Abstract
A major challenge of assisted reproduction technologies (ARTs) is to mimic the natural environment required to sustain oocyte and embryo survival. Herein, we show that the ceramide-metabolizing enzyme, acid ceramidase (AC), is expressed in human cumulus cells and follicular fluid, essential components of this environment, and that the levels of this enzyme are positively correlated with the quality of human embryos formed in vitro. These observations led us to develop a new approach for oocyte and embryo culture that markedly improved the outcome of in vitro fertilization (IVF). The addition of recombinant AC (rAC) to human and mouse oocyte culture medium maintained their healthy morphology in vitro. Following fertilization, the number of mouse embryos formed in the presence of rAC also was improved (from ∼40 to 88%), leading to ∼5-fold more healthy births. To confirm these observations, immature bovine oocytes were matured in vitro and subjected to IVF in the presence of rAC. Significantly more high-grade blastocysts were formed, and the number of morphologically intact, hatched embryos was increased from ∼24 to 70%. Overall, these data identify AC as an important component of the in vivo oocyte and embryo environment, and provide a novel technology for enhancing the outcome of assisted fertilization. Eliyahu, E., Shtraizent, N., Martinuzzi, K., Barritt, J., He, X., Wei, H., Chaubal, S., Copperman, A. B., Schuchman, E. H. Acid ceramidase improves the quality of oocytes and embryos and the outcome of in vitro fertilization.
Keywords: reproduction, apoptosis, ceramide
The normal development of oocytes and embryos in vivo is dependent on factors supplied by their local environment (e.g., cumulus cells, follicular fluid, blastocoel fluid, ovidutal secretions, and uterine secretions). An important goal of current research is, therefore, to identify these factors in order to improve the efficiency of assisted reproduction technologies (ARTs) and/or to predict their outcome (1, 2). Although significant advances have been made, mature [second meiotic metaphase (MII)] oocytes and preimplantation embryos can only be maintained in culture for short periods of time, and rapidly undergo programmed cell death (apoptosis) (3). Techniques to mature oocytes in vitro also are poor, necessitating controlled ovarian hyperstimulation with hormones to superovulate women so that an adequate cohort of MII oocytes can be obtained for in vitro fertilization (IVF). Because of inherent inefficiencies of human reproduction, it is not uncommon for infertile women to undergo multiple cycles of IVF in order to achieve reproductive success. To ensure success, multiple embryos also are routinely implanted. In addition to human IVF, the inability to efficiently mature and/or maintain oocytes and embryos in culture has important implications for agricultural and research IVF, animal cloning, and the preservation of endangered species (4, 5).
At birth, mammalian oocytes are arrested within ovarian follicles at the diplotene stage of the first meiotic prophase [i.e., the germinal vesicle (GV) stage]. The resumption of meiotic maturation, stimulated by a leutinizing hormone (LH) surge in sexually mature females, is manifested by germinal vesicle breakdown (GVBD), followed by chromatin condensation and microtubule reorganization. These events lead to the formation of the metaphase spindle, completion of the first meiotic division, and subsequent formation of the first polar body (PBI), after which oocytes enter the second meiotic arrest at MII (6). The completion of maturation requires synthesis and storage of RNAs and proteins, post-translational regulation of proteins, relocation of cytoplasmic organelles, and alterations in membrane transport systems, and requires mutual interactions between the oocyte and its surrounding somatic cells (follicular mural granulosa and cumulus oophorus) (7). During maturation, each oocyte is enclosed within a follicle, and follicular fluid is required to support and nourish the oocyte through maturation and ovulation. Failure to complete oocyte maturation can block development at fertilization, embryonic genome activation, blastocyst formation, or even implantation.
During the past decade, sphingolipids have emerged as essential second messengers in a variety of signal transduction pathways. Much of this research has focused on ceramide, an important inducer of programmed cell death, i.e., apoptosis (8,9,10). Ceramidases hydrolyze ceramide into sphingosine, which is converted to sphingosine-1-phosphate (S1P), another important signaling lipid that counteracts the effects of ceramide and promotes cell survival (11, 12). Notably, the only source of sphingosine in cells is via ceramide hydrolysis; thus, ceramidases act as “rheostats” that regulate the levels of ceramide and S1P in cells, and, as such, participate in the complex and delicate balance between cell death and survival.
Despite a large and rapidly growing literature on the role of sphingolipids in cell signaling, the specific involvement of these lipids in oocyte maturation and fertilization has not been examined in detail. Our recent study showed that in the absence of one ceramidase activity [i.e., acid ceramidase (AC)], mouse embryos could not survive beyond the 2-cell stage due to apoptotic death (13). These studies also revealed that AC was expressed at high levels in mature MII mouse oocytes and that during in vitro culture, the expression levels declined as apoptosis occurred. These apoptotic changes could be prevented by S1P. In addition, Tilly and colleagues have shown that in aged mice, ceramide is translocated from cumulus cells into the adjacent oocyte and induces apoptotic cell death (3).
Cell death in oocytes is exclusively attributed to apoptosis, defined by morphological criteria such as DNA double-stranded breaks and cytoplasm fragmentation, as well as the expression of caspase-2 and other apoptosis-related gene products. In vivo, two apoptotic cycles may occur in oocytes. The first occurs during menstruation if a mature MII oocyte fails to be fertilized, and the second is during menopause, when the cohort of immature oocytes remaining in the ovaries undergoes cell death. Thus, apoptosis is a normal component of the oocyte life cycle in vivo. Apoptotic death is further accelerated when oocytes are maintained in culture (13), probably due to the stress of the in vitro culture conditions and the lack of essential survival factors in the culture medium. Thus, many healthy oocytes and embryos are lost during the in vitro culture procedure, creating a major challenge for ARTs.
Herein, we show for the first time that AC is an essential component of the in vivo oocyte and embryo environment, and that its expression levels can be correlated with the quality of human embryos produced in vitro. In addition, we present a novel approach for improving the quality of cultured oocytes and embryos by supplementing medium with recombinant AC (rAC). Results are provided from three species (human, murine, and bovine). Notably, in mice, we also show that inclusion of rAC during oocyte/embryo culture increases the number of healthy pups obtained.
