Abstract
Purpose:
Reliable staging of the mediastinum determines TNM classification and directs therapy for non-small cell lung cancer (NSCLC). Our aim was to evaluate predictors of mediastinal lymph node metastasis in patients undergoing endobronchial ultrasound (EBUS).
Methods:
Patients with known or suspected lung cancer undergoing EBUS for staging were included. Lymph node radiographic characteristics on chest CT/PET scan and ultrasound characteristics of size, shape, border, echogenicity, and number were correlated with rapid on-site evaluation (ROSE) and final pathology. Logistic regression (estimated with generalized estimating equations to account for correlation across nodes within patients) was used with cancer (vs normal pathology) as the outcome. ORs compare risks across groups, and testing was performed with two-sided α of 0.05.
Results:
Two hundred twenty-seven distinct lymph nodes (22.5% positive for malignancy) were evaluated in 100 patients. Lymph node size, by CT scan and EBUS measurements, and round and oval shape were predictive of mediastinal metastasis. Increasing size of lymph nodes on EBUS was associated with increasing malignancy risk (P = .0002). When adjusted for CT scan size, hypermetabolic lymph nodes on PET scan did not predict malignancy. Echogenicity and border contour on EBUS and site of biopsy were not significantly associated with cancer. In 94.8% of lymph nodes with a clear diagnosis, the ROSE of the first pass correlated with subsequent passes.
Conclusions:
Lymph node size on CT scan and EBUS and round or oval shape by EBUS are predictors of malignancy, but no single characteristic can exclude a visualized lymph node from biopsy. Further, increasing the number of samples taken is unlikely to significantly improve sensitivity.
Appropriate staging of lung cancer is critical, as it predicts prognosis and dictates treatment. Radiographic staging with CT scan and PET scan can offer clues to the extent of disease, but pathologic confirmation of malignancy and determination of the TNM stage for non-small cell lung cancer (NSCLC) dictates the treatment choice.1 In practice, mediastinal lymph node involvement most often differentiates those who are surgical candidates from those who are not.2
The current methods available to adequately stage the mediastinum include mediastinoscopy, video-assisted thoracoscopy, endoscopic ultrasound (EUS), endobronchial ultrasound (EBUS), transthoracic needle aspiration, and transbronchial needle aspiration. The American College of Chest Physicians practice guidelines state that “tissue should be obtained by whatever method is easiest to perform” depending on the size and location of the lymph node, the availability of the technology, and expertise in the local facility.1
EBUS and EUS have gained acceptance as dependable procedures to stage lung cancer with comparable accuracy to surgical methods.1,3‐5 The use of ultrasound facilitates the direct visualization of the lymph node during biopsy and may offer information regarding nodal characteristics of malignant nodes.
Several studies exist that evaluate various ultrasound characteristics noted on EUS to predict malignancy.6‐8 Predictors have also been documented in the evaluation of cervical lymphadenopathy.9 Such assessment of mediastinal and hilar lymph nodes by EBUS may differ because of varying lymph node accessibility, imaging through the thicker-walled trachea, and use of a linear ultrasound probe. With this study we aimed to determine if any ultrasonographic or procedural characteristics in patients undergoing EBUS for diagnosis and/or staging of lung cancer predict malignant involvement of lymph nodes.
Materials and Methods
We prospectively enrolled 100 patients with known or suspected lung cancer who were referred by their primary physician to undergo EBUS at the Medical University of South Carolina. Patients met inclusion criteria if they were undergoing EBUS for diagnosis and/or staging of suspected or known lung cancer, had a PET scan prior to the procedure, and gave consent to participate. Patients were excluded if they were < 18 years old or were undergoing EBUS for suspicion of nonmalignant causes. The procedures were performed by a pulmonary fellow with one of four supervising physicians experienced in EBUS technique. Data collected on the ultrasound and procedural characteristics were agreed upon by at least two of the performing bronchoscopists; this optimized standardization of the recording and evaluation of nodal characteristics. The institutional review board (IRB; Committee 2, Approval # 17540) approved this study.
