Summary
Background
Human papillomaviruses (HPVs) cause genital warts and cancers in men. The natural history of HPV infection in men is largely unknown, and that information is needed to inform prevention strategies. The goal in this study was to estimate incidence and clearance of type-specific genital HPV infection in men, and to assess the associated factors.
Methods
Men (aged 18–70 years), residing in Brazil, Mexico, and the USA, who were HIV negative and reported no history of cancer were recruited from the general population, universities, and organised health-care systems. They were assessed every 6 months for a median follow-up of 27·5 months (18·0–31·2). Specimens from the coronal sulcus, glans penis, shaft, and scrotum were obtained for the assessment of the status of HPV genotypes.
Findings
In 1159 men, the incidence of a new genital HPV infection was 38·4 per 1000 person months (95% CI 34·3–43·0). Oncogenic HPV infection was significantly associated with having a high number of lifetime female sexual partners (hazard ratio 2·40, 1·38–4·18, for at least 50 partners vs not more than one partner), and number of male anal-sexual partners (2·57, 1·46–4·49, for at least three male partners vs no recent partners). Median duration of HPV infection was 7·52 months (6·80–8·61) for any HPV and 12·19 months (7·16–18·17) for HPV 16. Clearance of oncogenic HPV infection decreased in men with a high number of lifetime female partners (0·49, 0·31–0·76, for at least 50 female partners vs not more than one partner), and in men in Brazil (0·71, 0·56–0·91) and Mexico (0·73, 0·57–0·94) compared with the USA. Clearance of oncogenic HPV was more rapid with increasing age (1·02, 1·01–1·03).
Interpretation
The data from this study are useful for the development of realistic cost-effectiveness models for male HPV vaccination internationally.
Funding
National Cancer Institute.
Introduction
Infection with human papillomavirus (HPV) is the cause of several different diseases in men and women.1 In the USA, an estimated 32 000 cases of cancers in men and women in 2009 were attributable to HPV infection.2 These were cancers of the cervix, vagina, vulva, penis, oral cavity, head and neck, and anal canal. Anogenital warts are the most common outcome of HPV, with 205 cases per 100 000 diagnosed every year in the USA.3 In addition to the diseases HPV causes directly in men, the virus is readily transmitted from men to women and greatly affects the risk of disease in women.4–8 Because male sexual behaviour affects rates of HPV infection and disease in female partners, an improved understanding of the infection in men is an essential component of HPV-related cancer prevention.
Substantial progress has been made in our understanding of the efficacy of the interventions for prevention, such as vaccination to reduce HPV-related diseases in women.9 However, little is known about HPV infection in men, with only a few small prospective studies undertaken in Europe,10–12 Latin America,13 and the USA.14,15 Therefore, the optimum strategies for prevention of HPV infection in men are not known.
Our aim in this study was to define the incidence and clearance of type-specific HPV infection in a cohort of men who were residing in Brazil, Mexico, and the USA, and to assess infection-associated factors.
Methods
Study population
The HPV in Men (HIM) study was a continuous prospective study of the natural history of HPV infections in men in three countries. Men were eligible for participation if they were aged 18–70 years; residents of southern Florida, USA, São Paulo, Brazil, or Cuernavaca, Mexico; reported no previous diagnosis of penile or anal cancers; reported no previous diagnosis of genital or anal warts; had not participated in an HPV vaccine study; reported no previous diagnosis of HIV; reported no current penile discharge or burning during urination; were not being treated for sexually transmitted infection; had not been imprisoned or homeless during the past 6 months; had not received drug treatment during the past 6 months; had no plans to relocate in the next 4 years; and were willing to comply with ten scheduled visits every 6 months for 4 years. Men were recruited according to three age groups (18–30 years, 31–44 years, and 45–70 years) from three different population sources—general population, universities, and organised health-care systems. In Brazil, men were recruited from a large clinic in São Paulo that was providing genitourinary services, including tests for HIV and sexually transmitted infections, and the general population through television, radio, and newspaper advertisements. In Mexico, men were recruited in Cuernavaca and Morelos, through a large health plan, from factories and the military. In the USA, men were mainly recruited from the University of South Florida and the general community in Tampa, FL. A full description of cohort procedures, HPV prevalence, and factors associated with prevalent infections has already been reported.16,17
Men who were eligible to participate reviewed a written informed consent with a trained study team member who gave men the opportunity to ask questions and decline participation if they desired. Before study initiation, the human-subjects’ committees of the University of South Florida, FL, USA, the Ludwig Institute for Cancer Research, São Paulo, Brazil, the Centro de Referencia e Tratamento de Doencas Sexualmente Transmissiveis e AIDS, São Paulo, Brazil, and the National Institute of Public Health of Mexico, Cuernavaca, Mexico, approved all study procedures.
