and AD-type-mouse models, including Tg mouse systems and analyses after direct infusion of Aβ into brain parenchyma, have shown that activated microglia and astrocytes accumulate to the greatest extent in proximity to amyloid plaques (11, 47,48,49,50,51). In addition, many inflammatory mediators detected in AD brains are of microglial origin (13, 52). Neuroinflammation and microglial activation comprise the double-edged sword regulating the delicate balance in neuronal homeostasis. Both deficient and excessive responses will result in pathological conditions (11, 53,54,55). The innate immune responses of glia to injurious insults, foreign pathogen, or activating stimuli generally lead to beneficial outcomes, such as phagocytosis or production of reparative or protective factors. However, sustained activation of microglia and overproduction of proinflammatory mediators disturbs homeostasis, resulting in disease progression and exacerbation of induction factors that influence the severity of neuronal dysfunction and the progression of neuropathology (56, 57). It is possible that chronic administration of anti-inflammatory agents may have a therapeutic benefit in AD (52, 58, 59). The precise mechanisms by which Aβ mediates activation of microglia and astrocytes remain to be clarified. Our current study indicates an important role for RAGE-mediated signaling in microglia in neuronal dysfunction relevant to AD pathology.
RAGE is expressed by microglia and mediates, at least in part, the effects of Aβ on these cells. For example, we previously found that RAGE mediates, in large part, Aβ-induced microglial migration and cytokine induction in cultured human microglia from AD patients (20, 22, 60). The latter observations led us to propose that genetic manipulation of RAGE levels in microglia might affect AD pathogenesis in model systems. The results described here provide support for this hypothesis; increased microglial RAGE levels led to increased levels of proinflammatory cytokine transcripts and clusters of plaque-associated activated microglia and astrocytes. Consistent with the significance of these inflammatory changes, spatial learning/memory and the area occupied by AChE-positive neurites as well as neurite activity declined in animals with higher microglial levels of wild-type RAGE. In contrast, introduction of a DN-RAGE transgene into the Tg mAPP murine model suppressed these changes, often to levels below those seen in Tg mAPP mice. These data indicate that introduction of the DN-RAGE transgene affects the function of endogenous murine microglial RAGE, and, thus, further support the premise that this transgene evokes a dominant-negative effect in cells endogenously expressing the receptor.
An unexpected observation in our study was revealed by the analyses of plaque burden and Aβ levels in the brains of mice bearing the wild-type RAGE and DN-RAGE transgenes. Increased expression of wild-type RAGE was associated with elevated levels of Aβ, both Aβ (1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40), and Aβ (1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42). Consistent with these data, amyloid plaque burden was also significantly increased in Tg mAPP/RAGE mice beyond that observed in Tg mAPP. Our observations would suggest that in an Aβ-rich environment, RAGE action favors Aβ accumulation. Such accumulation could result from increased production of Aβ or mechanisms preventing its clearance. There were no significant differences in hAPP and endogenous mouse APP protein expression among Tg mAPP, Tg mAPP/RAGE, and Tg mAPP/DN-RAGE mice, indicating that increased Aβ accumulation in Tg mAPP/RAGE mice is not simply due to increased hAPP transgene or endogenous APP expression. There were also no significant differences in the expression of insulin-degrading enzyme (IDE, data not shown), which is involved in cerebral Aβ accumulation in mice (61). Our data, thus far, in microglia suggest that RAGE expression does not significantly affect hAPP transgene expression or Aβ clearance by IDE and that the other mechanisms may be responsible for the RAGE-dependent component of amyloid pathology. Of course, this does not rule out an increase in Aβ generation as an indirect effect of microglial mediators affecting neurons or other cell types (57). Another possibility is that neuroinflammation mediated by RAGE impairs protective clearance mechanisms, either by sequestering pathogenic Aβ species in an inflammatory environment or down-regulating specific clearance mechanisms (62, 63). Nevertheless, our results are consistent with an increasing body of evidence that correlates inflammation with levels of Aβ in Tg mice expressing mutant APP and AD patients (3, 51, 64). In this context, anti-inflammatory drugs, such as ibuprofen, have been shown to reduce plaque pathology and brain Aβ levels in animal models of AD (3, 23, 65, 66). Increased induction of proinflammatory mediators, such as TNF-α, IL-1β, or INF-γ, are also associated with neuronal damage in an Aβ-rich environment (10, 67,68,69,70), as well as Aβ accumulation (62, 71). In fact, polymorphisms in the regulatory regions of these cytokines are associated with a higher risk for developing AD (3, 58, 64, 72).
