Abstract
Background:
Impaired lung function has been linked to obesity and systemic inflammation. Pericardial fat has been shown to be associated with anomalies in cardiac structure, function, and atherosclerosis. We hypothesized that pericardial fat may have a similar role in the impairment of lung function.
Methods:
Cross-sectional associations of pericardial fat volumes, quantified by multidetector CT scan, with FEV1 and FVC assessed by spirometry, were investigated in 1,293 participants (54.5 ± 10.8 years; 66.4% women) in the Jackson Heart Study. We also examined whether these associations were independent of visceral adipose tissue (VAT).
Results:
Pericardial fat was associated with impaired lung function after multivariable adjustment, but these associations generally did not remain after adjustment for VAT. An exception was the FEV1/FVC ratio. Higher pericardial fat volumes were associated with higher odds of a restrictive lung pattern and lower odds of airway obstruction. Participants in the highest quartile had the highest odds of a restrictive lung pattern (OR, 1.85; 95% CI, 1.22-2.79, compared with quartile 1), even after adjustment for VAT. The odds of obstruction decreased across increasing quartiles of pericardial fat. These relationships were generally graded, suggesting dose-response trends.
Conclusions:
Pericardial fat is generally associated with lower lung function and independently associated with a restrictive lung pattern in middle-aged and elderly adults. Further research is needed to fully understand the mechanisms through which pericardial fat contributes to pulmonary anomalies.
Pericardial fat is an active endocrine organ found in the thoracic cavity1 that has paracrine and mechanical effects on neighboring anatomic structures. Pericardial fat has been shown to be associated with cardiac anomalies in left ventricular and right ventricular (RV) structure and function2,3 and coronary and aortic atherosclerosis,4,5 although many of these associations were not independent of visceral fat. Given the close proximity of pericardial fat to the pulmonary outflow tract and lungs, pericardial fat may have similar effects on the lungs. First, epicardial fat deposits6 and pulmonary artery constriction7 have been shown to increase RV end-diastolic pressure. Pericardial fat may compress the pulmonary artery, thereby increasing RV pressure and impairing lung function. Pericardial fat may also compress the vasculature of the lungs and contribute to pulmonary fibrotic processes and lung function impairment. Last, pericardial fat correlates with systemic inflammatory and oxidative stress biomarkers8 that have been shown to be associated with impaired lung function.9
The purpose of the present study was to examine the cross-sectional associations of pericardial fat, abdominal fat, and lung function as measured by spirometry. We hypothesized that higher pericardial fat volumes would be associated with impaired lung function, independent of visceral fat.
Methods and Materials
Study Population
The Jackson Heart Study is a large, population-based observational study evaluating the etiology of cardiovascular, renal, and respiratory diseases. The study design and recruitment protocol have been described previously.10,11 A total of 5,301 participants underwent clinical examinations (2000-2004) including spirometry, provided blood specimens, and completed medical and health histories. Of these participants, 1,414 participants (26.7%) underwent multidetector CT scanning of the chest and abdomen (2007-2009). Participants were excluded for the following reasons: spirometry did not meet American Thoracic Society (ATS) recommendations12 for acceptability of each maneuver and test repeatability (n = 72), inadequate pericardial fat volumes (n = 3), missing adiposity measures (n = 20), and an incomplete covariate profile (n = 26). This left 1,293 participants for analysis. The study protocol (project approval # 1998-6004) was approved by the institutional review boards of the three Jackson Heart Study institutions (Jackson State University, Tougaloo College, and the University of Mississippi Medical Center), and informed consent was obtained from all participants.