MATERIALS AND METHODS
Mouse oocyte and sperm collection
All experiments involving animals were approved by and performed in strict accordance with the guidelines of the appropriate institutional animal care and use committees. Seven- to 8-wk-old 129-SVIMJ and C57-Black/6 female mice (Jackson Laboratory, Bar Harbor, ME, USA) were superovulated with 10 IU of pregnant mare serum gonadotropin (PMSG; Syncro-part, Sanofi, France), followed by 10 IU of human chorionic gonadotropin (hCG; Sigma, St. Louis, MO, USA) 48 h later. Mature and aged MII oocytes were collected from the oviducal ampullae at 16 or 46 h after injection of hCG, respectively. Cumulus cells were removed by a brief exposure to 400 IU/ml of highly purified hyaluronidase (H-3631; Sigma) in M2 medium (Sigma) (14). Epididymal sperm from 10-wk-old mice were used for IVF of oocytes from the same strain.
Mouse fertilization and embryo culture
Microdrops of fertile sperm in Vitrofert solution (Vitrolife, Goteborg, Sweden) were prepared, and ∼10 oocytes were placed into each sperm microdrop. The fertilization process was performed for 6 h at 37°C in a humidified atmosphere of 5% CO2 and 95% air. After IVF, zygotes were washed 3 times with potassium simplex optimized medium (KSOM; Chemicon, Billerica, MA, USA) and then transferred into fresh KSOM and cultured for an additional 20–48 h at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Cleavage of the zygotes was observed and recorded throughout the in vitro culture.
Generation of mouse pups after rAC treatment
Collection of mouse embryos at the zygote stage was performed 20 h after hCG injection. Embryos were surgically retrieved and transferred into KSOM for in vitro culture with and without rAC for 24–48 h at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Two- to 4-cell embryos were then transferred into the oviduct of pseudopregnant female recipients; pregnancies were carried to full term, and the number of pups born and their development for ≥1 mo were recorded.
Bovine oocyte collection and maturation
Ovaries were collected from a local abattoir and transported to the lab within 2 h at a temperature of 25 to 30°C. Immature oocytes were retrieved from follicles with diameters from 2 to 8 mm using a syringe connected to an 18-gauge needle. Oocytes with >3 layers of granulous cells were selected and matured for 22 h in TCM 199 (Sigma M-4530) supplemented with 10% fetal calf serum (FCS; HyClone HI & GI, Waltham, MA, USA), 0.02 IU/ml of bovine follicle stimulating hormone (bFSH; cat. no. 715; Sioux Biochemical, Sioux Center, IA, USA), 0.02 IU/ml of bovine luteinizing hormone (bLH; cat. no. 725; Sioux Biochemical), and 1% of penicillin/streptomycin (15140-122; Life Technologies, Carlsbad, CA, USA), at 38.8°C with 5% CO2 and maximum humidity in air.
Bovine sperm preparation
Frozen semen from an Angus bull was used in this study. Semen straws were thawed in a water bath at 35°C. The semen was then loaded on the top of 2-layer Isolate solutions (99264; Irvine Scientific, Irvine, CA, USA) in a 15-ml centrifuge tube, and centrifuged at 650 g for 10 min. The pellet was washed in 4 ml of fertilization medium [Tyrode’s solution, lactate, and pyruvate (IVF-TALP) supplemented with 0.5% (w/v) bovine serum albumin (BSA) and 10 μg/ml of heparin] by centrifugation at 450 g for 5 min, and then moved to a centrifuge tube and used for fertilization within 5 min.
Bovine fertilization and embryo culture
After maturation, a group of 100 oocytes was inseminated with sperm at a final concentration of 1.5 × 106 sperm/ml in 500 μl of fertilization medium at 38.8°C in 5% CO2 in air at maximum humidity for 20 h. After fertilization, the cumulus cells were stripped from the zygotes by high-speed vortexing for 2 min. The zygotes were then transferred into synthetic oviductal fluid (SOF) medium (100 zygotes/500 μl) supplemented with 0.5% (w/v) BSA for 7 d at 38.8°C in 5% CO2 and 5% O2 in air at maximum humidity for embryo development culture.
Bovine embryo evaluation
In the morning of d 7 of embryo culture, embryos were evaluated according to the criteria of the International Embryo Transfer Society (15). After the evaluation, embryos were kept in the same culture dish and continuously cultured to d 10. The number of hatched embryos was recorded.
Collection of human materials
All human samples were obtained from patients who gave Institutional Review Board-approved consent to use discarded materials for research. hCG, either rec-hCG (250 μg s.c.; Serono, Rockland, MA, USA) or u-hCG (10,000 IU i.m.; Serono), was administered as a single dose. Cumulus cell–oocyte complexes were isolated 36 h after hCG injection. Cumulus cells were striped mechanically and by incubation in hyaluronidase (80 USP U/ml). Denuded oocytes were transferred into standard culture medium of 5% human serum albumin in Quinn’s cleavage medium (Sage Biopharma, Pasadena, CA, USA), and the stage of maturation was assessed. Oocytes that did not reach the MII stage at the time of evaluation, follicular fluid, and stripped cumulus cells were classified as discarded materials (at the patient’s request/consent) and used for research. Mature MII oocytes were inseminated and cultured for 16–18 h before being assessed for fertilization. All normally fertilized zygotes were then transferred to growth medium (Quinn’s Advantage cleavage medium supplemented with 10% synthetic serum supplement; Irvine Scientific).