It is important to note that the current findings do not exclude roles for other proinflammatory ligands of RAGE in an Aβ-enriched environment. Previous studies revealed that members of the S100/calgranulin ligand family of RAGE were up-regulated in microglia isolated and cultured from human AD brain. These considerations highlight the concept that RAGE focuses the effect of more than one family of proinflammatory mediators in AD brain and evokes maladaptive responses that amplify and enhance neuroinflammation, a process linked to neuronal dysfunction (18, 44).
Extensive supporting evidence indicates that the p38 MAPK signaling cascade contributes to cytokine overproduction and neurodegenerative sequelae observed in AD (13, 73). Activation of p38 MAPK was found in early stage AD brain (13, 74,75,76,77,78) and in an AD mouse model (74, 79, 80). Inhibition of p38 MAPK activation blocks Aβ-mediated cytokine production and neuronal death (58, 81). Levels of phosphorylation of p38 and ERK1/2 were significantly higher in brains of mAPP mice with increased expression of microglial RAGE than in brains of the single mAPP mice. In parallel, cytokine production and exaggerated neuronal stress were seen with increased microglial RAGE expression. However, expression of microglial DN-RAGE in mAPP mice attenuated Aβ-mediated detrimental effects. This suggests that RAGE-dependent activation of p38 and ERK1/2 MAPK pathways is responsible, at least in part, for Aβ-mediated microglial activation and the induction of proinflammatory mediators.
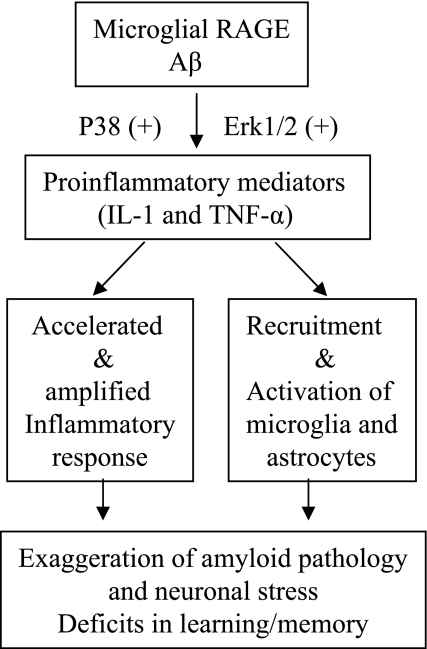
Taken together, our findings indicate that microglial RAGE contributes importantly to the amplification of neuroinflammation, accumulation of amyloid, and neuronal perturbation in an Aβ-rich environment. It is our hypothesis that the interaction of microglial RAGE with Aβ activates signal transduction cascades (MAP kinase, p38, and ERK1/2), enhances cytokine production (IL-β and TNF-α), accelerates or amplifies inflammatory response, leading to recruitment or activation of microglia and astrocytes. Consequently, RAGE-induced neuroinflammation has the potential to exaggerate neuronal stress and, ultimately, this contributes to deficits in learning/memory (Fig. 8). Furthermore, we conclude that RAGE-mediated induction of proinflammatory mediators enhances Aβ accumulation through a positive feedback loop stimulating the RAGE receptor, and the latter will further exaggerate neuroinflammation and amyloid pathology. The current study provides substantial evidence of a critical role of microglial RAGE in the pathogenesis of Alzheimer’s disease. Blockade of RAGE signaling in microglia may be a potentially attractive drug discovery target for halting neuronal damage in Alzheimer’s disease.