Men who provided consent underwent a clinical examination at a visit 2 weeks before the enrolment visit and every 6 months thereafter. Only men who returned for the enrolment visit were included in the study. Samples of penile and scrotal cells were obtained at each visit. Clinical and laboratory measurements were detection of HPV DNA by use of PCR, and genotyping. At each study visit, participants completed a computer-assisted self-interview questionnaire that we previously showed to elicit reliable responses.18 To encourage compliance with follow-up, men were given compensation for their participation.
Procedures
By use of Dacron (Digene, Gaithersburg, MD, USA) swabs prewetted with saline, three separate specimens were obtained from the coronal sulcus, glans penis, penile shaft, and scrotum, and placed in 450 μL of Specimen Transport Medium, and combined into one sample before DNA extraction. All specimens were stored at −70°C until PCR analyses and genotyping were undertaken. We have previously shown the validity of these three anatomical sites in the assessment of HPV status, and high sampling reproducibility for the detection of HPV DNA by use of this method.19 For HPV analyses, DNA was extracted with the Media Kit (QIAGen, Valencia, CA, USA) on a robotic system according to the manufacturer’s instructions. DNA was stored at 4°C until use. HPV testing was undertaken by use of PCR for amplification of a fragment of the L1 gene.20
Specimens were tested for the presence of HPV by amplification of 30 ng of DNA with the PGMY09/11 L1 consensus primer system.20 HPV genotyping was done with the linear array method21 on all samples irrespective of the HPV PCR result (Roche Molecular Diagnostics, Alameda, CA, USA). Only samples that tested positive for β globin (99% at enrolment) were judged to be adequate and included in the analysis. Before genotyping, the amplification products were run on 2% agarose gels to visualise a 450 bp band corresponding to HPV amplification for identification of samples that might have a HPV type other than the 37 types analysed in the genotyping assay. Samples in which HPV was amplified on PCR, but did not hybridise with a specific HPV type during the genotyping assay (eg, unclassified infections), were classified as HPV negative. The HPV types that were classified as oncogenic were 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66.22 The non-oncogenic types were 6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39, and CP6108.
Statistical analysis
Sociodemographic and sexual behavioural characteristics of participants, non-participants, and the full cohort were compared by use of Fisher’s exact test for categorical variables and Van der Waerden’s normal score test for continuous variables. Incidence and median time to clearance were estimated for any, oncogenic, and non-oncogenic HPV types, and for every specific HPV type. The classification of any HPV was defined as a positive test result for at least one of 37 HPV genotypes. HPV infections with single or multiple oncogenic virus types were classified as oncogenic. Non-oncogenic infections were those that tested positive for only non-oncogenic HPV type(s). For estimates of any or type-specific HPV incidence, only participants who were free of any or a specific HPV type, respectively, at enrolment were included. Since multiple infections are possible within an individual, multiple positive tests were judged as a separate event. Person time for newly acquired HPV infection was estimated by use of the time from study entry to the date of the first detection of HPV DNA, assuming a new infection arose at the date of detection. The calculation of the exact 95% CIs for incidence estimates was based on the number of events modelled as a Poisson variable for the total person months.23
Cumulative risk of acquisition of a new infection was estimated for any HPV, HPV 16, oncogenic HPV, and non-oncogenic HPV infections with the Kaplan-Meier method. Factors associated with these outcomes were assessed with proportional hazards regression. Backward-selection method, with a significance threshold of 0·05, was used to identify variables included in the final multivariable model. Candidate variables included race, education, marital status, smoking status, circumcision status, lifetime number of female partners, lifetime number of male anal-sex partners, number of female partners in the past 3 months, and number of male anal-sex partners in the past 3 months. Country (USA, Brazil, and Mexico) and age (years) were included in all models as design factors.