Embryos were analyzed for morphological criteria at d 3 and 5, and graded using standard assessment methods. Briefly, embryos that formed blastocysts were graded for degree of expansion of the blastocoel cavity, quality of the inner cell mass, and trophectoderm score by using a modified grading system. Blastocysts were given a numerical score from 1 to 6 on the basis of their degree of expansion and hatching status as follows: 1, an early blastocyst with a blastocoel that is less than half of the volume of the embryo; 2, a blastocyst with a blastocoel that is half of or greater than half of the volume of the embryo; 3, a blastocyst with a blastocoel completely filling the embryo; 4, an expanded blastocyst with a blastocoel volume larger than that of the early embryo, with a thinning zona pellucida (ZP); 5, a hatching blastocyst with the trophectoderm starting to herniate through the ZP; or 6, a hatched blastocyst, in which the blastocyst has completely escaped from the ZP. For blastocysts with expansions graded as 3–6, the development of the inner cell mass was assessed as follows: A, tightly packed, many cells; B, loosely grouped, several cells; C, few cells slightly disorganized; or D, very few disorganized and uneven cells. The trophectoderm of the blastocysts with expansion grades of 3–6 was assessed as follows: A, many cells forming a cohesive epithelium; B, few cells forming a loose epithelium; C, few large cells; or D, very few cells of an uneven nature. An appropriate number of suitable grade blastocysts was returned to the patients, and the remaining high- and low-grade embryos were classified as discarded materials (at the patient’s request/consent), and used for research.
Preparation of rAC
To prepare conditioned medium containing rAC, Chinese hamster ovary (CHO) cells overexpressing and secreting human AC (CHO6) (16) were grown to confluence at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Prior to rAC collection, the standard CHO medium (DMEM, Life Technologies), supplemented with 10% FBS (Cellgro, Manassas, VA, USA) and 0.1% Pen Strep (Life Technologies) was changed to IVF medium, and the cells were grown for an additional 5 d. The conditioned medium was then collected, concentrated by pressure filtration (molecular weight cutoff 30 kDa; Amicon, Billerica, MA, USA), and the amount of rAC was quantified by enzyme activity assay and by Western blot analysis. For some experiments, the concentrated, conditioned medium as used directly; for others, it was partially purified by Concanavalin A chromatography using an AKTA fast protein liquid chromatography (FPLC) system (Amersham Biosciences, Piscataway, NJ, USA) (16). The concentrated medium was slowly applied to a 10-ml Con A column that had been equilibrated with 10× bed volume of Con A wash buffer (20 mM Tris-HCl, 500 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, and 1 mM MnCl2, pH 7.2), attached to the AKTA FPLC. The washing, preelution, and elution steps were carried out with Con A wash buffer, preelution buffer (Con A wash buffer containing 10 mM methyl-α-d-glucopyranoside), and elution buffer (Con A wash buffer containing 1 M methyl-α-d-glucopyranoside), respectively, and monitored using a UV detector. Fractions from the elution step containing the highest AC activity were pooled, concentrated to <10 ml using a 30-kDa ultrafiltration membrane, and then diluted and reconcentrated 3 times in the presence of 20 mM Tris-HCl, pH 7.2, at 4°C (for buffer exchange). A final dilution and concentration were done using mnimal essential medium Eagle (MEME; Sigma), and the amount of rAC was quantified by enzyme activity assay and Western blot analysis.
AC expression analysis
For immunohistochemistry, oocytes and embryos were fixed in 3% paraformaldehyde (Thermo Scientific, Pittsburgh, PA, USA). ZPs were removed postfixation by Pronase (Sigma), and the ZP-free oocytes and embryos were permeabilized by Nonidet P-40 (Fluka, Pittsburgh, PA, USA), followed by incubation with different primary and secondary antibodies or DNA labeling by Hoechst 33342 (Sigma) or TUNEL assay (Promega, Madison, WI, USA). The fluorescent images were visualized and photographed with a Zeiss confocal laser-scanning microscope (Carl Zeiss, Oberkochen, Germany).
For Western blotting analyses, cumulus cells were subjected to lysis in buffer containing 50 mM Tris-HCL (Cellgro), 150 mM NaCl (Sigma), 2 mM EDTA (Sigma), 1% Nonidet P-40, 1 mM Vanadate (Sigma), 5 mM NaF (Sigma), and 10 μg/ml aprotinine (Sigma), pH 7.4. Protein extracts from cumulus cells and samples of follicular fluid were separated by SDS-PAGE using 12% precast Nupage Bis/Tris gels (Invitrogen, Carlsbad, CA, USA) under reducing conditions and MES running buffer (Invitrogen), and transferred onto a nitrocellulose membrane (Bio-Rad) using a semidry transfer apparatus and Nupage-MOPS transfer buffer (Invitrogen). For immunoblot analysis, membranes were blocked with Tris-buffered saline/Tween (Sigma) containing 5% dry milk, and then were incubated with specific monoclonal and polyclonal antibodies against AC (see below). Bound antibodies were recognized by secondary antibodies conjugated to horseradish peroxidase. Detection was performed using an enhanced chemiluminescence (ECL) detection reagent (Promega). Approximate molecular masses were determined by comparison with the migration of prestained protein standards (Bio-Rad, Hercules, CA, USA).
For AC activity assays, cell extracts and follicular fluid were incubated for 22 h at 37°C with 0.1 mM BODIPY-conjugated C12-ceramide (gift of Dr. Arie Dagan, Hebrew University, Jerusalem, Israel) in 0.1 M citrate/phosphate buffer (pH 4.5), 150 mM NaCl, 0.05% BSA, and 0.1% Igepal CA-630 (Sigma). After the reactions were complete, 5 μl of the assay mixtures were removed and added into 95 μl of ethanol, mixed, and then centrifuged for 5 min for 10,000 g. The supernatants were then transferred to a Waters glass sampling vial (Waters Corp., Milford, MA, USA), and 5 μl (2.5% of the original reaction mixture) was removed by a WIPS 712 (Waters) autosampler onto an HPLC equipped with a reverse-phase column (BetaBasic-18; 4.6×30 mm; Keystone Scientific, Bellefonte, PA, USA), and eluted isocratically with methanol/water (95:5 v/v) at a flow rate of 1 ml/min. Fluorescence was quantified using a Waters 474 fluorescence detector set to excitation and emission wavelengths of 505 and 540 nm, respectively. The undigested substrate and product (i.e., BODIPY-conjugated C12-ceramide and fatty acid, respectively) peaks were identified by comparing their retention times with standards, and the amount of product was calculated using a regression equation that was established from a standard curve using BODIPY-conjugated C12 fatty acid.
Antibodies
The following antibodies were used: anti-human AC goat polyclonal immunoglobulin G (IgG; T-20, sc-28486; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-human AC mouse monoclonal immunoglobulin M (IgM; 612302; BD Bioscience, San Jose, CA, USA), anti-human Lamp1 rat monoclonal IgG2a (sc-19992; Santa Cruz Biotechnology), and anti β-tubulin mouse monoclonal IgG (sc-5274; Santa Cruz Biotechnology).