Procedure
After informed consent was obtained, conscious sedation with IV fentanyl and midazolam was initiated, and EBUS bronchoscopy was performed per standard medical care.10 Vital signs (BP, heart rate, respiratory rate, and oxygen saturation) were monitored throughout the procedure. The EBUS bronchoscope (bronchoscope BF-UC180F, processor EU-ME1 or EU-C60; Olympus Medical; Tokyo, Japan) with an electronic linear array probe at 7.5 MHz was introduced into the oropharynx, and topical lidocaine anesthesia was administered. Additional doses of fentanyl, midazolam, and lidocaine were given as needed to maintain adequate sedation and cough control during the procedure.
The mediastinum was evaluated by imaging the lymph node stations per the Mountain and Dresler classification map.11 Ultrasonic lymph node characteristics were determined and recorded prior to biopsy. Sampling commenced with N3 lymph node stations (if visible) using a 21- or 22-gauge EBUS sampling cytology needle (NA-201SX-4021 or -4022; Olympus America Inc). Position of the needle within the lymph node and its relation to surrounding structures were monitored in real time, per EBUS protocol.12 In all cases, real-time ultrasound visualization was used to verify the needle position within the lymph node at the time of fine-needle aspiration. Rapid on-site evaluation (ROSE) by a cytopathologist was performed. An adequate biopsy was defined as the presence of lymphocytes by ROSE or if malignancy was diagnosed. Sampling continued until a diagnosis was made or three adequate samples had been obtained at a given lymph node station.
If no cancer was identified, then sampling proceeded to the N2, then the N1, lymph node stations until a diagnosis was made or three pathologically adequate passes had been performed at each site. At this point, the procedure was stopped, and the patient was monitored and discharged per standard of care.
Data Collection
Prior to the EBUS procedure, anonymized information describing the radiographic location and characteristics of tumors and lymph nodes was collected for each patient by one of the investigators. The lobar location within the thorax and the largest diameter of the primary tumor were measured and recorded. The greatest short-axis diameter of mediastinal and hilar lymph nodes visualized on CT scan or PET/CT scan was measured and recorded. The PET scan metabolic activity, described as increased or normal by the radiology report, was recorded. The pre-EBUS and post-EBUS TNM and stage were determined according to the International Association for the Study of Lung Cancer, seventh edition. Results of subsequent interventions performed following the EBUS were also recorded (ie, mediastinoscopy or surgery).
During the EBUS procedure, we documented the following nodal characteristics: shape (triangular, round, oval, draping), size (longest short-axis diameter based on the EBUS screen’s ruler markings), echogenicity (hyperechoic; hypoechoic; isoechoic as compared with the surrounding mediastinal soft tissues, which are hyperechoic, and the major vascular structures, which are hypoechoic), border definition (well-defined vs indistinct), and number of lymph nodes at the lymph node station (single vs multiple) (Figs 1, 2).
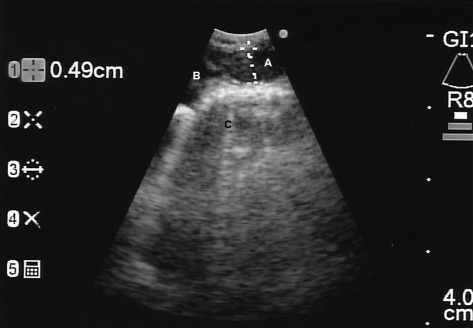
Figure 1.
Station 4R (right paratracheal) lymph node, described as oval shape, 4.9-mm size, hypoechoic, well-defined border, and single (A). Edge of azygos vein shown at B. Mediastinal soft tissue shown at C.
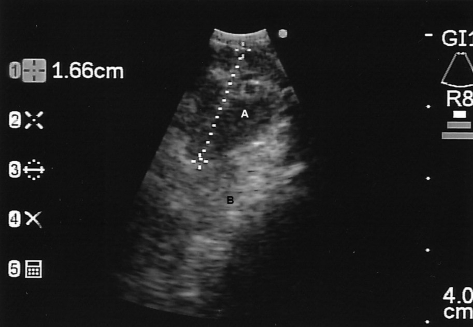
Figure 2.
