Abstract
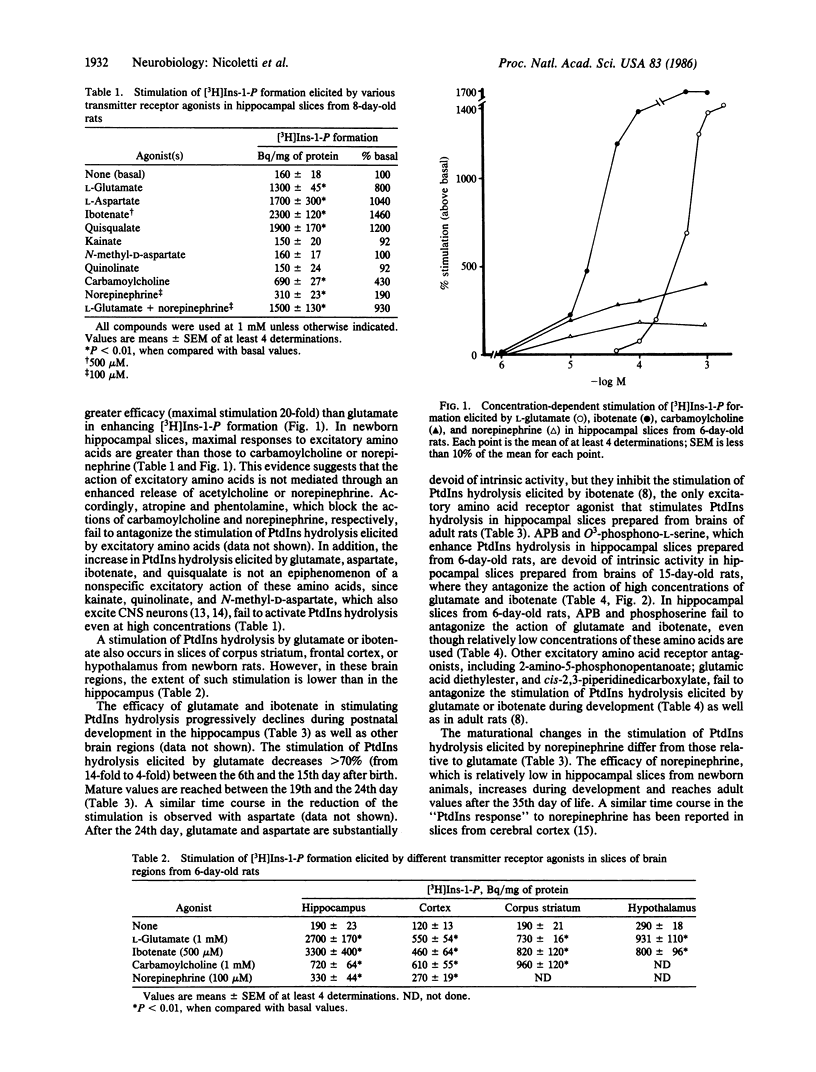
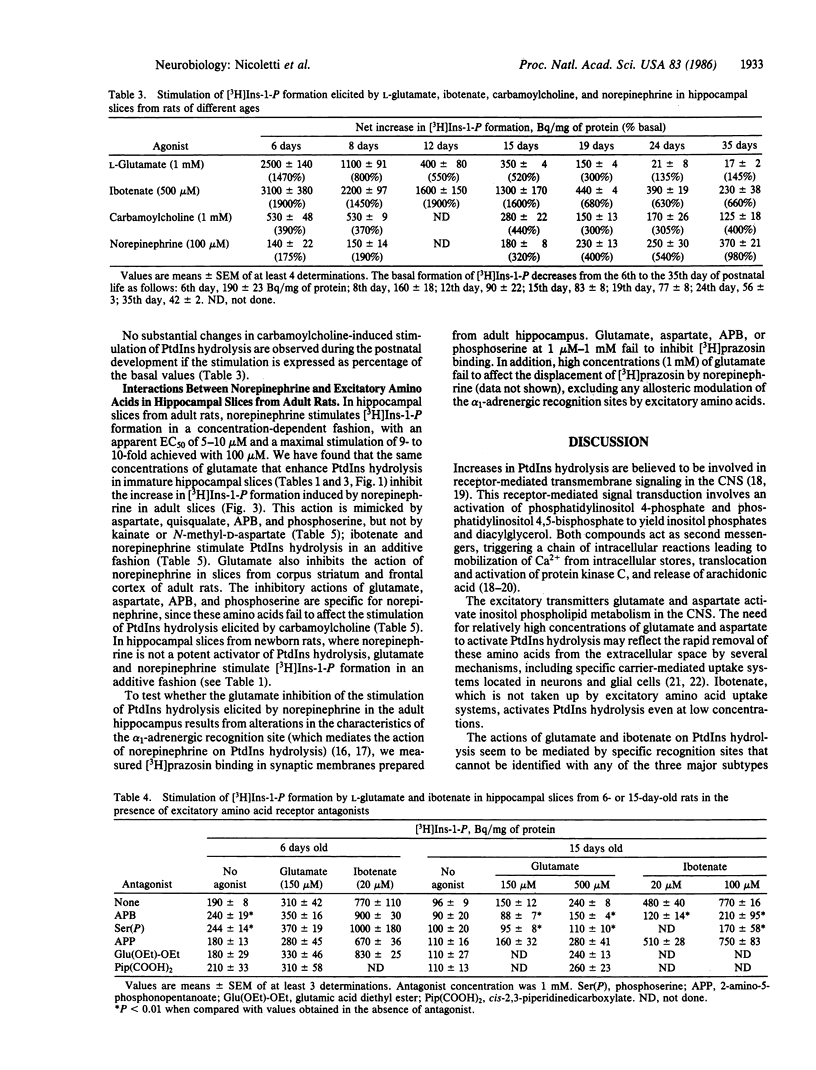
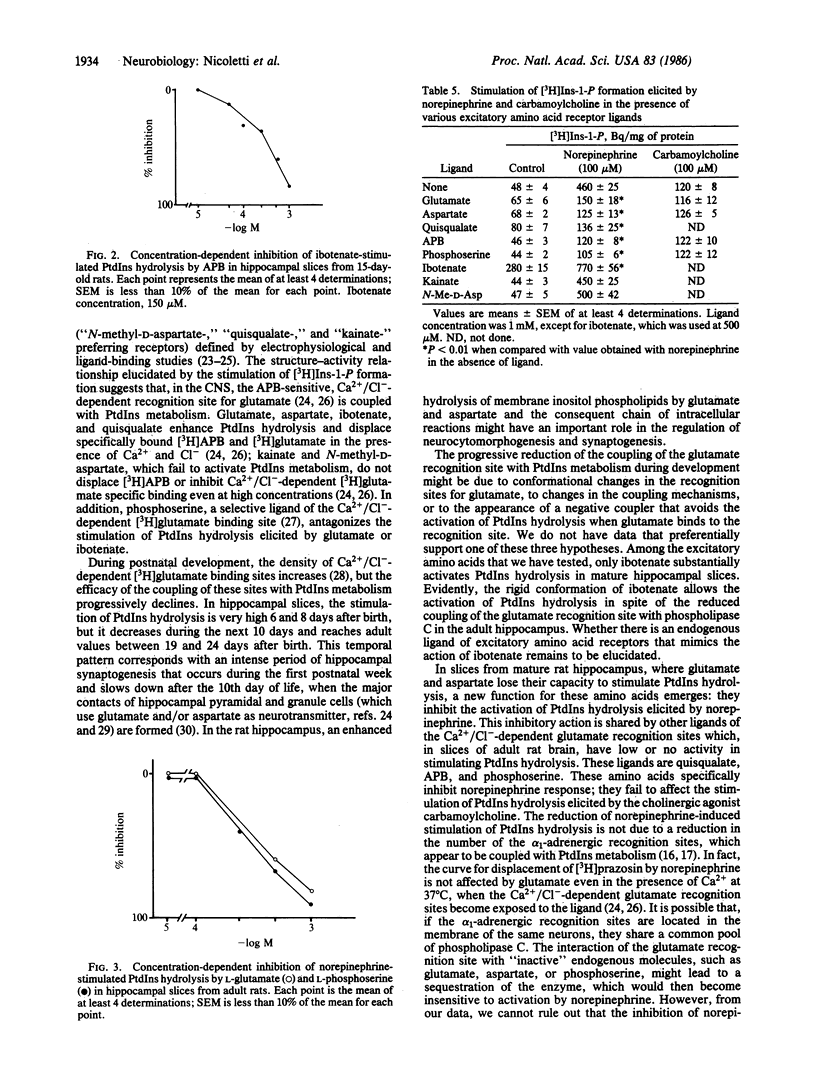
Glutamate, aspartate, ibotenate, and quisqualate activate inositol phospholipid hydrolysis in hippocampal slices prepared from brains of 6- to 8-day-old rats. The stimulation by glutamate and aspartate progressively declines during postnatal development and is negligible after the 24th day of life. In contrast, the stimulation of inositol phospholipid hydrolysis by norepinephrine is low in hippocampal slices from newborn animals and increases during development, reaching mature values after the 35th day of life. In adult hippocampal slices, the stimulation of inositol phospholipid hydrolysis elicited by norepinephrine is inhibited by glutamate in a concentration-dependent fashion. This inhibition can also be brought about by aspartate, 2-amino-4-phosphonobutanoate, and L-phosphoserine, a product of endogenous phosphatidylserine hydrolysis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. H., Blisner M. E., Holland W. H., Hipps P. P., Sherman W. R. Increased brain myo-inositol 1-phosphate in lithium-treated rats. Biochem Biophys Res Commun. 1976 Jul 26;71(2):664–670. doi: 10.1016/0006-291x(76)90839-1. [DOI] [PubMed] [Google Scholar]
- Balcar V. J., Johnston G. A. High affinity uptake of transmitters: studies on the uptake of L-aspartate, GABA, L-glutamate and glycine in cat spinal cord. J Neurochem. 1973 Feb;20(2):529–539. doi: 10.1111/j.1471-4159.1973.tb12152.x. [DOI] [PubMed] [Google Scholar]