Multidetector CT Scanning
Continuous scout images of cardiac and abdominal fat were undertaken by multidetector CT scanning (GE Healthcare Lightspeed 16 Pro; Milwaukee, Wisconsin) performed at the Jackson Medical Mall and assessed at the Wake Forest University CT Reading Center. Based on standard protocol,13 an average of 52 2.5-mm-thick slices were taken for cardiac gated CT scans of the coronary arteries in the supine position. The volume analysis software tool (GE Healthcare; Waukesha, Wisconsin) was used to discern fat from the remainder of the heart, with a threshold of −190 to −30 Hounsfield units. The pericardium was manually traced and pericardial fat was defined by any adipose tissue (AT) located in the pericardial sac.2‐4 Interreader reproducibility was assessed by two independent readers measuring pericardial fat on a subset of 60 randomly selected scans. The interclass correlation coefficient for pericardial fat was 0.96. Quantification of visceral AT (VAT) and subcutaneous AT (SAT) has been described previously.3,4
Spirometry
Computerized spirometry was performed in accord to ATS guidelines12 and measured using a dry rolling seal spirometer (Occupational Marketing; Houston, Texas). To adjust for age, age2, and height, we used published race- and sex-specific prediction equations for FEV1 and FVC.14 A restrictive lung pattern was defined as a FEV1/FVC ratio greater than the lower limit of normal (LLN) with either a normal or below the LLN FEV1 and FVC % predicted.15 Obstruction was defined as FEV1/FVC ratio < LLN with either a normal or below the LLN FEV1 and FVC % predicted.15
Covariates
BMI (kg/m2) was calculated as weight divided by height. Waist circumference (WC) was measured at the level of the umbilicus and rounded to the nearest centimeter. Cigarette smoking status was categorized as current, former, and never smoker, and pack-years of smoking (for former and current smokers) was defined as the number of years of smoking times the average number of cigarettes smoked per day divided by 20. Physical activity was defined as a summary score of the intensity, frequency, and duration of activities associated with active living, including transportation and leisure time activities.16 Respiratory medications used within 2 weeks of the baseline clinic visit were categorized as belonging to at least one of eight classes.17
Current asthma was defined as either (1) an affirmative response to the questions “Have you ever had asthma?”, “Has it been confirmed by a doctor?”, and “Do you still have asthma?” or (2) actual asthma medication use.17 Former asthma was defined as a negative response to the question “Do you still have asthma?” Self-reported lung disease was defined as an affirmative response to the question “Has a physician or doctor ever told you that you have lung disease such as emphysema or chronic bronchitis?”
C-reactive protein (CRP) was obtained in duplicate by immunoturbidimetric CRP-Latex assay (Kamiya Biomedical Company; Seattle, Washington) using a Hitachi 911 analyzer (Roche Diagnostics; Indianapolis, Indiana). Diabetes was defined as a fasting serum glucose ≥ 126 mg/dL or use of insulin or oral hypoglycemic medications within 2 weeks of the clinic examination. Prevalent cardiovascular disease (CVD) was defined as a history of a physician-diagnosed myocardial infarction, stroke, or coronary revascularization or evidence of a myocardial infarction by ECG by an expert panel of three cardiologists.
Statistical Analysis
The distribution of all adiposity measures were standardized to a mean of 0 and an SD of 1. Age-adjusted Pearson correlation coefficients were computed to assess the association between pericardial fat, abdominal fat, and FEV1 and FVC % predicted and FEV1/FVC ratio. Multivariable linear and logistic regression models were used to estimate the associations between pericardial fat and lung function and pattern. Models were generated in stages: Model 1 adjusted for sex, education, cigarette smoking status, pack-years of smoking, physical activity, and respiratory medication use (multivariable adjusted), and model 2 further adjusted for VAT. Two-way interactions between adiposity measures and sex with respect to the three lung function outcomes were formally tested by adding product terms in multivariable adjusted models. Since no statistically significant interactions with adiposity measures (in separate models) were observed in multivariable-adjusted models (all P > .10), subsequent analyses were pooled and adjusted for sex.
In secondary analyses, the multivariable models (model 1) were (1) adjusted for CRP and examined excluding (2) ever smokers and (3) prevalent diabetes and CVD. We examined these associations in participants with a stable BMI ( < 5% increase) from examination 1 to examination 2 in sensitivity analyses. All statistical analyses were conducted in the Statistical Analysis Software (SAS), version 9.2 (SAS Institute, Inc; Cary, North Carolina).
Results
Our sample consisted of 858 women and 435 men, with a mean age of 54.5 ± 10.8 years. Men had larger pericardial fat volumes than women (66.9 ± 28.3 cm3 vs 79.2 ± 35.1 cm3) (Table 1) as well as lower unadjusted mean % predicted and ratio values. Pericardial fat was most strongly correlated with VAT, followed by WC, BMI, and SAT in women and men (Table 2).
Table 1.