Station 7 (subcarinal) lymph node, described as round shape, 16.6-mm size, hypoechoic, poorly defined border, and single (A). Mediastinal soft tissue shown at B.
For each biopsy specimen, procedural characteristics were recorded: the location of the needle within the lymph node (center vs peripheral), number of passes, and the presence of lymphocytes in each specimen (numerous, scant, or absent). The ROSE diagnosis by the cytopathologist present at the time of bronchoscopy was recorded, and the final pathology data were entered when they became available after the procedure.
Statistical Analysis
Descriptive statistics were generated for demographic and clinical characteristics of patients. Generalized estimating equations (GEE) logistic regression models were used to assess the relationship between the malignancy status of lymph nodes and the ultrasound and radiographic characteristics of the nodes. Correlation between nodes from the same patients was accounted for by specifying an exchangeable correlation structure. The models were fit individually for each predictor. Additional GEE logistic regression models were performed to assess the relationship between cancer, PET scan activity, and lymph node size on CT scan of the chest. ORs and 95% CIs were presented. P values < .05 were considered statistically significant. Agreement between diagnostic approaches was estimated using proportions based on tabulations of the data.
Results
Patients were enrolled from September 2008 through March 2010. A total of 339 EBUS procedures were performed at the study facility during this time, and 100 patients with known or suspected lung cancer met enrollment criteria and consented for participation in this study. Table 1 lists the patient characteristics and primary tumor descriptions.
Table 1.
—Patient Characteristics
| Age, y, mean (range) | 64.3 (29-81) |
| Race | |
| White | 82 |
| Black | 15 |
| Unknown | 3 |
| Gender | |
| Male | 60 |
| Female | 40 |
| Tumor location | |
| RUL | 32 |
| RML | 5 |
| RLL | 18 |
| LUL | 17 |
| LLL | 9 |
| Right hilum | 6 |
| Left hilum | 5 |
| Other | 1 |
| No primary tumor visible | 7 |
| Tumor size, cm | |
| Mean (range) | 3.8 (0.8-11.6) |
| ≤ 1 | 7 |
| > 1, ≤ 2 | 22 |
| > 2, ≤ 3 | 26 |
| > 3, ≤ 4 | 14 |
| > 4 | 24 |
| No primary tumor visible | 7 |
| Primary diagnosis | |
| Cancer | |
| Primary lung | |
| Squamous cell | 31 |
| Adenocarcinoma | 27 |
| Undifferentiated NSCLC | 9 |
| Large cell | 3 |
| Small cell | 4 |
| Recurrent | 1 |
| Metastatic | 6 |
| Nonmalignant | 19 |
Data presented as No. unless otherwise noted. LLL = left lower lobe; LUL = left upper lobe; NSCLC = non-small cell lung cancer; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe.
Biopsies were performed on a total of 227 lymph nodes (Table 2). If nondiagnostic results are excluded, 28.3% were positive for malignancy, indicating metastatic disease. A definitive surgical procedure was performed in 26 patients: 22 had a negative EBUS, three had a nondiagnostic EBUS, and one was a candidate for surgical cure based on presence of only hilar metastasis. The remainder did not undergo surgery because the EBUS staging precluded the need for surgery, or performance status or lung function precluded the patient’s ability to undergo surgery safely. Based on surgical pathology or 6-month radiographic follow-up, the sensitivity, specificity, negative predictive value, and positive predictive value of EBUS in this study were 87%, 100%, 89%, and 100%, respectively (Table 3).
Table 2.
—Biopsy Results of All Lymph Nodes
| Nodes | Total | ≤ 10 mm | < 5 mm |
| Total No. visualized | 306 | 194 | 64 |
| No. sampled | 227 | 126 | 12 |
| Results, No. (%) | |||
| Positive | 51 (22.5) | 13 (10.3) | 1 (8.3) |
| Negative | 129 (56.8) | 82 (65.1) | 6 (50) |
| Unsatisfactory | 47 (20.7) | 31 (24.6) | 5 (41.7) |
Table 3.