Data presentation and statistical analysis
All experiments were independently replicated ≥3 times. The combined data from the replicate experiments were subjected to t test or Pearson’s χ2 test analyses, and results were considered statistically significant at P < 0.05. Graphs represent the means ± se of combined data from the replicate experiments. Representative photomicrographs are presented for the oocyte and embryo morphology, TUNEL labeling, and immunohistochemistry.
RESULTS
AC is expressed in human oocytes at all maturation stages
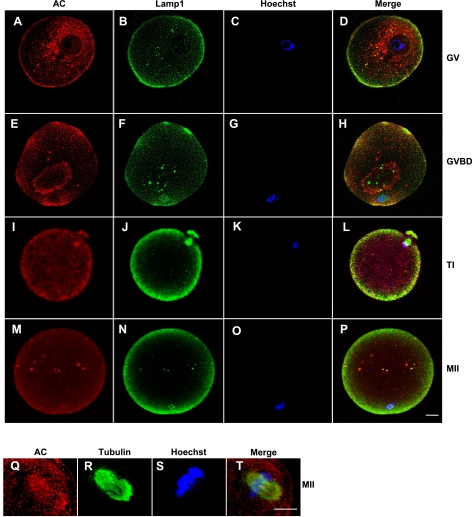
Our previous work has shown that MII mouse oocytes express AC at high levels and that the expression levels decline with aging (13). On the basis of these findings, we hypothesized that AC is required throughout all stages of oocyte maturation in order to maintain ceramide in a low range, thereby slowing or preventing apoptosis. To examine this hypothesis, coimmunohistochemistry of human immature GV (Fig. 1A–D), GV breakdown (GVBD; Fig. 1E–H), first telophase (TI; Fig. 1I–L), and mature MII (Fig. 1M–P) oocytes was performed by using specific antibodies against AC, a late endosome/lysosome marker (Lamp1), and the spindle marker (tubulin); and by DNA labeling using Hoechst stain. The fluorescence distribution of the antibodies and DNA labeling was visualized at the equator of the oocyte (Fig. 1A–P) or at the metaphase plate (Fig. 1Q–T).
Figure 1.
AC is highly expressed in human oocytes at different maturation stages. GV (A–D), GVBD (E–H), TI (I–L), and MII (M–T) oocytes. Oocytes were labeled using specific antibodies for AC (red; A, E, I, M, Q), Lamp1 (green; B, F, J, N), and tubulin (green; R), and for DNA using Hoechst stain (C, G, K, O, S). Localization of the primary antibodies was visualized using a fluorescent second antibody, Cy-3/2, and laser-scanning confocal microscopy. Merged images indicate colocalization of labeled proteins and/or DNA (D, H, L, P, T). As a control, oocytes were labeled with secondary antibodies only (data not shown). Scale bars = 10 μm. Images are representative of 3 independent experiments.
These analyses revealed that AC is expressed in the cortex and membrane at all stages of human oocyte maturation, and that some, but not all, of the AC was colocalized with Lamp1-positive vesicles at all maturation stages. In addition, colocalization of AC with the GV membrane was observed at the GVBD stage (Fig. 1E, F, H), and with the DNA at the TI stage (Fig. 1I, K, L). Finally, we observed colocalization of AC with the meiotic spindle at the MII stage (Fig. 1Q–T). These findings support our hypothesis that AC is important for oocyte survival throughout maturation and indicate that the enzyme may be involved in various cellular functions aside from its housekeeping function in lysosomes.
AC is present and active in human follicular fluid and cumulus cells
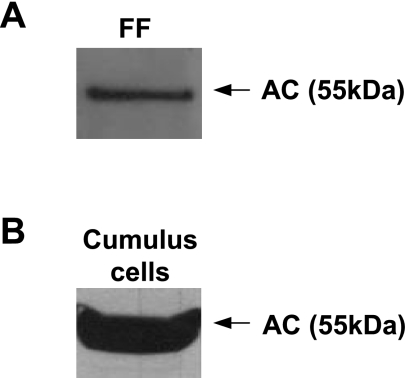
To determine whether AC was a component of the in vivo oocyte support environment, we determined the expression and activity of the enzyme in human follicular fluid and cumulus cell extracts. Forty-five samples of follicular fluid and 34 samples of cumulus cells were collected and analyzed by AC activity assays (Table 1), and representative samples also were analyzed by Western blot analysis using a specific antibody against AC (Fig. 2). The results clearly showed that AC is expressed and active in follicular fluid and cumulus cells, both of which are required to nourish oocytes in vivo. Most of the enzyme was in the 55-kDa precursor form, which can undergo autocatalytic cleavage and activation as a function of pH and other factors (17). These data further indicated that AC is required for oocyte survival and maturation, and suggested that supplementing culture medium with the recombinant enzyme could be beneficial for oocyte and embryo handling in vitro (see below).
TABLE 1.
AC is active in human follicular fluid and cumulus cells
| Sample | AC activity (fmol/μg) |
|---|---|
| Follicular fluid | 277.7 ± 30* |
| Cumulus cells | 285 ± 57* |
| Substrate blank | 10 ± 1.1 |
AC activity was assessed using a fluorescent substrate and HPLC separation (see Materials and Methods). Activity data represent analysis of 45 samples of follicular fluid and 34 samples of cumulus cells, collected from women undergoing assisted fertilization. Results shown are the averages ± sd
P < 0.001 compared to reactions containing substrate alone (BODIPY-conjugated C12-ceramide).
Figure 2.
AC is present in human follicular fluid (FF) and cumulus cells. AC expression was determined by Western blotting in 10 μl of human FF (diluted to 1 μg/μl total protein concentration) (A), and 24 μg of total protein from cumulus cell extracts (B). Western blot analysis was performed using mouse anti-human AC IgG, revealing the human AC precursor (at 55 kDa). Western blot images are representative of ≥3 independent experiments.