HPV clearance was defined as a participant testing HPV negative at two consecutive visits after testing positive. The probability of maintaining an HPV infection present at the first visit or an incident infection acquired during the study period was assessed by use of the Kaplan-Meier method. Because multiple infections were possible per man, analyses were undertaken for individual type-specific HPV clearance. The robust sandwich estimator was used for the covariance matrix to account for within-subject correlations.24 The proportional hazards assumption for the Cox models was tested25 and although no gross violations were shown, HPV status at enrolment, country, age, and total number of female partners were not proportional over time in some of the HPV incidence or clearance models. However, because these covariates might be important factors in the epidemiology of HPV infection, they were retained in the final models.
All analyses were done with SAS (version 9.2), tests were two sided, and had a significance level of 0·05.
Role of the funding source
Study sponsors had no role in the study design, data collection, or analysis. The corresponding author had full access to all data in the study, and had final responsibility for the decision to submit for publication.
Results
Study recruitment began in July, 2005, and ended in September, 2009, and 4299 men provided consent— 1443 from Brazil, 1429 from Mexico, and 1427 from the USA. The first 1159 men (subcohort) recruited from June, 2005, to December, 2006, who completed a minimum of 2 weeks of follow-up (median 27·5 months, range 0·5 to 40·5; mean 23·6 months, IQR 18·0–31·2) were followed up until March, 2009. 132 (11%) of 1159 men completed 2 weeks of follow-up, 94 (8%) completed 6 months, 82 (7%) completed 12 months, 118 (10%) completed 18 months, 247 (21%) completed 24 months, 373 (32%) completed 30 months, and 113 (10%) completed 36 months. Participants and non-participants, and men in the subcohort and full cohort were similar for most demographic and behavioural characteristics (table 1). Mean age of participants was 32·1 years (SD 10·8). In the subcohort, the most common self-reported race was white, most men were uncircumcised, and most were non-smokers at enrolment (table 1). 46 (15%) of 306 men who reported no female sexual partners in the past 3–6 months reported sexual intercourse with men.
Table 1.
Baseline characteristics of men enrolled and not enrolled
| Subcohort (n=1159) | Did not enrol (n=50) | Full cohort (n=4074) | |
|---|---|---|---|
| Age (years)*
| |||
| Median (range) | 30 (18–70) | 28 (18–68) | 31 (18–70) |
| Mean (SD) | 32·1 (10·8) | 31·3 (10·8) | 32·5 (11·1) |
| 18–30 | 598 (52%) | 30 (60%) | 1989 (49%) |
| 31–44 | 452 (39%) | 15 (30%) | 1558 (38%) |
| 45–70 | 109 (9·4%) | 5 (10%) | 527 (13%) |
|
| |||
| Country of residence*
| |||
| USA | 417 (36%) | 24 (48%) | 1343 (33%) |
| Brazil | 382 (33%) | 9 (18%) | 1401 (34%) |
| Mexico | 360 (31%) | 17 (34%) | 1330 (33%) |
|
| |||
| Race
| |||
| White | 535 (46%) | 17 (34%) | 1825 (45%) |
| Black | 171 (15%) | 8 (16%) | 636 (16%) |
| Asian | 25 (2%) | 3 (6%) | 112 (3%) |
| American Indian or Alaskan | 23 (2%) | 0 | 80 (2%) |
| Mixed | 389 (34%) | 20 (40%) | 1358 (33%) |