—EBUS Results of All Patients
| EBUS Result |
|||
| Mediastinal/Hilar Metastasis | Positive | Negative | Total |
| Present | 41 | 6 | 47 |
| Absent | 0 | 49 | 49 |
| Total | 41 | 55 | 96a |
Sensitivity, 87%; specificity, 100%; negative predictive value, 89%; positive predictive value, 100%. EBUS = endobronchial ultrasound.
Four patients excluded because true stage unknown: two with metastasis found at surgery in lymph nodes not undergoing biopsy at time of EBUS, one lost to follow-up, one with radiographically larger lymph node but no tissue verification of metastasis.
Radiographically, lymph nodes > 10 mm were predictive of malignancy, and the likelihood increased as the size increased (Table 4). Increased metabolic PET scan activity also predicted malignancy (OR, 3.48; 95% CI, 1.40-8.64) when evaluated alone, but when adjusted for CT scan lymph node size, PET scan activity no longer remained significant (P = .11; OR, 2.36; 95% CI, 0.81-6.83) (Table 5).
Table 4.
—ORs Describing the Risk of Malignancy by Nodal Characteristics
| EBUS Pathology | Predictor | Variable | OR (95% CI) | P Value |
| Radiographic characteristics | PET scan activity | Normal | Ref | … |
| Increased | 3.48 (1.40-8.64) | .0072a | ||
| CT scan lymph node size | < 10 mm | Ref | … | |
| 10-20 mm | 2.89 (1.11-7.52) | .029a | ||
| > 20 mm | 34.38 (6.02-196.48) | < .0001a | ||
| Ultrasound characteristics | Size | Continuous: change of 5 mm | 1.57 (1.23-1.99) | .0002a |
| < 10 mm | Ref | … | ||
| 10-20 mm | 3.39 (1.77-6.46) | .0002a | ||
| > 20 mm | 10.28 (4.31-24.50) | < .0001a | ||
| Shape | Triangular | Ref | … | |
| Oval | 3.50 (1.54-7.96) | .0028a | ||
| Round | 4.16 (1.67-10.36) | .0022a | ||
| Draping | 1.49 (0.46-4.89) | .51 | ||
| Echogenicity | Hyperechoic | Ref | … | |
| Hypoechoic | 1.47 (0.51-4.30) | .48 | ||
| Isoechoic | 0.61 (0.11-3.32) | .56 | ||
| Borders | Well-defined | Ref | … | |
| Indistinct | 0.98 (0.58-1.66) | .93 | ||
| Procedural characteristics | Biopsy location | Center | Ref | … |
| Periphery | 0.97 (0.56-1.66) | .91 |
See Table 3 legend for expansion of abbreviation. GEE = generalized estimating equation; Ref = referent.
Indicates statistical significance using α of 0.05. Estimates were calculated using a GEE logistic regression model as described in the “Statistical Analysis” section.
Table 5.
—ORs Describing the Risk of Malignancy by Nodal Characteristics
| Adjusted Model |
|||
| Variable | Categories | OR (95% CI) | P Value |
| PET scan activity | Normal | Ref | … |
| Increased | 2.36 (0.81-6.83) | .11 | |
| Lymph node size on CT scan of the chest | < 10 mm | Ref | … |
| 10-20 mm | 2.84 (1.07-7.51) | .0359a | |
| > 20 mm | 26.61 (4.60-153.96) | .0002a | |
Adjusted models included both PET scan activity and lymph node size on CT scan of the chest. See Table 4 legend for expansion of abbreviation.
Indicates statistical significance using α of 0.05. Estimates were calculated using a GEE logistic regression model as described in the “Statistical Analysis” section.