AC expression in human embryos corresponds with embryo quality
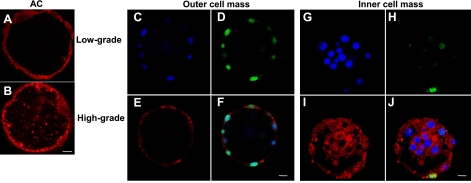
In the absence of AC activity, mouse embryos cannot survive beyond the 2-cell stage due to apoptotic death (13). On the basis of this observation, we hypothesized that AC must also be expressed in healthy human embryos at the earliest developmental stages. To examine this aspect of our hypothesis, we preformed immunohistochemistry of low- and high-grade human blastocysts using a specific antibody against AC (Fig. 3). The enzyme was found in both the inner and outer cell masses of human embryos, as well as in the blastocoel containing the embryonic fluid. Notably, AC levels were correlated with embryo quality, as high-grade embryos had higher expression levels in the blastocele fluid than low-grade embryos (compare Fig. 3A, B). Moreover, in low-grade embryos, TUNEL staining, indicating apoptotic cells, was most evident in the outer cell mass, where AC expression levels were lowest (Fig. 3C–F). In contrast, the inner cell mass had higher levels of AC expression and less apoptosis (Fig. 3G–J).
Figure 3.
AC expression in human embryos. A, B) Low AC expression in the blastocele fluid and outer cell mass of a representative low-grade human embryo (A) compared with high AC expression in a high-grade embryo (B), detected using goat IgG against human AC. C–J), Lower AC expression and higher apoptosis (TUNEL) in the outer cell mass of a low-grade embryo compared to the inner cell mass of the same embryo, which had higher AC expression and less apoptosis. AC was detected using goat IgG against human AC (E, I) and TUNEL assay (D, H); DNA labeling by Hoechst (C, G); merged images indicate colocalization (F, J). Scale bars = 10 μm. Data are representative of 3 independent experiments.
AC improves the quality of oocytes cultured in vitro
On the basis of the fact that AC is required for mouse embryo survival (13), as well as our current results showing expression of AC in healthy human oocytes, embryos, and surrounding cells and fluids, we hypothesized that AC could have a protective effect during oocyte maturation and fertilization in vitro. We attempted to mimic the physiological levels of AC observed in the follicular fluid by supplementing rAC into the culture medium during in vitro oocyte and embryo handling. AC may be incorporated into cells through the mannose-6-phosphate receptor-mediated uptake pathway (18).
For the mouse studies, mature oocytes were retrieved 14 h after hCG injection, followed by in vitro culture for 24 h in the presence or absence of rAC-supplemented oocyte culture medium (M2). Human oocytes (immature or mature) were retrieved 36 h after hCG injection, followed by in vitro culture for 24 h in the presence or absence of rAC-supplemented cleavage medium. The apoptosis process was analyzed by DNA morphology using Hoechst labeling and TUNEL assay, and by assessment of cellular morphology by light imaging.
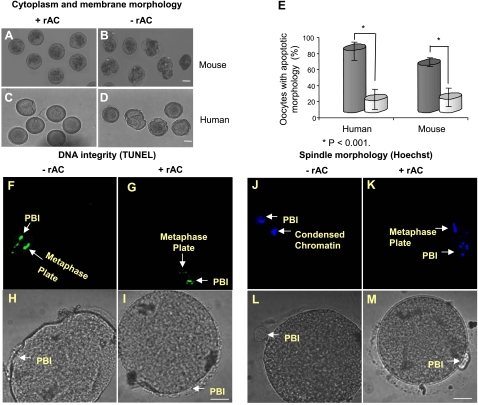
As shown in Fig. 4, rAC supplementation into oocyte culture medium effectively suppressed the appearance of structural changes characteristic of apoptotic cell death (3, 13, 19, 20). For example, most of the mouse and human oocytes cultured in the presence of rAC maintained intact membranes and cytoplasm (Fig. 4A, C), and the DNA remained nonfragmented and was aligned on the metaphase plate (Fig. 4G, K). In the absence of rAC, we observed cytoplasmic fragmentation and membrane blebbing, detected by light microscopy (Fig. 4B, D), as well as DNA nicking and condensation, detected by TUNEL and Hoechst staining, respectively (Fig. 4F, J). The number of apoptotic oocytes in each group was assessed by these characteristics and summarized (Fig. 4E). Overall, these results demonstrated that rAC had a positive effect on the quality of human and mouse oocytes during in vitro culture.
Figure 4.
rAC supplementation improves the quality of oocytes cultured in vitro. Mature MII mouse oocytes (A, B) or immature and mature human oocytes (C, D, F–M), retrieved following superovulation, were incubated in vitro for 24 h in the absence or presence of rAC. Oocyte quality and apoptosis were assessed on the basis of membrane, cytoplasm, and DNA morphology using light imaging, TUNEL assay, and Hoechst DNA labeling. Fluorescence was visualized using confocal microscopy. A–D) Representative images show the apoptotic appearance of mouse and human oocytes cultured in the absence of rAC (B, D) compared to those cultured with rAC (A, C). E) Percentages of apoptotic mouse and human oocytes cultured with and without rAC. Data represent analysis of 100 oocytes from each group (P<0.001 between groups with and without rAC). Black bars, without rAC; gray bars, with rAC. F–I) DNA fragmentation (apoptosis) assessed by TUNEL staining in a representative human oocyte incubated in the absence of rAC (F) compared to an oocyte cultured in the presence of rAC (G). PBI, first polar body (positive control for apoptosis). J–M) Condensed appearance of DNA in a human oocyte incubated in the absence of rAC (J), compared to an intact metaphase plate in an oocyte cultured in the presence of rAC (K). Scale bars = 10 μm. Images represent analysis of 3 different oocytes with and without rAC treatment.
AC enhances the outcome of murine and bovine IVF
To study the effect of rAC supplementation on the quality of embryos derived by IVF, MII mouse oocytes were collected and subjected to IVF in the absence or presence of rAC-supplemented fertilization medium (VG-MOPS-plus, Vitrolife). To mimic the conditions of human IVF, the mouse oocytes were maintained in culture for 6 h before they were exposed to sperm for 3 h (aged oocytes), followed by embryo culture for 2 d. Fertilization rates and the progression of embryos through various stages of early embryo development (2-cell, 4-cell) were visualized by light imaging (Fig. 5 and Table 2). In another set of experiments, mouse oocytes were fertilized in vivo; the zygotes (or unfertilized oocytes) were collected, and their development in vitro was followed during 24–48 h of culture in the presence or absence of rAC (Table 3).