Figure 8.
Hypothesis for microglial RAGE involved in neuroinflammation, neuronal stress, and impaired learning/memory.
Supplementary Material
Acknowledgments
This work was supported by grants from the U.S. Public Health Service (PO1AG17490). L-F.L. and D.G.W. were supported by a grant from the Arizona Alzheimer’s Consortium. D.S. is a consultant of TransTech Pharma.
References
- Uchihara T., Akiyama H., Kondo H., Ikeda K. Activated microglial cells are colocalized with perivascular deposits of amyloid-beta protein in Alzheimer’s disease brain. Stroke. 1997;28:1948–1950. doi: 10.1161/01.str.28.10.1948. [DOI] [PubMed] [Google Scholar]
- Giulian D., Li J., Leara B., Keenen C. Phagocytic microglia release cytokines and cytotoxins that regulate the survival of astrocytes and neurons in culture. Neurochem Int. 1994;25:227–233. doi: 10.1016/0197-0186(94)90066-3. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Rogers J., McGeer E. G. Inflammation, anti-inflammatory agents and Alzheimer disease: the last 12 years. J Alzheimers Dis. 2006;9:271–276. doi: 10.3233/jad-2006-9s330. [DOI] [PubMed] [Google Scholar]
- Giulian D., Haverkamp L. J., Li J., Karshin W. L., Yu J., Tom D., Li X., Kirkpatrick J. B. Senile plaques stimulate microglia to release a neurotoxin found in Alzheimer brain. Neurochem Int. 1995;27:119–137. doi: 10.1016/0197-0186(95)00067-i. [DOI] [PubMed] [Google Scholar]
- Minghetti L. Role of inflammation in neurodegenerative diseases. Curr Opin Neurol. 2005;18:315–321. doi: 10.1097/01.wco.0000169752.54191.97. [DOI] [PubMed] [Google Scholar]
- Britschgi M., Wyss-Coray T. Immune cells may fend off Alzheimer disease. Nat Med. 2007;13:408–409. doi: 10.1038/nm0407-408. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Horiba M., Buescher J. L., Huang D., Gendelman H. E., Ransohoff R. M., Ikezu T. Overexpression of monocyte chemotactic protein-1/CCL2 in beta-amyloid precursor protein transgenic mice show accelerated diffuse beta-amyloid deposition. Am J Pathol. 2005;166:1475–1485. doi: 10.1016/s0002-9440(10)62364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Groen T., Kadish I. Transgenic AD model mice, effects of potential anti-AD treatments on inflammation and pathology. Brain Res Rev. 2005;48:370–378. doi: 10.1016/j.brainresrev.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Heneka M. T., Sastre M., Dumitrescu-Ozimek L., Hanke A., Dewachter I., Kuiperi C., O'Banion K., Klockgether T., Van Leuven F., Landreth G. E. Acute treatment with the PPARγ agonist pioglitazone and ibuprofen reduces glial inflammation and Aβ1-42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- Floden A. M., Li S., Combs C. K. Beta-amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor alpha and NMDA receptors. J Neurosci. 2005;25:2566–2575. doi: 10.1523/JNEUROSCI.4998-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik L. J., Thompson W. L., Ranaivo H. R., Behanna H. A., Watterson D. M. Glia proinflammatory cytokine upregulation as a therapeutic target for neurodegenerative diseases: function-based and target-based discovery approaches. Int Rev Neurobiol. 2007;82:277–296. doi: 10.1016/S0074-7742(07)82015-0. [DOI] [PubMed] [Google Scholar]
- Weninger S. C., Yankner B. A. Inflammation and Alzheimer disease: the good, the bad, and the ugly. Nat Med. 2001;7:527–528. doi: 10.1038/87839. [DOI] [PubMed] [Google Scholar]
- Hensley K., Floyd R. A., Zheng N. Y., Nael R., Robinson K. A., Nguyen X., Pye Q. N., Stewart C. A., Geddes J., Markesbery W. R., Patel E., Johnson G. V., Bing G. p38 kinase is activated in the Alzheimer’s disease brain. J Neurochem. 1999;72:2053–2058. doi: 10.1046/j.1471-4159.1999.0722053.x. [DOI] [PubMed] [Google Scholar]