—Selected Baseline Characteristics by Sex Among Jackson Heart Study Participants Who Have Valued Multidetector CT Scan and Acceptable Spirometry
| Characteristic | Women (n = 858) | Men (n = 435) |
| Age, y | 55.1 (10.9) | 53.3 (10.7) |
| Pericardial fat, cm3 | 66.9 (28.3) | 79.2 (35.1) |
| Visceral adipose tissue, cm3 | 790.6 (360.6) | 850.4 (397.4) |
| Subcutaneous adipose tissue, cm3 | 2,636.0 (945.8) | 1,724.1 (808.0) |
| Waist circumference, cm | 100.7 (15.3) | 102.8 (12.8) |
| BMI, kg/m2 | 32.6 (6.9) | 29.8 (4.9) |
| Total body weight, kg | 87.5 (19.3) | 94.5 (17.5) |
| FEV1 % predicted | 94.0 (17.5) | 91.6 (16.2) |
| FVC % predicted | 92.1 (16.3) | 90.7 (14.7) |
| FEV1/FVC ratio | 81.6 (8.9) | 80.1 (8.5) |
| Restrictive lung pattern, % | 8.9 (76) | 8.5 (37) |
| Obstruction, % | 5.0 (43) | 7.8 (34) |
| Current asthma,a % | 7.0 (60) | 3.3 (14) |
| Former asthma,a % | 3.0 (26) | 4.9 (21) |
| Lung disease, % | 7.7 (66) | 5.1 (22) |
| < High school diploma, % | 10.8 (93) | 12.0 (52) |
| Current smoker, % | 7.3 (63) | 12.9 (56) |
| Former smoker, % | 14.6 (125) | 25.3 (110) |
| Pack-years of smoking | 4.3 (12.1) | 9.3 (17.0) |
| Physical activity score | 2.1 (0.8) | 2.2 (0.8) |
| Respiratory medication use, % | 7.1 (61) | 2.8 (12) |
| C-reactive protein,a,b mg/dL | 1.4 | 1.2 |
| Diabetes,a % | 15.5 (131) | 13.3 (57) |
| Cardiovascular disease,a % | 6.3 (54) | 7.4 (32) |
Data represent mean (SD) or % (No.).
Missing values: asthma: n = 1,287; C-reactive protein: n = 1,273; diabetes: n = 1,275; cardiovascular disease: n = 1,285.
Geometric mean.
Table 2.
—Correlation Coefficients Between Indicators of Adiposity by Sex Among Jackson Heart Study Participants Who Have Valued Multidetector CT Scan and Acceptable Spirometry
| Indicator | Pericardial Fat | VAT | SAT | WC | BMI |
| Pericardial fat | 1.0 | 0.667 | 0.299 | 0.495 | 0.404 |
| VAT | 0.770 | 1.0 | 0.420 | 0.675 | 0.570 |
| SAT | 0.407 | 0.391 | 1.0 | 0.753 | 0.826 |
| WC | 0.532 | 0.574 | 0.876 | 1.0 | 0.858 |
| BMI | 0.448 | 0.492 | 0.847 | 0.891 | 1.0 |
The upper portion is for women and the lower portion is for men. All P < .001. SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue; WC = waist circumference.
Adiposity was correlated with the lung function measures, with the exception of VAT and FEV1/FVC ratio (Table 3). Among women, the negative correlations ranged from −0.12 to −0.19 (FEV1 % predicted) and −0.18 to −0.24 (FVC % predicted), and the positive correlations ranged from 0.06 to 0.12 (FEV1/FVC ratio). Similar correlations were observed among men.
Table 3.
—Age-Adjusted Pearson Correlation Coefficients Between Cardiac and Visceral Adiposity and Obesity and Lung Function by Sex Among Jackson Heart Study Participants Who Have Valued Multidetector CT Scan and Acceptable Spirometry
| Adiposity Measure | FEV1 % Predicted | FVC % Predicted | FEV1/FVC Ratio |
| Women | |||
| Pericardial fat, cm3 | −0.12a | −0.20a | 0.12a |
| Visceral adipose tissue, cm3 | −0.18a | −0.22a | 0.06 |
| Subcutaneous adipose tissue, cm3 | −0.17a | −0.23a | 0.08b |
| Waist circumference, cm | −0.19a | −0.24a | 0.08c |
| BMI, kg/m2 | −0.13a | −0.18a | 0.08c |
| Men | |||
| Pericardial fat, cm3 | −0.14a | −0.21a | 0.11c |
| Visceral adipose tissue, cm3 | −0.17a | −0.21a | 0.06 |
| Subcutaneous adipose tissue, cm3 | −0.21a | −0.31a | 0.13b |
| Waist circumference, cm | −0.20a | −0.29a | 0.12c |
| BMI, kg/m2 | −0.10c | −0.21a | 0.17a |
P < .001.