For the ultrasonographic size, as with radiographic size measurements, lymph nodes that are 10 to 20 mm and > 20 mm at the time of EBUS are more likely to be positive for malignancy than those < 10 mm (OR, 3.39; 95% CI, 1.77-6.46; and OR, 10.28; 95% CI, 4.31-24.50, respectively) (Table 4). Lymph nodes that were oval and round in shape were more likely to be malignant than triangular-shaped lymph nodes (OR, 3.50; 95% CI, 1.54-7.96; and OR, 4.16; 95% CI, 1.67-10.36, respectively). Although we found that triangular shape was less likely to be malignant, two (4.8%) of the triangular lymph nodes (n = 42) were positive on biopsy. Neither echogenicity nor lymph node border definition were predictive of cancer.
Among the lymph nodes biopsies performed, 126 (55.5%) measured ≤ 10 mm by ultrasound and were sampled despite their size (Table 2). Thirteen (10.3%) were positive for malignancy. Another 68 lymph nodes that measured ≤ 10 mm were visualized on ultrasound but did not undergo biopsy. Biopsies were performed on 12 lymph nodes < 5 mm. Of those sampled, one (8.3%) was positive for malignancy.
Procedurally, there was no significant difference in positivity when the biopsy was obtained from the center or periphery of the lymph node. Additionally, when the ROSE diagnosis for each pass within each individual lymph node was reviewed, the diagnosis of the first pass correlated with the diagnosis of all subsequent passes in 94.8% of cases (n = 211; for 16 lymph nodes, only one pass was performed).
Discussion
This study has several important findings. First, lymph nodes that were larger and were round or oval shaped by ultrasound had an increased chance of being malignant. Second, a clinically significant proportion of normal-sized lymph nodes had metastases, which has implications on our approach to EBUS. Finally, the ROSE findings on the first pass were highly accurate, whether the finding was positive or negative.
With improvements in radiographic imaging and advances in endoscopic and ultrasonographic technology, the evaluation and staging of thoracic tumors has continued to expand.13 EUS was developed in the 1980s to evaluate GI cancers. The first EBUS was described in 199214 and led to the development of a linear EBUS scope that allows for direct guidance for transbronchial needle aspiration.3 The usefulness of this procedure in the diagnosis and staging of lung cancer is beyond question, with a weighted sensitivity of 93% and virtually no complications.1 As the literature expands, several unanswered questions remain, specifically whether any ultrasonographic features or procedural characteristics could predict malignancy or benignity prior to biopsy. The ability to predict those could allow proceduralists to perform biopsy on only some, rather than all, visualized mediastinal and hilar lymph nodes.
Lymph node features that predict malignancy on EUS in head and neck and GI cancers are round shape, size > 1 cm, sharp borders, and hypoechoic density.6‐9 For cervical lymph nodes in head and neck cancers and lymphoma, round shape and hypoechoic density without an echogenic hilus were noted to be evidence of metastasis, and ill-defined borders suggest extracapsular spread.9 Bhutani et al6 and Catalano et al,7 though, concluded that ultrasonographic features are not reliable independently and cannot conclusively allow the practitioner not to perform biopsy of a lymph node. Our findings support this conclusion in lung cancer using EBUS.
Our results have some similarities and differences as compared with a recent retrospective study by Fujiwara et al,15 who evaluated ultrasound features in patients with lung cancer undergoing EBUS. They found that size > 1 cm, round shape, heterogenous echogenicity, and coagulation necrosis sign (hypoechoic area within the lymph node that has no blood flow) suggested malignancy. We did find that round shape was predictive of malignancy, but distinct margins were not. We were unable to evaluate the other two ultrasound characteristics—heterogenous echogenicity and the presence of the coagulation necrosis sign—as our study was in process when this retrospective was published. The study by Fujiwara et al15 did find that 96% of the lymph nodes that lacked all of these characteristics were benign and may predict benignity. However, the authors concluded that an “EBUS classification system will need to be validated in a prospective study,” which was done in our study. We also described the characteristics during real-time ultrasonography and biopsy, not postprocedure from a fixed ultrasound photograph of the lymph node.
The most widely accepted criterion to define an abnormal lymph node suspicious for malignancy is a short-axis diameter of > 1 cm and/or an increased fluorine-18 fluorodeoxyglucose on PET scan.2 Despite the criterion, no size can reliably predict malignancy, and large, PET scan-active lymph nodes should be “proven” by pathologic sampling/resection. Our results show that increased size did predict an increased likelihood of malignancy, but when adjusted for size, PET scan activity did not. This may indicate that the increased size of lymph nodes may be the reason for increased metabolic activity and not the presence of malignancy.