- Baudry M., Arst D., Oliver M., Lynch G. Development of glutamate binding sites and their regulation by calcium in rat hippocampus. Brain Res. 1981 Jan;227(1):37–48. doi: 10.1016/0165-3806(81)90092-4. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggio G., Guidotti A. Climbing fiver activation and 3', 5'-cyclic guanosine monophosphate (cGMP) content in cortex and deep nuclei of cerebellum. Brain Res. 1976 May 7;107(2):365–373. doi: 10.1016/0006-8993(76)90233-x. [DOI] [PubMed] [Google Scholar]
- Coyle J. T. Neurotoxic action of kainic acid. J Neurochem. 1983 Jul;41(1):1–11. doi: 10.1111/j.1471-4159.1983.tb11808.x. [DOI] [PubMed] [Google Scholar]
- Davies J., Evans R. H., Jones A. W., Smith D. A., Watkins J. C. Differential activation and blockade of excitatory amino acid receptors in the mammalian and amphibian central nervous systems. Comp Biochem Physiol C. 1982;72(2):211–224. doi: 10.1016/0306-4492(82)90086-7. [DOI] [PubMed] [Google Scholar]
- Fagg G. E., Foster A. C. Amino acid neurotransmitters and their pathways in the mammalian central nervous system. Neuroscience. 1983 Aug;9(4):701–719. doi: 10.1016/0306-4522(83)90263-4. [DOI] [PubMed] [Google Scholar]
- Ferrendelli J. A., Chang M. M., Kinscherf D. A. Elevation of cyclic GMP levels in central nervous system by excitatory and inhibitory amino acids. J Neurochem. 1974 Apr;22(4):535–540. doi: 10.1111/j.1471-4159.1974.tb06890.x. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Foster G. A., Roberts P. J. Kainic acid stimulation of cerebellar cyclic GMP levels: potentiation by glutamate and related amino acids. Neurosci Lett. 1981 Apr 9;23(1):67–70. doi: 10.1016/0304-3940(81)90188-9. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Cellular uptake disguises action of L-glutamate on N-methyl-D-aspartate receptors. With an appendix: diffusion of transported amino acids into brain slices. Br J Pharmacol. 1985 May;85(1):297–307. doi: 10.1111/j.1476-5381.1985.tb08860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. M., Michell R. H. Stimulus-response coupling at alpha-adrenergic receptors. Biochem Soc Trans. 1978;6(3):673–688. doi: 10.1042/bst0060673. [DOI] [PubMed] [Google Scholar]
- Kendall D. A., Brown E., Nahorski S. R. Alpha 1-adrenoceptor-mediated inositol phospholipid hydrolysis in rat cerebral cortex: relationship between receptor occupancy and response and effects of denervation. Eur J Pharmacol. 1985 Aug 7;114(1):41–52. doi: 10.1016/0014-2999(85)90518-7. [DOI] [PubMed] [Google Scholar]
- Koerner J. F., Cotman C. W. Micromolar L-2-amino-4-phosphonobutyric acid selectively inhibits perforant path synapses from lateral entorhinal cortex. Brain Res. 1981 Jul 6;216(1):192–198. doi: 10.1016/0006-8993(81)91288-9. [DOI] [PubMed] [Google Scholar]
- Luini A., Goldberg O., Teichberg V. I. Distinct pharmacological properties of excitatory amino acid receptors in the rat striatum: study by Na+ efflux assay. Proc Natl Acad Sci U S A. 1981 May;78(5):3250–3254. doi: 10.1073/pnas.78.5.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C. C., Guidotti A., Costa E. The regulation of cyclic guanosine monophosphate in rat cerebellum: possible involvement of putative amino acid neurotransmitters. Brain Res. 1974 Oct 25;79(3):510–514. doi: 10.1016/0006-8993(74)90449-1. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., McMills M. C., Chamberlin A. R., Cotman C. W. Synthesis of [3H]2-amino-4-phosphonobutyric acid and characterization of its binding to rat brain membranes: a selective ligand for the chloride/calcium-dependent class of L-glutamate binding sites. Brain Res. 1983 Nov 14;278(1-2):137–144. doi: 10.1016/0006-8993(83)90232-9. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Barbaccia M. L., Iadarola M. J., Pozzi O., Laird H. E., 2nd Abnormality of alpha 1-adrenergic receptors in the frontal cortex of epileptic rats. J Neurochem. 1986 Jan;46(1):270–273. doi: 10.1111/j.1471-4159.1986.tb12957.x. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Meek J. L., Iadarola M. J., Chuang D. M., Roth B. L., Costa E. Coupling of inositol phospholipid metabolism with excitatory amino acid recognition sites in rat hippocampus. J Neurochem. 1986 Jan;46(1):40–46. doi: 10.1111/j.1471-4159.1986.tb12922.x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Puil E. S-Glutamate: its interactions with spinal neurons. Brain Res. 1981 Dec;228(3):229–322. doi: 10.1016/0165-0173(81)90007-2. [DOI] [PubMed] [Google Scholar]
- Schoepp D. D., Rutledge C. O. Comparison of postnatal changes in alpha 1-adrenoceptor binding and adrenergic stimulation of phosphoinositide hydrolysis in rat cerebral cortex. Biochem Pharmacol. 1985 Aug 1;34(15):2705–2711. doi: 10.1016/0006-2952(85)90571-4. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Whetsell W. O., Jr, Mangano R. M. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983 Jan 21;219(4582):316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]