P < .01.
P < .05.
All adiposity measures were inversely associated with FEV1 and FVC % predicted and positively associated with FEV1/FVC ratio (except for VAT) in multivariable adjusted models (Table 4). The mean difference in FEV1 and FVC % predicted per SD increase in VAT, SAT, WC, and BMI was significantly lower (greater reduction) than the mean difference per SD increase of pericardial fat (all P < .05). The mean difference in FEV1/FVC ratio per SD increase in SAT and BMI was significantly higher than the mean difference per SD of pericardial fat (all P < .05). Only the significant association between pericardial fat and FEV1/FVC ratio persisted after controls for VAT.
Table 4.
—Standardized Multivariable Adjusted Mean Differences in Lung Function per SD of Adiposity and Obesity Among Jackson Heart Study Participants Who Have Valued Multidetector CT Scan and Acceptable Spirometry
| Measure | MV Adjusteda | P Value | MV Adjusted + VAT | P Value | P Value for Sex Interaction |
| FEV1 % predicted | |||||
| Pericardial fat, cm3 | −1.03 ± 0.47 | .029 | 0.67 ± 0.63 | .289 | .272 |
| VAT,b cm3 | −2.11 ± 0.47 | < .001 | … | … | .676 |
| SAT, cm3 | −3.02 ± 0.46 | < .001 | −2.61 ± 0.50 | < .001 | .662 |
| WC, cm | −2.88 ± 0.46 | < .001 | −2.58 ± 0.59 | < .001 | .860 |
| BMI, kg/m2 | −1.91 ± 0.46 | < .001 | −1.11 ± 0.55 | .043 | .563 |
| FVC % predicted | |||||
| Pericardial fat, cm3 | −2.29 ± 0.44 | < .001 | −0.75 ± 0.58 | .198 | .249 |
| VAT,b cm3 | −2.80 ± 0.43 | < .001 | … | … | .618 |
| SAT, cm3 | −3.94 ± 0.42 | < .001 | −3.38 ± 0.46 | < .001 | .342 |
| WC, cm | −3.72 ± 0.42 | < .001 | −3.27 ± 0.54 | < .001 | .486 |
| BMI, kg/m2 | −2.87 ± 0.43 | < .001 | −1.95 ± 0.51 | < .001 | .790 |
| FEV1/FVC ratio | |||||
| Pericardial fat, cm3 | 0.85 ± 0.25 | < .001 | 1.10 ± 0.33 | < .001 | .836 |
| VAT,b cm3 | 0.35 ± 0.24 | .150 | … | … | .807 |
| SAT, cm3 | 1.12 ± 0.24 | < .001 | 1.18 ± 0.26 | < .001 | .659 |
| WC, cm | 0.89 ± 0.24 | < .001 | 1.11 ± 0.31 | < .001 | .655 |
| BMI, kg/m2 | 1.19 ± 0.24 | < .001 | 1.42 ± 0.28 | < .001 | .186 |
MV = multivariable. See Table 2 legend for expansion of other abbreviations.
MV adjustment includes sex, education, cigarette smoking status, pack-years of smoking, respiratory medication use, and physical activity.
Models considering VAT as the independent variable were not further adjusted for VAT in Model 2.
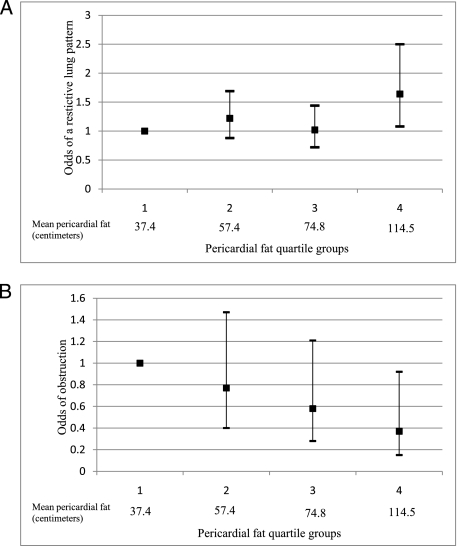
Pericardial fat was positively associated with the odds of a restrictive lung pattern: participants in the highest quartile had the highest odds of a restrictive pattern (OR, 1.85; 95% CI, 1.22-2.79, compared with quartile 1), even after adjustment for VAT (Fig 1). The odds of obstruction decreased across increasing quartiles of pericardial fat; participants in the highest quartile had the lowest odds of obstruction (OR, 0.37; 95% CI, 0.15-0.92). These relationships were generally graded, suggesting dose-response trends.