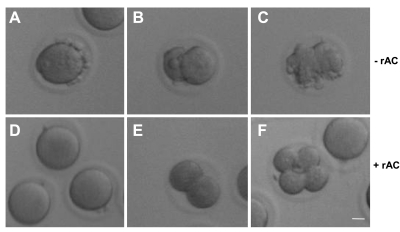
Figure 5.
rAC improves the quality and number of mouse embryos derived by IVF and the number of mouse pups born following reimplantation. MII mouse oocytes were retrieved following superovulation and incubated in vitro for 6 h (aged oocytes) in order to mimic the conditions of human IVF, followed by fertilization in the absence (A–C) or presence (D–F) of rAC-supplemented fertilization medium. Representative images were recorded, and percentages of oocytes developing to the 2- to 4-cell stage were determined. Images illustrate the apoptotic morphology of aged oocytes (A) and embryos (B, C) derived from IVF in the absence of rAC, compared to the normal appearance of oocytes (D) and embryos (E, F) derived by IVF in the presence of rAC, assessed by light microscopy. Scale bar = 10 μm. Images are representative of ≥3 independent experiments.
TABLE 2.
Number of nonapoptotic mouse embryos derived from IVF of aged oocytes in the presence or absence of rAC
| Group | +rAC | −rAC |
|---|---|---|
| Total aged oocytes | 107 | 65 |
| 2-cell (nonapoptotic) | 21 | 1 |
| 4-cell (nonapoptotic) | 6 | 0 |
| Fertilization % | 25.2 | 0.02* |
Data represent analysis of embryos obtained from 4 female mice.
P < 0.002.
TABLE 3.
Number of mouse pups derived from zygotes/oocytes retrieved following IVF
| Treatment | Starting oocytes/zygotes | 2- to 4-cell embryos formed and transferred [n (%)] | Pups born [n (%)] |
|---|---|---|---|
| −rAC | 80 | 32 (40) | 5 (6.25) |
| +rAC | 89 | 78 (87.6) | 30 (33.7) |
| P value | <0.003 | <0.02 |
Superovulated female mice were fertilized in vivo, and the zygotes (or unfertilized oocytes) were cultured in the presence or absence of rAC for 24–48 h. The number of 2- to 4-cell embryos formed was recorded. For both groups, the 2- to 4-cell embryos were then implanted into hosts, and the number of pups born was recorded. Percentages (in parentheses) are relative to the starting number of zygotes/oocytes. P values compare treatment with and without rAC.
The results demonstrated that rAC supplementation significantly improved the quality and number of mouse embryos derived by IVF. As illustrated in Fig. 5 and summarized in Table 2, in the absence of rAC, none of 65 fertilized, aged oocytes developed to the 4-cell stage, and only 1 developed to the 2-cell stage. In contrast, in the presence of rAC, 25% of the aged mouse oocytes (27/107) underwent successful fertilization and reached the 2- to 4-cell stage (P<0.002). Apoptosis was assessed using established morphological criteria, including cell shrinkage, membrane blebbing, and cytoplasm fragmentation, as described previously (3, 13, 19, 20).
To further assess the effect of rAC treatment on embryo development, mouse oocytes or zygotes were retrieved following in vivo fertilization, and their development was followed during 24–48 h of culture in the presence or absence of rAC. All of the 2- and 4-cell embryos from both groups were then transferred into pseudopregnant female recipients, and the birth rates were recorded. As shown in Table 3, in the presence of rAC, 87.6% of the oocytes/zygotes (78/89) reached the 2- to 4-cell stage, in contrast to only 40% (32/80) in the absence of rAC (P<0.003). Notably, 33.7% (30/89) of the treated oocytes/zygotes developed into live pups, while only 6.3% (5/80) of those without rAC treatment did (P<0.02). The birth rate of implanted 2- to 4-cell embryos from the rAC-treated group (30/78, 38.5%) was even higher than that without rAC treatment (5/32, 15.7%), indicating no deleterious effect of the enzyme treatment on implantation or development. The pups derived from the rAC-treated embryos were followed for up to 1 mo, and all had a normal appearance and motor function (data not shown).
In addition to demonstrating the positive effect of rAC on embryo quality in humans and mice, we confirmed this effect in the bovine model. Immature bovine oocytes were obtained, and rAC was maintained during in vitro maturation and fertilization, as well as during embryo culture for an additional 7–10 d. Significantly more high-grade blastocysts (66.5%, 111/167) were observed in the rAC-treated group as compared to those without rAC treatment (48.8%, 78/160) (P<0.05). The number of collapsed embryos after hatching (i.e., shedding of the ZP envelope at d 10) also was recorded, and it was observed that most of the embryos without rAC treatment (71.8%, 28/39) collapsed after hatching, as compared to those with rAC (23.8% collapsed, 10/42). Overall, these results reveal a new method to improve the number of high-quality embryos and animals derived by IVF.
DISCUSSION
The demand for ARTs has been rapidly growing during the past decade, leading to the birth of >3 million children worldwide following IVF (21, 22). However, conditions for the ex vivo handling of human oocytes and embryos are not fully developed, leading to the reimplantation of poor-quality embryos and contributing to less than optimal birth rates after IVF (22). To increase the live birth rate, multiple embryos are often returned to a woman for implantation, which may at times result in potentially dangerous multiple gestation.
Improving the quality of cultured oocytes, and most importantly, embryos derived by IVF, can potentially improve the efficiency of ARTs, and decrease the costs and health risks associated with the hormonal stimulation and multiple-fetus gestation. In addition, improving the conditions of in vitro maturation (IVM) could allow successful fertilization of oocytes that are immature at the time of the retrieval, providing a cost-effective alternative to ovarian stimulation and an option for recruiting oocytes without hormonal treatment (23).
