Abstract
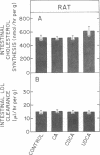
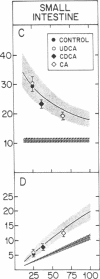
The effect of different bile acids on receptor-dependent and receptor-independent low density lipoprotein (LDL) uptake in the liver and intestine was investigated. When fed at the 0.1% level for three weeks, cholic acid and chenodeoxycholic acid suppressed hepatic cholesterol synthesis in the rat by 80% and 50%, respectively, while ursodeoxycholic acid had no effect. In contrast, hepatic cholesteryl ester levels, rates of hepatic LDL transport, and concentrations of plasma LDL-cholesterol were not affected by bile acid feeding in this species. Cholic acid and chenodeoxycholic acid also suppressed hepatic cholesterol synthesis in the hamster. However, since basal rates of hepatic cholesterol synthesis in this species, as in man, are very low, the absolute reduction in hepatic synthesis could not compensate for the change in hepatic sterol balance induced by bile acid feeding. Hence, in the hamster the feeding of cholic acid and chenodeoxycholic acid increased hepatic cholesteryl ester levels 660% and 39%, respectively, reduced hepatic receptor-dependent LDL uptake by 50% and 32%, respectively, and elevated plasma LDL-cholesterol levels by 160% and 50%, respectively. Ursodeoxycholic acid feeding did not alter any of these processes, and none of the bile acids changed the rate of hepatic receptor-independent LDL transport. In the intestine, none of the bile acids altered rates of cholesterol synthesis or LDL uptake. When cholic acids, chenodeoxycholic acid, or ursodeoxycholic acid was infused continuously for 8 hr in supranormal amounts into control hamsters or rats or into animals pretreated with cholestyramine, there were no changes in LDL transport or any other parameter of hepatic cholesterol metabolism. Thus, these studies indicated that cholic acid and chenodeoxycholic acid have no acute, direct effect on rates of receptor-dependent LDL transport or cholesterol synthesis but do alter these processes indirectly by inducing changes in cholesterol balance across the liver. Ursodeoxycholic acid, in contrast, does not affect these processes either directly or indirectly and so causes no change in plasma LDL levels.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. M., Dietschy J. M. Relative importance of high and low density lipoproteins in the regulation of cholesterol synthesis in the adrenal gland, ovary, and testis of the rat. J Biol Chem. 1978 Dec 25;253(24):9024–9032. [PubMed] [Google Scholar]
- Angelin B., Raviola C. A., Innerarity T. L., Mahley R. W. Regulation of hepatic lipoprotein receptors in the dog. Rapid regulation of apolipoprotein B,E receptors, but not of apolipoprotein E receptors, by intestinal lipoproteins and bile acids. J Clin Invest. 1983 Apr;71(4):816–831. doi: 10.1172/JCI110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Watanabe Y., Kita T. Impaired receptor-mediated catabolism of low density lipoprotein in the WHHL rabbit, an animal model of familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1982 May;79(10):3305–3309. doi: 10.1073/pnas.79.10.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison W. G., Grundy S. M. Effect of ursodeoxycholate and its taurine conjugate on bile acid synthesis and cholesterol absorption. Gastroenterology. 1984 Jul;87(1):130–135. [PubMed] [Google Scholar]
- Jeske D. J., Dietschy J. M. Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using [3H]water. J Lipid Res. 1980 Mar;21(3):364–376. [PubMed] [Google Scholar]
- Kesaniemi Y. A., Witztum J. L., Steinbrecher U. P. Receptor-mediated catabolism of low density lipoprotein in man. Quantitation using glucosylated low density lipoprotein. J Clin Invest. 1983 Apr;71(4):950–959. doi: 10.1172/JCI110849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiss O., von Bergmann K., Streicher U., Strotkoetter H. Effect of three different dihydroxy bile acids on intestinal cholesterol absorption in normal volunteers. Gastroenterology. 1984 Jul;87(1):144–149. [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H. An electrophoretic method for the quantitative isolation of human and swine plasma lipoproteins. Biochemistry. 1974 Apr 23;13(9):1964–1969. doi: 10.1021/bi00706a600. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Melchior G. W., Innerarity T. L., Holcombe K. S. Inhibition of receptor-mediated clearance of lysine and arginine-modified lipoproteins from the plasma of rats and monkeys. Proc Natl Acad Sci U S A. 1980 Jan;77(1):225–229. doi: 10.1073/pnas.77.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Andersen J. M., Dietschy J. M. Sites of tissue binding and uptake in vivo of bacterial lipopolysaccharide-high density lipoprotein complexes: studies in the rat and squirrel monkey. J Clin Invest. 1981 Dec;68(6):1503–1513. doi: 10.1172/JCI110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman R. C., Green S. R., Attie A. D., Steinberg D. Radiolabeled sucrose covalently linked to protein. A device for quantifying degradation of plasma proteins catabolized by lysosomal mechanisms. J Biol Chem. 1979 Aug 10;254(15):6876–6879. [PubMed] [Google Scholar]
- Ponz de Leon M., Carulli N., Loria P., Iori R., Zironi F. The effect of chenodeoxycholic acid (CDCA) on cholesterol absorption. Gastroenterology. 1979 Aug;77(2):223–230. [PubMed] [Google Scholar]
- Raicht R. F., Cohen B. I., Mosbach E. H. Effects of sodium taurochenodeoxycholate and sodium taurocholate on cholesterol absorption in the rat. Gastroenterology. 1974 Dec;67(6):1155–1161. [PubMed] [Google Scholar]
- Raicht R. F., Cohen B. I., Sarwal A., Takahashi M. Ursodeoxycholic acid. Effects on sterol metabolism in rats. Biochim Biophys Acta. 1978 Oct 25;531(1):1–8. doi: 10.1016/0005-2760(78)90175-3. [DOI] [PubMed] [Google Scholar]
- Schoenfield L. J., Lachin J. M. Chenodiol (chenodeoxycholic acid) for dissolution of gallstones: the National Cooperative Gallstone Study. A controlled trial of efficacy and safety. Ann Intern Med. 1981 Sep;95(3):257–282. doi: 10.7326/0003-4819-95-3-257. [DOI] [PubMed] [Google Scholar]
- Spady D. K., Bilheimer D. W., Dietschy J. M. Rates of receptor-dependent and -independent low density lipoprotein uptake in the hamster. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3499–3503. doi: 10.1073/pnas.80.11.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Dietary saturated triacylglycerols suppress hepatic low density lipoprotein receptor activity in the hamster. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4526–4530. doi: 10.1073/pnas.82.13.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Turley S. D., Dietschy J. M. Rates of low density lipoprotein uptake and cholesterol synthesis are regulated independently in the liver. J Lipid Res. 1985 Apr;26(4):465–472. [PubMed] [Google Scholar]
- Spady D. K., Turley S. D., Dietschy J. M. Receptor-independent low density lipoprotein transport in the rat in vivo. Quantitation, characterization, and metabolic consequences. J Clin Invest. 1985 Sep;76(3):1113–1122. doi: 10.1172/JCI112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley S. D., Dietschy J. M. Re-evaluation of the 3 alpha-hydroxysteroid dehydrogenase assay for total bile acids in bile. J Lipid Res. 1978 Sep;19(7):924–928. [PubMed] [Google Scholar]
- von Bergmann K., Epple-Gutsfeld M., Leiss O. Differences in the effects of chenodeoxycholic and ursodeoxycholic acid on biliary lipid secretion and bile acid synthesis in patients with gallstones. Gastroenterology. 1984 Jul;87(1):136–143. [PubMed] [Google Scholar]