- Yankner B. A. Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron. 1996;16:921–932. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- Lue L. F., Walker D. G., Rogers J. Modeling microglial activation in Alzheimer’s disease with human postmortem microglial cultures. Neurobiol Aging. 2001;22:945–956. doi: 10.1016/s0197-4580(01)00311-6. [DOI] [PubMed] [Google Scholar]
- Yan S. D., Chen X., Fu J., Chen M., Zhu H., Roher A., Slattery T., Zhao L., Nagashima M., Morser J., Migheli A., Nawroth P., Stern D., Schmidt A. M. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Chen X., Walker D. G., Schmidt A. M., Arancio O., Lue L. F., Yan S. D. RAGE: a potential target for Abeta-mediated cellular perturbation in Alzheimer’s disease. Curr Mol Med. 2007;7:735–742. doi: 10.2174/156652407783220741. [DOI] [PubMed] [Google Scholar]
- Hofmann M. A., Drury S., Fu C., Qu W., Taguchi A., Lu Y., Avila C., Kambham N., Bierhaus A., Nawroth P., Neurath M. F., Slattery T., Beach D., McClary J., Nagashima M., Morser J., Stern D., Schmidt A. M. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- Lue L. F., Walker D. G., Brachova L., Beach T. G., Rogers J., Schmidt A. M., Stern D. M., Yan S. D. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Exp Neurol. 2001;171:29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- Du Yan S., Zhu H., Fu J., Yan S. F., Roher A., Tourtellotte W. W., Rajavashisth T., Chen X., Godman G. C., Stern D., Schmidt A. M. Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: a proinflammatory pathway in Alzheimer disease. Proc Natl Acad Sci U S A. 1997;94:5296–5301. doi: 10.1073/pnas.94.10.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. C., Tavares R., Johanson C. E., Hovanesian V., Donahue J. E., Gonzalez L., Silverberg G. D., Stopa E. G. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer’s disease. Brain Res. 2008;1230:273–280. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancio O., Zhang H. P., Chen X., Lin C., Trinchese F., Puzzo D., Liu S., Hegde A., Yan S. F., Stern A., Luddy J. S., Lue L. F., Walker D. G., Roher A., Buttini M., Mucke L., Li W., Schmidt A. M., Kindy M., Hyslop P. A., Stern D. M., Du S. S. Yan. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004;23:4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R., Du S. Yan, Submamaryan R. K., LaRue B., Jovanovic S., Hogg E., Welch D., Manness L., Lin C., Yu J., Zhu H., Ghiso J., Frangione B., Stern A., Schmidt A. M., Armstrong D. L., Arnold B., Liliensiek B., Nawroth P., Hofman F., Kindy M., Stern D., Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Origlia N., Righi M., Capsoni S., Cattaneo A., Fang F., Stern D. M., Chen J. X., Schmidt A. M., Arancio O., Yan S. D., Domenici L. Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-beta-mediated cortical synaptic dysfunction. J Neurosci. 2008;28:3521–3530. doi: 10.1523/JNEUROSCI.0204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N., Toki S., Chowei H., Saito T., Nakano N., Hayashi Y., Takeuchi M., Makita Z. Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer’s disease. Brain Res. 2001;888:256–262. doi: 10.1016/s0006-8993(00)03075-4. [DOI] [PubMed] [Google Scholar]