Figure 1.
A, Fully adjusted odds of a restrictive lung pattern among participants in the Jackson Heart Study. B, Fully adjusted odds of airway obstruction among participants in the Jackson Heart Study. A restrictive lung pattern was defined as a FEV1/FVC ratio greater than the lower limit of normal (LLN) with either a normal or below the LLN FEV1 and FVC % predicted. Obstruction was defined as a FEV1/FVC ratio less than the LLN with either a normal or below the LLN FEV1 and FVC % predicted. The fully adjusted model includes education, cigarette smoking status, pack-years of cigarette smoking, physical activity, respiratory medication use, and visceral adipose tissue volume.
Adjustment for CRP did not attenuate the associations between pericardial fat and FVC % predicted or the FEV1/FVC ratio (Table 5). In multivariable models excluding ever-smokers, pericardial fat was associated with lung function (except FEV1 % predicted). Similarly, pericardial fat was associated with lung function (except FEV1 % predicted) in analyses excluding prevalent type 2 diabetes and CVD. Slight sex differences were observed between pericardial fat and FEV1 % predicted in models excluding ever smokers and prevalent diabetes and CVD (e-Table 1). Additional adjustment for self-reported physician-diagnosed asthma and lung disease or restricting these analyses to participants with a stable BMI did not materially alter these associations (results not shown).
Table 5.
—Standardized Mean Differences in Lung Function per SD Increase in Pericardial Fat in Multivariable Adjusted Models Among Jackson Heart Study Participants Who Have Valued Multidetector CT and Acceptable Spirometry
| Measure | MV Adjusted | P Value | P Value for Sex Interaction |
| CRP adjustmenta (n = 1,273) | |||
| FEV1 % predicted | −0.54 ± 0.49 | .265 | .320 |
| FVC % predicted | −1.78 ± 0.45 | < .001 | .293 |
| FEV1/FVC ratio | 0.81 ± 0.25 | .001 | .808 |
| Excluding ever smokersb (n = 939) | |||
| FEV1 % predicted | −1.02 ± 0.59 | .083 | .659 |
| FVC % predicted | −2.25 ± 0.54 | < .001 | .295 |
| FEV1/FVC ratio | 0.79 ± 0.31 | .011 | .828 |
| Excluding T2D and CVDa (n = 1,064) | |||
| FEV1 % predicted | −0.83 ± 0.53 | .116 | .066 |
| FVC % predicted | −1.89 ± 0.49 | < .001 | .062 |
| FEV1/FVC ratio | 0.68 ± 0.28 | .015 | .870 |
CRP = C reactive protein; CVD = cardiovascular disease; T2D = type 2 diabetes. See Table 4 legend for expansion of other abbreviation.
Multivariable adjusted model (model 1) includes sex, education, cigarette smoking status, pack-years of smoking, respiratory medication use, and physical activity.
Multivariable adjusted model (model 1) includes sex, education, respiratory medication use, and physical activity.
Discussion
This is the first study, to our knowledge, to investigate associations between pericardial fat, abdominal fat, and lung function and pattern among middle-aged and elderly adults in the general population with standardized spirometry. In both women and men, greater pericardial fat volumes were associated with lower FEV1 and FVC % predicted values and a higher FEV1/FVC ratio. After further adjustment for VAT and CRP, in separate models, higher pericardial fat volumes were associated with a lower FVC % predicted (in models adjusted for CRP) and a higher FEV1/FVC ratio. In multivariable models excluding ever smokers and prevalent type 2 diabetes and CVD, pericardial fat was generally inversely associated with FVC % predicted and positively associated with FEV1/FVC ratio. Greater pericardial fat volumes were also associated with higher odds of a restrictive lung pattern and lower odds of obstruction, even after controlling for VAT. These results indicate that pericardial fat may not be a better correlate of impaired lung function than the systemic effects of VAT or other generalized adiposity measures but suggest that pericardial fat is associated with a restrictive lung pattern rather than airway obstruction, independent of VAT.