| Refused to answer | 16 (1%) | 2 (4%) | 63 (2%) |
|
| |||
| Marital status
| |||
| Single or never married | 543 (47%) | 24 (48%) | 1838 (45%) |
| Married | 385 (33%) | 16 (32%) | 1384 (34%) |
| Cohabiting | 133 (11%) | 5 (10%) | 484 (12%) |
| Divorced, separated, or widowed | 88 (8%) | 5 (10%) | 357 (9%) |
| Refused to answer | 10 (<1%) | 0 | 11 (<1%) |
|
| |||
| Education (years)*
| |||
| <12 | 259 (22%) | 13 (26%) | 900 (22%) |
| 12 | 283 (24%) | 9 (18%) | 1089 (27%) |
| 13–15 | 330 (28%) | 20 (40%) | 1038 (25%) |
| 16 | 218 (19%) | 6 (12%) | 785 (19%) |
| ≥17 | 59 (5%) | 2 (4%) | 248 (6%) |
| Refused to answer | 10 (<1%) | 0 | 14 (<1%) |
|
| |||
| Sexual orientation
| |||
| Had no sex | 56 (5%) | 2 (4%) | 237 (6%) |
| MSW | 976 (84%) | 43 (86%) | 3429 (84%) |
| MSM | 122 (11%) | 3 (6%) | 393 (10%) |
| Refused to answer | 5 (<1%) | 2 (4%) | 15 (<1%) |
|
| |||
| Circumcision*
| |||
| No | 673 (58%) | 27 (54%) | 2583 (63%) |
| Yes | 486 (42%) | 23 (46%) | 1491 (37%) |
|
| |||
| Current smoker
| |||
| No | 868 (75%) | 36 (72%) | 3104 (76%) |
| Yes | 289 (25%) | 14 (28%) | 963 (24%) |
| Refused to answer | 2 (<1%) | 0 | 7 (<1%) |
|
| |||
| Lifetime female sex partners
| |||
| Median (range) | 6 (0–1000) | 5 (0–25) | 6 (0–5000) |
| Mean (SD) | 14·8 (50·5) | 7·1 (6·4) | 17 (100·1) |
| 0–1 | 218 (19%) | 8 (16%) | 718 (18%) |
| 2–9 | 480 (41%) | 24 (48%) | 1622 (40%) |
| 10–49 | 336 (29%) | 14 (28%) | 1272 (31%) |
| ≥50 | 58 (5%) | 0 | 229 (6%) |
| Refused to answer | 67 (6%) | 4 (8%) | 233 (6%) |
|
| |||
| Female sex partners in past 3–6 months*†
| |||
| None | 306 (26%) | 7 (14%) | 1243 (31%) |
| 1 | 474 (41%) | 15 (30%) | 1655 (41%) |
| 2 | 142 (12%) | 17 (34%) | 519 (13%) |
| ≥3 | 115 (10%) | 9 (18%) | 531 (13%) |
| Refused to answer | 122 (11%) | 2 (4%) | 126 (3%) |
|
| |||
| Lifetime male anal-sex partners*
| |||
| Median (range) | 0 (0–2000) | 0 (0–30) | 0 (0–2500) |
| Mean (SD) | 7·6 (82·5) | 0·9 (4·5) | 4·0 (58·5) |
| 0–1 | 987 (85%) | 42 (84%) | 3600 (88%) |
| 2–9 | 76 (7%) | 2 (4%) | 228 (6%) |
| 10–49 | 21 (2%) | 1 (2%) | 110 (3%) |
| ≥50 | 23 (2%) | 0 | 50 (1%) |
| Refused to answer | 52 (4%) | 5 (10%) | 86 (2%) |
|
| |||
| Male anal-sex partners in past 3 months*
| |||
| None | 1069 (92%) | 48 (96%) | 3807 (93%) |
| 1 | 36 (3%) | 1 (2%) | 100 (2%) |
| 2 | 10 (<1%) | 0 | 47 (1%) |
| ≥3 | 37 (3%) | 1 (2%) | 95 (2%) |
| Refused to answer | 7 (<1%) | 0 | 25 (<1%) |
|
| |||
| HPV status (positive for at least one genotype)
| |||
| Negative | 575 (50%) | 28 (56%) | 1993 (50%) |
| Positive | 584 (50%) | 22 (44%) | 2081 (51%) |
|
| |||
| Diagnosis of sexually transmitted infectious disease, ever
| |||
| Yes | 169 (15%) | 5 (10%) | 658 (16%) |
| No | 957 (83%) | 44 (88%) | 3288 (81%) |
| Do not know | 30 (3%) | 1 (2%) | 120 (3%) |
| Refused to answer | 3 (<1%) | 0 | 8 (<1%) |
Data are number (%), unless otherwise indicated. MSW=men who have sex with women. MSM=men who have sex with men.
Significant difference between subcohort and men from the full cohort not included in this analysis at p<0·05 with Fisher’s exact test.
Significant difference between subcohort and did not enrol group at p<0·05 with Fisher’s exact test. Responses that were refusals for each characteristic were not included for calculations of p values.