- Wilson J. S., Mruthinti S., Buccafusco J. J., Schade R. F., Mitchell M. B., Harrell D. U., Gulati N. K., Stephen L. Miller. Anti-RAGE and Aβ immunoglobulin levels are related to dementia level and cognitive performance. J Gerontol. 2009;64:264–271. doi: 10.1093/gerona/gln002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruthinti S., Sood A., Humphrey C. L., Swamy-Mruthinti S., Buccafusco J. J. The induction of surface beta-amyloid binding proteins and enhanced cytotoxicity in cultured PC-12 and IMR-32 cells by advanced glycation end products. Neuroscience. 2006;142:463–473. doi: 10.1016/j.neuroscience.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Mruthinti S., Schade R. F., Harrell D. U., Gulati N. K., Swamy-Mruthinti S., Lee G. P., Buccafusco J. J. Autoimmunity in Alzheimer’s disease as evidenced by plasma immunoreactivity against RAGE and Abeta42: complication of diabetes. Curr Alzheimer Res. 2006;3:229–235. doi: 10.2174/156720506777632899. [DOI] [PubMed] [Google Scholar]
- Mruthinti S., Hill W. D., Swamy-Mruthinti S., Buccafusco J. J. Relationship between the induction of RAGE cell-surface antigen and the expression of amyloid binding sites. J Mol Neurosci. 2003;20:223–232. doi: 10.1385/JMN:20:3:223. [DOI] [PubMed] [Google Scholar]
- Mruthinti S., Capito N., Sood A., Buccafusc J. J. Cytotoxicity of Aβ1-42, RAGE23–54, and an Aβ-RAGE complex in PC-12 cells. Curr Alzheimer Res. 2007;4:581–586. doi: 10.2174/156720507783018325. [DOI] [PubMed] [Google Scholar]
- Mruthinti S., Buccafusco J. J., Hill W. D., Waller J. L., Jackson T. W., Zamrini E. Y., Schade R. F. Autoimmunity in Alzheimer’s disease: increased levels of circulating IgGs binding Aβ and RAGE peptides. Neurobiol Aging. 2004;25:1023–1032. doi: 10.1016/j.neurobiolaging.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Giri R., Shen Y., Stins M., Du S. Yan, Schmidt A. M., Stern D., Kim K. S., Zlokovic B., Kalra V. K. β-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol Cell Physiol. 2000;279:C1772–C1781. doi: 10.1152/ajpcell.2000.279.6.C1772. [DOI] [PubMed] [Google Scholar]
- Giri R., Selvaraj S., Miller C. A., Hofman F., Yan S. D., Stern D., Zlokovic B. V., Kalra V. K. Effect of endothelial cell polarity on β-amyloid-induced migration of monocytes across normal and AD endothelium. Am J Physiol Cell Physiol. 2002;283:C895–C904. doi: 10.1152/ajpcell.00293.2001. [DOI] [PubMed] [Google Scholar]
- Hadding A., Kaltschmidt B., Kaltschmidt C. Overexpression of receptor of advanced glycation end products hypersensitizes cells for amyloid β peptide-induced cell death. Biochim Biophys Acta. 2004;1691:67–72. doi: 10.1016/j.bbamcr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Sturchler E., Galichet A., Weibel M., Leclerc E., Heizmann C. W. Site-specific blockade of RAGE-Vd prevents amyloid-β oligomer neurotoxicity. J Neurosci. 2008;28:5149–5158. doi: 10.1523/JNEUROSCI.4878-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong L. L., Yan S. F., Wendt T., Hans D., Pachydaki S., Bucciarelli L. G., Adebayo A., Qu W., Lu Y., Kostov K., Lalla E., Yan S. D., Gooch C., Szabolcs M., Trojaborg W., Hays A. P., Schmidt A. M. RAGE modulates peripheral nerve regeneration via recruitment of both inflammatory and axonal outgrowth pathways. FASEB J. 2004;18:1818–1825. doi: 10.1096/fj.04-1900com. [DOI] [PubMed] [Google Scholar]
- Floden A. M., Combs C. K. Microglia repetitively isolated from in vitro mixed glial cultures retain their initial phenotype. J Neurosci Methods. 2007;164:218–224. doi: 10.1016/j.jneumeth.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustbader J. W., Cirilli M., Lin C., Xu H. W., Takuma K., Wang N., Caspersen C., Chen X., Pollak S., Chaney M., Trinchese F., Liu S., Gunn-Moore F., Lue L. F., Walker D. G., Kuppusamy P., Zewier Z. L., Arancio O., Stern D., Yan S. S., Wu H. ABAD directly links Aβ to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Rong L. L., Gooch C., Szabolcs M., Herold K. C., Lalla E., Hays A. P., Yan S. F., Yan S. S., Schmidt A. M. RAGE: a journey from the complications of diabetes to disorders of the nervous system—striking a fine balance between injury and repair. Restor Neurol Neurosci. 2005;23:355–365. [PubMed] [Google Scholar]