Comparison With Previous Studies
There are no existing studies in which to directly contrast the findings with pericardial fat. Recent studies have found that abdominal obesity is associated with impaired lung function18,19 and a restrictive lung pattern.20 The findings we observed with abdominal adiposity are consistent with previous epidemiologic studies exploring associations of fat distribution and overall adiposity with respiratory function in children21 and adults.18,19,22,23
Potential Mechanisms
Pericardial fat may mechanistically influence pulmonary function and a restrictive lung pattern through compression of the pulmonary artery. RV diastolic pressure has been shown to be positively associated with epicardial fat in humans,6 and in anesthetized dogs, constriction of the pulmonary artery increased RV diastolic pressure.7 Pericardial fat may indeed compress the pulmonary artery, thereby increasing pulmonary artery systolic pressure (PASP) and contributing to a restrictive lung pattern. A study of obese subjects undergoing autopsy demonstrated a higher frequency of pulmonary edema and pulmonary hypertensive changes, including venous hypertension and capillary hemangiomatosis, than healthy age-matched control subjects.24 PASP has been shown to be associated with lower FEV1 and FVC, although this association was not independent of age and systemic circulation.25 Future analyses are warranted to determine whether pericardial fat is associated with PASP and lung function impairment.
Pericardial fat may also reduce lung function and contribute to a restrictive lung pattern through the development of pulmonary fibrotic diseases.24,26 Idiopathic pulmonary fibrosis is the pathologic scarring of lung tissue in response to microscopic injury, which results in the loss of lung contractility and a restrictive lung pattern. This hypoxic vasoconstriction may lead to the obliteration of the vasculature and pulmonary hypertension.27 A recent review of respiratory disorders, including pulmonary fibrosis, documented a number of pulmonary vascular abnormalities within these conditions.26 Vessel compression, for example, may lead to fibrous organization of vessels.28 Therefore, future research should investigate whether pericardial fat may contribute to pulmonary vascular abnormalities and a restrictive lung pattern.
Pericardial fat shares the same blood supply as the lungs and may exert a locally adverse effect on lung function through the expression of inflammatory biomarkers. Cardiac adiposity biopsies from patients undergoing coronary artery bypass grafting demonstrated that epicardial fat is a source of chronic inflammatory biomarkers.29 Recent epidemiologic studies have demonstrated that pericardial fat is correlated with inflammatory and oxidative stress biomarkers.8 The present data extend the literature by providing the first population-based evidence that pericardial fat is associated with lung function, generally independent of CRP, suggesting that pericardial fat may exert deleterious effects on the lungs through local inflammatory processes. Additional work is needed to understand the secretion of these cytokines directly into the lumen of pulmonary arteries and the effects on the alveolar-capillary network of the lungs.
We cannot dismiss the possibility that obstruction is masked by obesity30 through reduction of FVC or rule out the influence of sitting during spirometry. In a sample of obese men, obesity similarly reduced FEV1 and FVC (% predicted) and resulted in normal FEV1/FVC ratio and static lung volumes.31 However, the FEV1/FVC ratio has been shown to decrease with increasing BMI in overweight (25 ≤ BMI < 30 kg/m2), obese (BMI ≥ 30 kg/m2),32 and morbidly obese (BMI ≥ 40 kg/m2) individuals.33 No significant effect modification of BMI on the association between pericardial fat and lung function was observed (all P > .10) in the present study, and stratification by BMI category did not materially change the associations among normal weight, overweight, and obese participants (results not shown). Moreover, participants performed spirometry in the seated position. In a study among obese individuals, small but statistically significant differences have been observed in FVC in seated spirometry.34
Study strengths include a comprehensive and highly reproducible volumetric, rather than thickness, technique for pericardial fat and VAT quantification; the large sample size with wide ranges in age and BMI (although 64% of the sample population was classified as obese), which reduces the risk of ascertainment bias; and adjustment for a large panel of potential confounders. The current study is specific for African Americans and may not be generalizable to other racial or less obese populations. The cross-sectional design precludes determination of causal pathways between pericardial fat and lung function. In this study, we were unable to classify airway restriction according to ATS guidelines, and misclassification of pericardial fat may be due to combined measurement of AT in the pericardium. Additional limitations include the absence of lung volumes and carbon monoxide diffusing capacity to more appropriate classify COPD, especially in obese persons.