Our results also support the recommendation that biopsy of radiographically nonmetabolic, small lymph nodes cannot be excluded. Several studies have shown the inaccuracy of radiographic methods, both CT and PET scans, to predict metastatic involvement of the mediastinum in NSCLC. The pooled sensitivity and specificity of PET scanning is 74% and 85%, respectively, which is better than CT scanning (51% and 86%, respectively), but PET scan is less sensitive in normal-sized lymph nodes.2 By EUS, Wallace et al16 found 14 patients out of 69 (20.3%) with lymph nodes < 1 cm on CT scan of the chest who had mediastinal metastasis of NSCLC. Histologic type was not associated with or predictive of positivity. Gonzalez-Stawinski et al17 retrospectively evaluated the efficacy of PET scanning compared with mediastinoscopy and found that PET scanning had a false-negative rate of 35.6%. Additionally, in the EBUS literature, two studies by Herth et al18,19 support the need to sample lymph nodes despite radiographic negativity. In the first study done before the routine use of PET scans, 19 of 100 patients evaluated with CT-scan “normal” ( < 1 cm) lymph nodes were found to have metastasis by EBUS.18 Additionally, lymph nodes that underwent biopsy were 5 to 10 mm on ultrasound. In the second study, Herth et al19 studied the same population, but patients also had normal PET scans. Eight of the 100 patients studied were upstaged because of EBUS lymph node positivity compared with radiographic staging alone, and one additional patient had lymph node positivity at surgical staging.
Although we found that lymph nodes > 10 mm on ultrasound are more likely to be malignant, lymph nodes ≤ 10 mm cannot be ignored and presumed to be benign. Fifty-five percent of the lymph nodes sampled in our study were ≤ 10 mm, and 13 (10%) of those were positive for malignancy. The final EBUS pathology in the eight patients with those positive “normal”-sized lymph nodes were adenocarcinoma (n = 4), squamous cell (n = 2), undifferentiated NSCLC (n = 1), and small cell (n = 1). Of the 12 lymph nodes < 5 mm that underwent biopsy, 8.3% (n = 1) were positive for malignancy. We did not perform biopsy on a 4-mm, subcarinal lymph node in one patient who subsequently underwent surgery. At the time of surgical resection, that node was found to be positive for malignancy. Additionally, despite our data showing that triangular shape has a lower risk of malignancy, a small fraction (5%) of triangular nodes was positive for malignancy. These data support the need to perform biopsy on lymph nodes at every station visualized despite the size or shape and to view EBUS for lung cancer staging as a systematic procedure, which is approached and performed the same way each time.
The number of samples needed at each lymph node station to optimize sensitivity has previously been studied. Lee et al20 found that sample adequacy was 90.1% for the first pass, and reached 100% by pass three, but they did not have the benefit of ROSE. The sensitivity was 95.3% by pass three and did not increase with a subsequent pass. They concluded that three passes was optimal. A recent study by Trisolini et al21 found that having ROSE available can decrease the number of biopsies needed without affecting the accuracy. Our data strongly support the conclusion described here. When the first lymph node biopsy pass was definitively negative or positive, subsequent passes correlated in 95% of lymph nodes we sampled. From our study, we found that diagnostic accuracy did not change significantly after the first pass, making more than three passes unnecessary. Additionally, since we had the benefit of ROSE, only one pass was needed in 7% (n = 16) of lymph nodes, because the diagnosis was made without further biopsy.