- Takuma K., Yao J., Huang J., Xu H., Chen X., Luddy J., Trillat A. C., Stern D. M., Arancio O., Yan S. S. ABAD enhances Aβ-induced cell stress via mitochondrial dysfunction. FASEB J. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- Origlia N., Capsoni S., Cattaneo A., Fang F., Arancio O., Yan S. D., Domenici L. Aβ-dependent inhibition of LTP in different intracortical circuits of the visual cortex: the role of RAGE. J Alzheimers Dis. 2009;17:59–68. doi: 10.3233/JAD-2009-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H., Guo L., Zhang W., Rydzewska M., Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model [E-pub ahead of print] Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.003. doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H., Guo L., Fang F., Chen D., Sosunov A. A., McKhann G. M., Yan Y., Wang C., Zhang H., Molkentin J. D., Gunn-Moore F. J., Vonsattel J. P., Arancio O., Chen J. X., Yan S. D. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue L. F., Yan S. D., Stern D. M., Walker D. G. Preventing activation of receptor for advanced glycation endproducts in Alzheimer’s disease. Curr Drug Targets CNS Neurol Disord. 2005;4:249–266. doi: 10.2174/1568007054038210. [DOI] [PubMed] [Google Scholar]
- Yan S. D., Stern D. M. Mitochondrial dysfunction and Alzheimer’s disease: role of amyloid-beta peptide alcohol dehydrogenase (ABAD) Int J Exp Pathol. 2005;86:161–171. doi: 10.1111/j.0959-9673.2005.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieven G. L. The biology of p38 kinase: a central role in inflammation. Curr Topics Med Chem. 2005;5:921–928. doi: 10.2174/1568026054985902. [DOI] [PubMed] [Google Scholar]
- Frautschy S. A., Yang F., Irrizarry M., Hyman B., Saido T. C., Hsiao K., Cole G. M. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T., Sastre M., Dumitrescu-Ozimek L., Dewachter I., Walter J., Klockgether T., Van Leuven F. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J Neuroinflammation. 2005;2:22. doi: 10.1186/1742-2094-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehmas A. K., Kawas C. H., Stewart W. F., Troncoso J. C. Immune reactive cells in senile plaques and cognitive decline in Alzheimer’s disease. Neurobiol Aging. 2003;24:321–331. doi: 10.1016/s0197-4580(02)00090-8. [DOI] [PubMed] [Google Scholar]
- Stalder M., Phinney A., Probst A., Sommer B., Staufenbiel M., Jucker M. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am J Pathol. 1999;154:1673–1684. doi: 10.1016/S0002-9440(10)65423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D., Gordon M. N., Tan J., Wilcock D., Rojiani A. M. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer therapeutics. J Neuropathol Exp Neurol. 2005;64:743–753. doi: 10.1097/01.jnen.0000178444.33972.e0. [DOI] [PubMed] [Google Scholar]
- Ralay Ranaivo H., Craft J. M., Hu W., Guo L., Wing L. K., Van Eldik L. J., Watterson D. M. Glia as a therapeutic target: selective suppression of human amyloid-beta-induced upregulation of brain proinflammatory cytokine production attenuates neurodegeneration. J Neurosci. 2006;26:662–670. doi: 10.1523/JNEUROSCI.4652-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J., Toft M., Hickman S. E., Means T. K., Terada K., Geula C., Luster A. D. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Tesseur I., Zou K., Esposito L., Bard F., Berber E., Can J. V., Lin A. H., Crews L., Tremblay P., Mathews P., Mucke L., Masliah E., Wyss-Coray T. Deficiency in neuronal TGF-β signaling promotes neurodegeneration and Alzheimer’s pathology. J Clin Invest. 2006;116:3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. G., Lue L. F. Investigations with cultured human microglia on pathogenic mechanisms of Alzheimer’s disease and other neurodegenerative diseases. J Neurosci Res. 2005;81:412–425. doi: 10.1002/jnr.20484. [DOI] [PubMed] [Google Scholar]