ARTs also have been widely used for the in vitro production of embryos from commercially important animals, and are being increasingly used for the preservation of animals at risk of extinction (4, 5, 24). A major concern of agricultural IVF (in cattle particularly) is large offspring syndrome (LOS) (25), resulting from exposure of IVF-derived embryos to BSA. Under current conditions, in the absence of serum supplement, the efficiency of bovine IVF ranges from ∼20 to 50%. Notably, for this species, the number of embryos that can be reimplanted is restricted to 1; therefore, small increases in IVF efficiency can have a major effect on production (26). The recent decision by the Food and Drug Administration to allow the sale of food products derived from cloned animals (http://www.fda. gov/default.htm) raises the likelihood that ARTs will be used more frequently in the future for this and other commercially important species. In addition, genetic material (DNA, sperm, oocytes, and embryos) from species in danger of extinction are increasingly being preserved cryogenically so that they can be used to generate offspring following IVF, embryo culture, and transfer into surrogate recipients (24). Lastly, although IVF is routinely used in rodents for research, there is a large degree of strain and species variation, and some of the most commonly used research strains are not amenable to IVF unless invasive and time consuming, single-cell manipulation procedures such as zona drilling are used (27).
The research described in the present work was guided by our underlying hypothesis that one important factor leading to poor oocyte and embryo quality during in vitro culture is the accumulation of the proapoptotic lipid, ceramide, due to a decrease in the expression of its metabolizing enzyme, AC. We have previously shown that AC is highly expressed in mature mouse oocytes, presumably to support the zygote until embryonic genome activation (13). Increase of ceramide in aging oocytes in vivo also has been shown, and attributed to the tight network between the oocyte and the surrounding multilayer of cumulus cells, leading to the transfer of ceramide from the cumulus cells into the oocyte (10).
Herein, we demonstrate for the first time that AC is widely expressed throughout human, mouse, and bovine oocyte maturation and embryo development, presumably to maintain low ceramide levels. In addition, we show that human cumulus cells and follicular fluid, which represent the natural environment supporting the oocyte, also express AC. Notably, AC expression in the blastocyst cavity (blastocoel) was positively correlated with human embryo quality, and its decrease was associated with the onset of apoptotic changes within the embryo outer cell mass. Taken together, these results support the hypothesis that AC expression is essential for in vivo oocyte and embryo development, and suggested that supplementing rAC into culture media might mimic this natural environment and assist in maintaining oocytes and embryos in vitro by slowing/preventing the apoptotic process.
Indeed, the addition of rAC to the culture medium significantly improved the quality of cultured oocytes (human and murine), and enhanced the outcome of IVF (murine and bovine). Specifically, rAC decreased the occurrence of DNA nicking and cytoplasm fragmentation, and stabilized the metaphase plate of human oocytes. Bovine and murine IVF performed in the presence of rAC also resulted in increased numbers of high-quality embryos and, most important, mouse zygotes cultured to the 2- to 4-cell stage in the presence of rAC resulted in ∼5-fold more births than embryos without rAC treatment. These effects could be due to uptake and delivery of rAC to an intracellular compartment, or to activity of the enzyme at the cell surface. In the future, it will be of interest to determine the exact mechanism of uptake of rAC by cultured oocytes and embryos, and to follow the subcellular trafficking in more detail.
Previously, ceramide has been described as a key regulator of morphological changes and checkpoints, interfering with spindle assembly and DNA transcription factor binding (28, 29). Ceramide production within cell membranes also leads to reorganization of signaling platforms, frequently resulting in cell death (30). Together, these changes in ceramide can lead to cell cycle arrest and/or cell death, preventing the normal development of embryos produced in vitro. Our results show for the first time that the ceramide-metabolizing enzyme, AC, colocalizes with the membranes, meiotic spindle, and the DNA, supporting its potential role in preventing cell cycle arrest, and further suggested that this enzyme might have a protective function during the in vitro culture of oocytes and embryos.
CONCLUSIONS
The data presented here identify AC as an essential component of the in vivo oocyte and embryo environment, and describe a novel approach for improving oocyte and embryo handling in vitro. Because AC is a factor normally required for oocyte and embryo survival, an important aspect of our approach is that we are simply providing a natural component to in vitro culture systems that we hypothesize is missing from most commercial media. Notably, exposure of developing embryos to rAC in culture had no deleterious effects and, in fact, increased the number of healthy animals born. In addition, the fact that rAC is expressed at high levels in follicular fluid and cumulus cells, and can be secreted from embryos produced by IVF into the culture medium (data not shown), suggests that AC might be a useful biomarker to guide selection of superior embryos for transfer.
Acknowledgments
This research was supported, in part, by a grant from the U.S. National Institutes of Health to E.H.S. (R01 DK 54830). The authors also acknowledge the Mouse Genetics Shared Resource Facility at the Mount Sinai School of Medicine for assistance with the mouse surgeries. E.H.S., E.E., N.S. and X.H. are coinventors on a patent application describing the use of AC for cell survival.