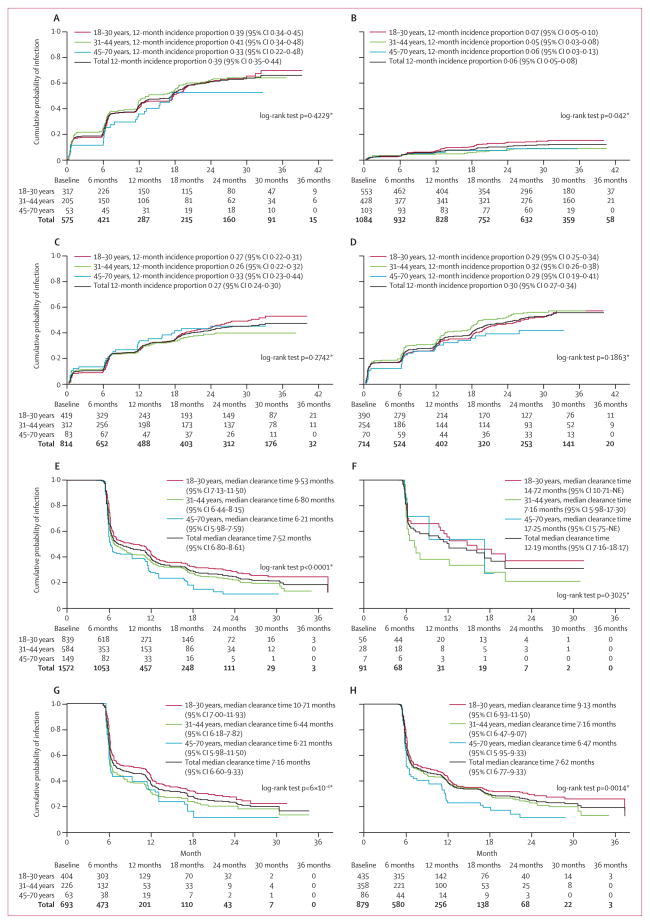
Oncogenic HPV types with the highest incidences per 1000 person months were 16, 51, 52, and 59 (table 2). Non-oncogenic HPV types with the highest incidences per 1000 person months were 6, 62, 84, and CP6108 (table 2). The incidences of any, non-oncogenic, or oncogenic HPV infections did not vary with age (figure). Differences in cumulative incidences were also noted by country (webappendix pp 2–4). At 12 months, Brazil had the highest incidence of oncogenic infection (p=0·0109) and non-oncogenic (p<0·0001) infection; however, the risk of HPV 16 infection was highest in Mexico (p=0·0195; webappendix p 2).
Table 2.
Prevalence at enrolment, and incidence and clearance of human papillomavirus (HPV) infections in men
| Prevalence (n=1159) | Incident infections | Person months | Incidence per 1000 person months (95% CI) | 12-month Incidence (95% CI) | New infections* | Cleared infections | Median time to clearance (months; 95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Any HPV | 584 (50%) | 311 | 8090 | 38·4 (34·3–43·0) | 39·3% (34·9–43·4) | 1572 | 1038 | 7·5 (6·8–8·7) |
|
| ||||||||
| Oncogenic | 345 (30%) | 311 | 13 978 | 22·2 (19·8–24·9) | 27·1% (23·8–30·2) | 693 | 465 | 7·2 (6·7–9·5) |
| 16 | 75 (6%) | 105 | 23 929 | 4·4 (3·6–5·3) | 6·2% (4·6–7·7) | 91 | 49 | 12·2 (7·4–20·2) |
| 18 | 20 (2%) | 49 | 26 090 | 1·9 (1·4–2·5) | 2·4% (1·5–3·4) | 44 | 30 | 6·3 (6·0–12·7) |
| 31 | 19 (2%) | 35 | 26 304 | 1·3 (0·9–1·9) | 1·9% (1·1–2·8) | 30 | 22 | 6·7 (6·4–15·9) |
| 33 | 2 (<1%) | 8 | 27 087 | 0·3 (0·1–0·6) | 0·5% (0·1–0·9) | 8 | 7 | 6·4 (6·1–NE) |
| 35 | 21 (2%) | 27 | 26 337 | 1·0 (0·7–1·5) | 1·3% (0·6–1·9) | 24 | 17 | 11·6 (8·6–18·1) |
| 39 | 42 (4%) | 65 | 25 305 | 2·6 (2·0–3·3) | 3·6% (2·4–4·8) | 56 | 37 | 7·4 (6·2–18·4) |
| 45 | 13 (1%) | 43 | 26 331 | 1·6 (1·2–2·2) | 2·1% (1·2–2·9) | 40 | 26 | 6·5 (6·1–18·2) |
| 51 | 72 (6%) | 108 | 23 753 | 4·5 (3·7–5·5) | 6·4% (4·8–7·9) | 96 | 62 | 10·3 (6·5,13·8) |
| 52 | 85 (7%) | 104 | 23 507 | 4·4 (3·6–5·4) | 7·4% (5·7–9·0) | 93 | 68 | 7·6 (6·3–12·0) |