One of the drawbacks of this study is that the size measurements and description of other ultrasound characteristics were made during real-time ultrasound observation and may be more subjective. The shape (and size) of the lymph nodes was based on the visual relationship of the long to short axis and was agreed on by the two bronchoscopists performing the procedure. Rigorous objective measurements of the ultrasound image with the EBUS software or postprocedure calculations of long-axis to short-axis ratios to determine round or oval shape were not routinely performed. Such measurements require manipulation of the EBUS output while the bronchoscopist attempts to maintain the image at the largest diameter. Although our determinations of the ultrasound characteristics are more subjective, it is more applicable to general practice, as definitive size measurements can be cumbersome and time consuming during the actual procedure. Additionally, excluding size, shape was the only feature that had any significant predictive value. No measurement can be made by the EBUS software to objectively define triangular shape, and triangular shape did not preclude the need to perform biopsy.
Another limitation to this study is that a clear correlation between the radiographic characteristics on CT/PET scans cannot be made with the EBUS characteristics. We did not perform biopsy on every lymph node that was seen on CT or PET scan. According to our protocol, biopsies were performed on N3 nodes first if visualized, even if not seen on CT or PET scan. If malignancy was present, the procedure was stopped and biopsy was not always performed on other lymph nodes that were positive radiographically. Thus, not all lymph nodes seen on CT or PET scan underwent biopsy, making it impossible to determine the relationship of radiographic imaging to the EBUS result by each individual lymph node.
In conclusion, this study shows that increasing size is indicative of malignancy, and oval or round shape has an increased risk of malignancy. No characteristics exist that allow the proceduralist to exclude lymph nodes for biopsy, because at least 10% of metastatic disease could be missed if patients with small, PET scan-negative lymph nodes do not undergo biopsy. Additionally, although more than one pass may be necessary (we still recommend three), the diagnostic yield is not likely to increase greatly after the initial needle aspiration. In patients with large, PET scan-avid lymph nodes, it makes sense clinically to aggressively perform biopsy on those lymph nodes, as the yield for cancer is likely to be higher.
Acknowledgments
Author contributions: Dr Wang Memoli is the guarantor of the manuscript.
Dr Wang Memoli: contributed to study conception and design, analysis and interpretation, and drafting the manuscript.
Dr El-Bayoumi: contributed to study conception and design and drafting the manuscript.
Dr Pastis: contributed to study conception and design and drafting the manuscript.
Dr Tanner: contributed to study conception and design, analysis and interpretation, and drafting the manuscript.
Dr Gomez: contributed to study conception and design and drafting the manuscript.
Dr Huggins: contributed to study conception and design and drafting the manuscript.
Ms Onicescu: contributed to analysis and interpretation and drafting the manuscript.
Dr Garrett-Mayer: contributed to study conception and design, analysis and interpretation, and drafting the manuscript.
Mr Armeson: contributed to analysis and interpretation and drafting the manuscript.
Ms Taylor: contributed to study conception and design and drafting the manuscript.
Dr Silvestri: contributed to study conception and design, analysis and interpretation, and drafting the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Pastis provided a lecture on EBUS for the sales force from Olympus. Drs Wang Memoli, El-Bayoumi, Tanner, Gomez, Huggins, Garrett-Mayer, and Silvestri; Mss Onicescu and Taylor; and Mr Armeson have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Abbreviations
- EBUS
endobronchial ultrasound
- EUS
endoscopic ultrasound
- GEE
generalized estimating equations
- NSCLC
non-small cell lung cancer
- ROSE
rapid on-site evaluation