- Craft J. M., Watterson D. M., Hirsch E., Van Eldik L. J. Interleukin 1 receptor antagonist knockout mice show enhanced microglial activation and neuronal damage induced by intracerebroventricular infusion of human beta-amyloid. J Neuroinflammation. 2005;2:15. doi: 10.1186/1742-2094-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Munoz L., Ranaivo H. R., Roy S. M., Hu W., Craft J. M., McNamara L. K., Chico L. W., Van Eldik L. J., Watterson D. M. A novel p38 alpha MAPK inhibitor suppresses brain proinflammatory cytokine up-regulation and attenuates synaptic dysfunction and behavioral deficits in an Alzheimer’s disease mouse model. J Neuroinflammation. 2007;4:21. doi: 10.1186/1742-2094-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K., Launer L. J., Ott A., Hoes A. W., Breteler M. M., Hofman A. Do nonsteroidal anti-inflammatory drugs decrease the risk for Alzheimer’s disease? The Rotterdam Study. Neurology. 1995;45:1441–1445. doi: 10.1212/wnl.45.8.1441. [DOI] [PubMed] [Google Scholar]
- Lue L. F., Walker D. G. Modeling Alzheimer’s disease immune therapy mechanisms: interactions of human postmortem microglia with antibody-opsonized amyloid beta peptide. J Neurosci Res. 2002;70:599–610. doi: 10.1002/jnr.10422. [DOI] [PubMed] [Google Scholar]
- Farris W., Mansourian S., Chang Y., Lindsley L., Eckman E. A., Frosch M. P., Eckman C. B., Tanzi R. E., Selkoe D. J., Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Kiyota T., Horiba M., Buescher J. L., Walsh S. M., Gendelman H. E., Ikezu T. Interferon-gamma and tumor necrosis factor-alpha regulate amyloid-beta plaque deposition and beta-secretase expression in Swedish mutant APP transgenic mice. Am J Pathol. 2007;170:680–692. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. J., Son S. M., Jin S. M., Hong H. S., Shin D. H., Kim S. J., Huh K., Mook-Jung I. RAGE regulates BACE1 and Aβ generation via NFAT1 activation in Alzheimer’s disease animal model. FASEB J. 2009;23:2639–2349. doi: 10.1096/fj.08-126383. [DOI] [PubMed] [Google Scholar]
- Blalock E. M., Chen K. C., Stromberg A. J., Norris C. M., Kadish I., Kraner S. D., Porter N. M., Landfield P. W. Harnessing the power of gene microarrays for the study of brain aging and Alzheimer’s disease: statistical reliability and functional correlation. Ageing Res Rev. 2005;4:481–512. doi: 10.1016/j.arr.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Javadov S. A., Lim K. H., Kerr P. M., Suleiman M. S., Angelini G. D., Halestrap A. P. Protection of hearts from reperfusion injury by propofol is associated with inhibition of the mitochondrial permeability transition. Cardiovasc Res. 2000;45:360–369. doi: 10.1016/s0008-6363(99)00365-x. [DOI] [PubMed] [Google Scholar]
- Haq S., Choukroun G., Lim H., Tymitz K. M., del Monte F., Gwathmey J., Grazette L., Michael A., Hajjar R., Force T., Molkentin J. D. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. doi: 10.1161/01.cir.103.5.670. [DOI] [PubMed] [Google Scholar]
- Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G. M., Cooper N. R., Eikelenboom P., Emmerling M., Fiebich B. L., Finch C. E., Frautschy S., Griffin W. S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I. R., McGeer P. L. M. K., O'Banion, Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van F. L., Muiswinkel Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski E., Andreasen N., Tarkowski A., Blennow K. Intrathecal inflammation precedes development of Alzheimer’s disease. J Neurol Neurosurg Psych. 2003;74:1200–1205. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudal S., Krzywkowski P., Paquette J., Morissette C., Lacombe D., Tremblay P., Gervais F. Inflammation occurs early during the Aβ deposition process in TgCRND8 mice. Neurobiol Aging. 2004;25:861–871. doi: 10.1016/j.neurobiolaging.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Li Y., Liu L., Barger S. W., Griffin W. S. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23:1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y. F., Wang B. J., Cheng H. T., Kuo L. H., Wolfe M. S. Tumor necrosis factor-α, interleukin-1β, and interferon-γ stimulate γ-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J Biol Chem. 2004;279:49523–49532. doi: 10.1074/jbc.M402034200. [DOI] [PubMed] [Google Scholar]
- Griffin W. S., Liu L., Li Y., Mrak R. E., Barger S. W. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J Neuroinflammation. 2006;3:5. doi: 10.1186/1742-2094-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. V., Bailey C. D. The p38 MAP kinase signaling pathway in Alzheimer’s disease. Exp Neurol. 2003;183:263–268. doi: 10.1016/s0014-4886(03)00268-1. [DOI] [PubMed] [Google Scholar]
- Giovannini M. G., Scali C., Prosperi C., Bellucci A., Vannucchi M. G., Rosi S., Pepeu G., Casamenti F. Beta-amyloid-induced inflammation and cholinergic hypofunction in the rat brain in vivo: involvement of the p38MAPK pathway. Neurobiol Dis. 2002;11:257–274. doi: 10.1006/nbdi.2002.0538. [DOI] [PubMed] [Google Scholar]
- Sun A., Liu M., Nguyen X. V., Bing G. P38 MAP kinase is activated at early stages in Alzheimer’s disease brain. Exp Neurol. 2003;183:394–405. doi: 10.1016/s0014-4886(03)00180-8. [DOI] [PubMed] [Google Scholar]
- Pei J. J., Braak E., Braak H., Grundke-Iqbal I., Iqbal K., Winblad B., Cowburn R. F. Localization of active forms of C-jun kinase (JNK) and p38 kinase in Alzheimer’s disease brains at different stages of neurofibrillary degeneration. J Alzheimers Dis. 2001;3:41–48. doi: 10.3233/jad-2001-3107. [DOI] [PubMed] [Google Scholar]
- Swatton J. E., Sellers L. A., Faull R. L., Holland A., Iritani S., Bahn S. Increased MAP kinase activity in Alzheimer’s and Down syndrome but not in schizophrenia human brain. Eur J Neurosci. 2004;19:2711–2719. doi: 10.1111/j.0953-816X.2004.03365.x. [DOI] [PubMed] [Google Scholar]
- Zhu X., Rottkamp C. A., Hartzler A., Sun Z., Takeda A., Boux H., Shimohama S., Perry G., Smith M. A. Activation of MKK6, an upstream activator of p38, in Alzheimer’s disease. J Neurochem. 2001;79:311–318. doi: 10.1046/j.1471-4159.2001.00597.x. [DOI] [PubMed] [Google Scholar]
- Savage M. J., Lin Y. G., Ciallella J. R., Flood D. G., Scott R. W. Activation of c-Jun N-terminal kinase and p38 in an Alzheimer’s disease model is associated with amyloid deposition. J Neurosci. 2002;22:3376–3385. doi: 10.1523/JNEUROSCI.22-09-03376.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I. Stress kinases involved in tau phosphorylation in Alzheimer’s disease, tauopathies and APP transgenic mice. Neurotox Res. 2004;6:469–475. doi: 10.1007/BF03033283. [DOI] [PubMed] [Google Scholar]
- Zhu X., Mei M., Lee H. G., Wang Y., Han J., Perry G., Smith M. A. P38 activation mediates amyloid-beta cytotoxicity. Neurochem Res. 2005;30:791–796. doi: 10.1007/s11064-005-6872-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.