Pericardial fat shares the same traits as VAT.35 Our novel results suggest that pericardial fat volumes are inversely associated with lung function, although not independent of VAT, and are associated with higher odds of a restrictive pattern of lung function. Impaired lung function has been linked to increased cardiovascular risk,36,37 and pericardial fat may be an important mediator between impaired pulmonary function and CVD mortality.
Supplementary Material
Acknowledgments
Author contributions: Dr Hickson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Hickson: contributed to study concept and design, analysis and interpretation of data, drafting of the manuscript, and statistical analysis.
Dr Liu: contributed to study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content, and obtaining funding.
Dr Bidulescu: contributed to study concept and design and critical revision of the manuscript for important intellectual content.
Dr Burchfiel: contributed to analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
Dr Taylor: contributed to acquisition of data, critical revision of the manuscript for important intellectual content, and obtaining funding.
Dr Petrini: contributed to study supervision and critical revision of the manuscript for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Nationals Institutes of Health or the National Institute for Occupational Safety and Health. The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We thank the Jackson Heart Study participants, staff, and interns for their long-term commitment to the study. This work was performed at Jackson State University and the University of Mississippi Medical Center.
Additional information: The e-Table can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/140/6/1567/DC1.
Abbreviations
- AT
adipose tissue
- ATS
American Thoracic Society
- CRP
C-reactive protein
- CVD
cardiovascular disease
- LLN
lower limit of normal
- PASP
pulmonary artery systolic pressure
- RV
right ventricular
- SAT
subcutaneous adipose tissue
- VAT
visceral adipose tissue
- WC
waist circumference
Footnotes
Funding/Support: This work was supported by National Institutes of Health: National Heart Lung, and Blood Institute, and the National Center on Minority Health and Health Disparities [Contracts N01-HC-95170, N01-HC-95171, and N01-HC-95172].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153(6):907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Gona P, Hoffmann U, et al. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009;119(12):1586–1591. doi: 10.1161/CIRCULATIONAHA.108.828970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Fox CS, Hickson DA, et al. Pericardial fat and echocardiographic measures of cardiac abnormalities: the Jackson Heart Study. Diabetes Care. 2011;34(2):341–346. doi: 10.2337/dc10-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Fox CS, Hickson D, et al. Pericardial adipose tissue, atherosclerosis, and cardiovascular disease risk factors: the Jackson heart study. Diabetes Care. 2010;33(7):1635–1639. doi: 10.2337/dc10-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117(5):605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 6.Iacobellis G. Relation of epicardial fat thickness to right ventricular cavity size in obese subjects. Am J Cardiol. 2009;104(11):1601–1602. doi: 10.1016/j.amjcard.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 7.Nelson GS, Sayed-Ahmed EY, Kroeker CA, et al. Compression of interventricular septum during right ventricular pressure loading. Am J Physiol Heart Circ Physiol. 2001;280(6):H2639–H2648. doi: 10.1152/ajpheart.2001.280.6.H2639. [DOI] [PubMed] [Google Scholar]
- 8.Tadros TM, Massaro JM, Rosito GA, et al. Pericardial fat volume correlates with inflammatory markers: the Framingham Heart Study. Obesity (Silver Spring) 2010;18(5):1039–1045. doi: 10.1038/oby.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aronson D, Roterman I, Yigla M, et al. Inverse association between pulmonary function and C-reactive protein in apparently healthy subjects. Am J Respir Crit Care Med. 2006;174(6):626–632. doi: 10.1164/rccm.200602-243OC. [DOI] [PubMed] [Google Scholar]
- 10.Taylor HA, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005 15(4)(suppl 6):S6-4-17. [PubMed] [Google Scholar]
- 11.Fuqua SR, Wyatt SB, Andrew ME, et al. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005 15(4)(suppl 6):S6-18-29. [PubMed] [Google Scholar]