Funding/Support: The research in this article was supported by National Institutes of Health K-24 Mid-Career Investigator Award in Patient-Oriented Research [Grant 5K24CA120494-02]. It was also supported in part by the Biostatistics Shared Resource as part of the Hollings Cancer Center at the Medical University of South Carolina, which is funded by a Cancer Center Support Grant [Grant P30 CA138313].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA, American College of Chest Physicians Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(suppl 3):202S–220S. doi: 10.1378/chest.07-1362. [DOI] [PubMed] [Google Scholar]
- 2.Silvestri GA, Gould MK, Margolis ML, et al. American College of Chest Physicians Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132(suppl 3):178S–201S. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 3.Herth FJ, Eberhardt R. Actual role of endobronchial ultrasound (EBUS) Eur Radiol. 2007;17(7):1806–1812. doi: 10.1007/s00330-006-0497-6. [DOI] [PubMed] [Google Scholar]
- 4.Vincent BD, El-Bayoumi E, Hoffman B, et al. Real-time endobronchial ultrasound-guided transbronchial lymph node aspiration. Ann Thorac Surg. 2008;85(1):224–230. doi: 10.1016/j.athoracsur.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Wallace MB, Pascual JM, Raimondo M, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA. 2008;299(5):540–546. doi: 10.1001/jama.299.5.540. [DOI] [PubMed] [Google Scholar]
- 6.Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45(6):474–479. doi: 10.1016/s0016-5107(97)70176-7. [DOI] [PubMed] [Google Scholar]
- 7.Catalano MF, Sivak MV, Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40(4):442–446. doi: 10.1016/s0016-5107(94)70206-3. [DOI] [PubMed] [Google Scholar]
- 8.Schmulewitz N, Wildi SM, Varadarajulu S, et al. Accuracy of EUS criteria and primary tumor site for identification of mediastinal lymph node metastasis from non-small-cell lung cancer. Gastrointest Endosc. 2004;59(2):205–212. doi: 10.1016/s0016-5107(03)02692-0. [DOI] [PubMed] [Google Scholar]
- 9.Ahuja AT, Ying M. Sonographic evaluation of cervical lymph nodes. AJR Am J Roentgenol. 2005;184(5):1691–1699. doi: 10.2214/ajr.184.5.01841691. [DOI] [PubMed] [Google Scholar]
- 10.Herth FJ, Krasnik M, Yasufuku K, et al. Endobronchial ultrasound-guided transbronchial needle aspiration. Journal of Bronchology. 2006;13(2):84–91. [Google Scholar]
- 11.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111(6):1718–1723. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 12.Becker H, Herth F. Endobronchial ultrasound of the airways and the mediastinum. In: Bollinger C, Mathur PN, editors. Interventional Bronchoscopy. Basel, Switzerland: Karger; 2000. pp. 80–93. [Google Scholar]
- 13.Gomez M, Silvestri GA. Endobronchial ultrasound for the diagnosis and staging of lung cancer. Proc Am Thorac Soc. 2009;6(2):180–186. doi: 10.1513/pats.200808-081LC. [DOI] [PubMed] [Google Scholar]
- 14.Hürter T, Hanrath P. Endobronchial sonography: feasibility and preliminary results. Thorax. 1992;47(7):565–567. doi: 10.1136/thx.47.7.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujiwara T, Yasufuku K, Nakajima T, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest. 2010;138(3):641–647. doi: 10.1378/chest.09-2006. [DOI] [PubMed] [Google Scholar]
- 16.Wallace MB, Ravenel J, Block MI, et al. Endoscopic ultrasound in lung cancer patients with a normal mediastinum on computed tomography. Ann Thorac Surg. 2004;77(5):1763–1768. doi: 10.1016/j.athoracsur.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Stawinski GV, Lemaire A, Merchant F, et al. A comparative analysis of positron emission tomography and mediastinoscopy in staging non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;126(6):1900–1905. doi: 10.1016/s0022-5223(03)01036-5. [DOI] [PubMed] [Google Scholar]
- 18.Herth FJ, Ernst A, Eberhardt R, Vilmann P, Dienemann H, Krasnik M. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically normal mediastinum. Eur Respir J. 2006;28(5):910–914. doi: 10.1183/09031936.06.00124905. [DOI] [PubMed] [Google Scholar]
- 19.Herth FJ, Eberhardt R, Krasnik M, Ernst A. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest. 2008;133(4):887–891. doi: 10.1378/chest.07-2535. [DOI] [PubMed] [Google Scholar]
- 20.Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest. 2008;134(2):368–374. doi: 10.1378/chest.07-2105. [DOI] [PubMed] [Google Scholar]
- 21.Trisolini R, Cancellieri A, Tinelli C, et al. Rapid on-site evaluation of transbronchial aspirates in the diagnosis of hilar and mediastinal adenopathy: a randomized trial. Chest. 2011;139(2):395–401. doi: 10.1378/chest.10-1521. [DOI] [PubMed] [Google Scholar]