References
- Kolialexi A., Mavrou A., Spyrou G., Tsangaris G. T. Mass spectrometry-based proteomics in reproductive medicine. Mass Spectrom Rev. 2008;27:624–634. doi: 10.1002/mas.20181. [DOI] [PubMed] [Google Scholar]
- Revelli A., Delle Piane L., Casano S., Molinari E., Massobrio M., Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40–53. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez G. I., Tao X. J., Tilly J. L. Fragmentation and death (aka apoptosis) of ovulated oocytes. Mol Hum Reprod. 1999;5:414–420. doi: 10.1093/molehr/5.5.414. [DOI] [PubMed] [Google Scholar]
- Galli C., Lazzari G. The manipulation of gametes and embryos in farm animals. Reprod Domest Anim. 2008;43(Suppl. 2):1–7. doi: 10.1111/j.1439-0531.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- Pukazhenthi B. S., Wildt D. E. Which reproductive technologies are most relevant to studying, managing and conserving wildlife? Reprod Fertil Dev. 2004;16:33–46. doi: 10.10371/RD03076. [DOI] [PubMed] [Google Scholar]
- De La Fuente R. Chromatin modifications in the germinal vesicle (GV) of mammalian oocytes. Dev Biol. 2006;292:1–12. doi: 10.1016/j.ydbio.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Zheng P. Effects of in vitro maturation of monkey oocytes on their developmental capacity. Anim Reprod Sci. 2007;98:56–71. doi: 10.1016/j.anireprosci.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke N., Hannun Y. A. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50:S91–S96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li X., Becker K. A., Gulbins E. Ceramide-enriched membrane domains–structure and function. Biochim Biophys Acta. 2009;1788:178–183. doi: 10.1016/j.bbamem.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Perez G. I., Jurisicova A., Matikainen T., Moriyama T., Kim M. R., Takai Y., Pru J. K., Kolesnick R. N., Tilly J. L. A central role for ceramide in the age-related acceleration of apoptosis in the female germline. FASEB J. 2005;19:860–862. doi: 10.1096/fj.04-2903fje. [DOI] [PubMed] [Google Scholar]
- Mao C., Obeid L. M. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta. 2008;1781:424–434. doi: 10.1016/j.bbalip.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait N. C., Oskeritzian C. A., Paugh S. W., Milstien S., Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Eliyahu E., Park J. H., Shtraizent N., He X., Schuchman E. H. Acid ceramidase is a novel factor required for early embryo survival. FASEB J. 2007;21:1403–1409. doi: 10.1096/fj.06-7016com. [DOI] [PubMed] [Google Scholar]
- Ben-Yosef D., Oron Y., Shalgi R. Low temperature and fertilization-induced Ca2+ changes in rat eggs. Mol Reprod Dev. 1995;42:122–129. doi: 10.1002/mrd.1080420116. [DOI] [PubMed] [Google Scholar]
- Wright J. M. Non-surgical embryo transfer in cattle embryo-recipient interactions. Theriogenology. 1981;15:43–56. doi: 10.1016/s0093-691x(81)80017-9. [DOI] [PubMed] [Google Scholar]
- He X., Okino N., Dhami R., Dagan A., Gatt S., Schulze H., Sandhoff K., Schuchman E. H. Purification and characterization of recombinant, human acid ceramidase. J Biol Chem. 2003;278:32978–32986. doi: 10.1074/jbc.M301936200. [DOI] [PubMed] [Google Scholar]
- Shtraizent N., Eliyahu E., Park J. H., He X., Shalgi R., Schuchman E. H. Autoproteolytic cleavage and activation of human acid ceramidase. J Biol Chem. 2008;283:11253–11259. doi: 10.1074/jbc.M709166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlinz K., Kopal G., Bernardo K., Linke T., Bar J., Breiden B., Neumann U., Lang F., Schuchman E. H., Sandhoff K. Human acid ceramidase: processing, glycosylation, and lysosomal targeting. J Biol Chem. 2001;276:35352–35360. doi: 10.1074/jbc.M103066200. [DOI] [PubMed] [Google Scholar]
- Wu J., Zhang L., Wang X. Maturation and apoptosis of human oocytes in vitro are age-related. Fertil Steril. 2000;74:1137–1141. doi: 10.1016/s0015-0282(00)01597-1. [DOI] [PubMed] [Google Scholar]
- Chaube S. K., Prasad P. V., Thakur S. C., Srivastava T. G. Hydrogen peroxide modulates meiotic cell cycle and induces morphological features characteristics of apoptosis in rat oocytes cultured in vitro. Apoptosis. 2005;10:863–875. doi: 10.1007/s10495-005-0367-8. [DOI] [PubMed] [Google Scholar]
- Wright V. C., Schieve L. A., Reynolds M. A., Jeng G. Assisted reproductive technology surveillance. Morbid Mortal Wkly Rep. 2003;52:1–16. [PubMed] [Google Scholar]
- Caperton L., Murphey P., Yamazaki Y., McMahan C. A., Walter C. A., Yanagimachi R., McCarrey J. R. Assisted reproductive technologies do not alter mutation frequency or spectrum. Proc Natl Acad Sci U S A. 2007;104:5085–5090. doi: 10.1073/pnas.0611642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sutter P., Dhont M. Poor response after hormonal stimulation for in vitro fertilization is not related to ovarian aging. Fertil Steril. 2003;79:1294–1298. doi: 10.1016/s0015-0282(03)00264-4. [DOI] [PubMed] [Google Scholar]
- Swanson W. F. Application of assisted reproduction for population management in felids: The potential and reality for conservation of small cats. Theriogenology. 2006;66:49–58. doi: 10.1016/j.theriogenology.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Young L. E., Sinclair K. D., Wilmut I. Large offspring syndrome in cattle and sheep. Rev Reprod. 1998;3:155–163. doi: 10.1530/ror.0.0030155. [DOI] [PubMed] [Google Scholar]
- Scherzer J., Fayrer-Hosken R. A., Ray L., Hurley D. J., Heusner G. L. Advancements in large animal embryo transfer and related biotechnologies. Reprod Domest Anim. 2008;43:371–376. doi: 10.1111/j.1439-0531.2007.00921.x. [DOI] [PubMed] [Google Scholar]
- Meng Q., Li X., Wu T., Dinnyés A., Zhu S. Piezo-actuated zona-drilling improves the fertilisation of OPS vitrified mouse oocytes. Acta Vet Hung. 2007;55:369–378. doi: 10.1556/AVet.55.2007.3.11. [DOI] [PubMed] [Google Scholar]
- Ogretmen B., Kraveka J. M., Schady D., Usta J., Hannun Y. A., Obeid L. M. Molecular mechanisms of ceramide-mediated telomerase inhibition in the A549 human lung adenocarcinoma cell line. J Biol Chem. 2001;276:32506–32514. doi: 10.1074/jbc.M101350200. [DOI] [PubMed] [Google Scholar]
- Swanton C., Marani M., Pardo O., Warne P. H., Kelly G., Sahai E., Elustondo F., Chang J., Temple J., Ahmed A. A., Brenton J. D., Downward J., Nicke B. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11:498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Grassmé H., Riethmüller J., Gulbins E. Biological aspects of ceramide-enriched membrane domains. Prog Lipid Res. 2007;46:161–170. doi: 10.1016/j.plipres.2007.03.002. [DOI] [PubMed] [Google Scholar]