- 12.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 13.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144(5):1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 16.Smitherman TA, Dubbert PM, Grothe KB, et al. Validation of the Jackson Heart Study physical activity survey in African Americans. J Phys Act Health. 2009;6(suppl 1):S124–S132. doi: 10.1123/jpah.6.s1.s124. [DOI] [PubMed] [Google Scholar]
- 17.Hickson DA, Wilhite RL, Petrini MF, White WB, Burchfiel C. Asthma and asthma severity among African American adults in the Jackson Heart Study. J Asthma. 2009;46(4):421–428. doi: 10.1080/02770900902846307. [DOI] [PubMed] [Google Scholar]
- 18.Leone N, Courbon D, Thomas F, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179(6):509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 19.Ochs-Balcom HM, Grant BJ, Muti P, et al. Pulmonary function and abdominal adiposity in the general population. Chest. 2006;129(4):853–862. doi: 10.1378/chest.129.4.853. [DOI] [PubMed] [Google Scholar]
- 20.Jung DH, Shim JY, Ahn HY, Lee HR, Lee JH, Lee YJ. Relationship of body composition and C-reactive protein with pulmonary function. Respir Med. 2010;104(8):1197–1203. doi: 10.1016/j.rmed.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Rennie D, Cormier Y, Dosman JA. Waist circumference associated with pulmonary function in children. Pediatr Pulmonol. 2009;44(3):216–221. doi: 10.1002/ppul.20854. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Rennie D, Cormier YF, Dosman J. Waist circumference is associated with pulmonary function in normal-weight, overweight, and obese subjects. Am J Clin Nutr. 2007;85(1):35–39. doi: 10.1093/ajcn/85.1.35. [DOI] [PubMed] [Google Scholar]
- 23.Steele RM, Finucane FM, Griffin SJ, Wareham NJ, Ekelund U. Obesity is associated with altered lung function independently of physical activity and fitness. Obesity (Silver Spring) 2009;17(3):578–584. doi: 10.1038/oby.2008.584. [DOI] [PubMed] [Google Scholar]
- 24.Haque AK, Gadre S, Taylor J, Haque SA, Freeman D, Duarte A. Pulmonary and cardiovascular complications of obesity: an autopsy study of 76 obese subjects. Arch Pathol Lab Med. 2008;132(9):1397–1404. doi: 10.5858/2008-132-1397-PACCOO. [DOI] [PubMed] [Google Scholar]
- 25.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119(20):2663–2670. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han MK, McLaughlin VV, Criner GJ, Martinez FJ. Pulmonary diseases and the heart. Circulation. 2007;116(25):2992–3005. doi: 10.1161/CIRCULATIONAHA.106.685206. [DOI] [PubMed] [Google Scholar]
- 27.Nathan SD, Noble PW, Tuder RM. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med. 2007;175(9):875–880. doi: 10.1164/rccm.200608-1153CC. [DOI] [PubMed] [Google Scholar]
- 28.Crystal RG, Gadek JE, Ferrans VJ, Fulmer JD, Line BR, Hunninghake GW. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med. 1981;70(3):542–568. doi: 10.1016/0002-9343(81)90577-5. [DOI] [PubMed] [Google Scholar]
- 29.Baker AR, Silva NF, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams WT, Petrini MF. Spirometry in obese and normal weight smokers [abstract] Chest. 2008;134(4):s50002. [Google Scholar]
- 31.Sahebjami H, Gartside PS. Pulmonary function in obese subjects with a normal FEV1/FVC ratio. Chest. 1996;110(6):1425–1429. doi: 10.1378/chest.110.6.1425. [DOI] [PubMed] [Google Scholar]
- 32.Lazarus R, Sparrow D, Weiss ST. Effects of obesity and fat distribution on ventilatory function: the normative aging study. Chest. 1997;111(4):891–898. doi: 10.1378/chest.111.4.891. [DOI] [PubMed] [Google Scholar]
- 33.Biring MS, Lewis MI, Liu JT, Mohsenifar Z. Pulmonary physiologic changes of morbid obesity. Am J Med Sci. 1999;318(5):293–297. doi: 10.1097/00000441-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Gudmundsson G, Cerveny M, Shasby DM. Spirometric values in obese individuals. Effects of body position. Am J Respir Crit Care Med. 1997;156(3 pt 1):998–999. doi: 10.1164/ajrccm.156.3.9609089. [DOI] [PubMed] [Google Scholar]
- 35.Ho RJ, Fan CC, Barrera LA. Comparison of adipose glycerol kinase of hyperglycemic obese mice and lean litter-mates. Mol Cell Biochem. 1979;27(2):89–96. doi: 10.1007/BF00218353. [DOI] [PubMed] [Google Scholar]
- 36.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107(11):1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 37.Engström G, Lind P, Hedblad B, et al. Lung function and cardiovascular risk: relationship with inflammation-sensitive plasma proteins. Circulation. 2002;106(20):2555–2560. doi: 10.1161/01.cir.0000037220.00065.0d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.