| 56 | 24 (2%) | 45 | 25 995 | 1·7 (1·3–2·3) | 1·7% (0·9–2·5) | 39 | 29 | 6·2 (6·0–11·6) |
| 58 | 27 (2%) | 42 | 25 945 | 1·6 (1·2–2·2) | 2·8% (1·8–3·8) | 38 | 29 | 6·7 (6·0–12·0) |
| 59 | 62 (5%) | 95 | 24 577 | 3·9 (3·1–4·7) | 4·8% (3·5–6·2) | 69 | 46 | 6·2 (6·1–11·3) |
| 66 | 58 (5%) | 79 | 24 674 | 3·2 (2·5–4·0) | 4·5% (3·2–5·8) | 65 | 43 | 6·7 (6·1–16·9) |
|
| ||||||||
| Non-oncogenic | 445 (38%) | 313 | 11 263 | 27·8 (24·8–31·0) | 30·0% (26·3–33·5) | 879 | 573 | 7·6 (6·8–9·3) |
| 6 | 77 (7%) | 85 | 23 941 | 3·6 (2·8–4·4) | 4·8% (3·4–6·1) | 71 | 54 | 6·4 (6·1–10·4) |
| 11 | 16 (1%) | 23 | 26 608 | 0·9 (0·5–1·3) | 1·0% (0·4–1·6) | 17 | 10 | 11·8 (6·0–NE) |
| 26 | 4 (<1%) | 9 | 27 033 | 0·3 (0·2–0·6) | 0·5% (0·1–0·9) | 9 | 5 | 6·3 (5·9–NE) |
| 40 | 15 (1%) | 18 | 26 558 | 0·7 (0·4–1·1) | 1·1% (0·4–1·7) | 18 | 10 | 12·3 (6·1–NE) |
| 42 | 14 (1%) | 34 | 26 420 | 1·3 (0·9–1·8) | 1·7% (0·9–2·5) | 28 | 14 | 11·2 (7·9–NE) |
| 53 | 23 (2%) | 49 | 25 903 | 1·9 (1·4–2·5) | 2·2% (1·3–3·1) | 40 | 22 | 12·0 (6·5–NE) |
| 54 | 55 (5%) | 84 | 24 594 | 3·4 (2·7–4·2) | 4·5% (3·2–5·8) | 73 | 51 | 7·1 (6·0–12·4) |
| 55 | 31 (3%) | 59 | 25 582 | 2·3 (1·8–3·0) | 3·7% (2·5–4·8) | 52 | 32 | 11·3 (6·6–17) |
| 61 | 59 (5%) | 65 | 24 754 | 2·6 (2·0–3·3) | 4·1% (2·8–5·3) | 59 | 42 | 6·2 (6·0–9·3) |
| 62 | 85 (7%) | 87 | 23 721 | 3·7 (2·9–4·5) | 6·2% (4·6–7·7) | 79 | 49 | 12·2 (7·9–17·1) |
| 64 | 1 (<1%) | 4 | 27 213 | 0·1 (0·0–0·4) | 0·3% (0–0·6) | 4 | 3 | 6·5 (5·5–NE) |
| 67 | 4 (<1%) | 22 | 26 997 | 0·8 (0·5–1·2) | 0·8% (0·2–1·3) | 16 | 14 | 6·0 (5·8–11·7) |
| 68 | 31 (3%) | 55 | 25 741 | 2·1 (1·6–2·8) | 2·5% (1·6–3·5) | 43 | 35 | 6·3 (6·0–7·7) |
| 69 | 3 (<1%) | 4 | 27 182 | 0·1 (0·0–0·4) | 0·1% (0·0–0·3) | 4 | 3 | 5·8 (5·5–NE) |
| 70 | 26 (2%) | 33 | 25 981 | 1·3 (0·9–1·8) | 2·1% (1·2–3·0) | 30 | 18 | 12·0 (6·2–NE) |
| 71 | 13 (1%) | 17 | 26 743 | 0·6 (0·4–1·0) | 1·2% (0·5–1·8) | 17 | 11 | 12·1 (6·8–NE) |
| 72 | 14 (1%) | 31 | 26 466 | 1·2 (0·8–1·7) | 1·8% (1·0–2·6) | 27 | 16 | 6·2 (6·1–NE) |
| 73 | 15 (1%) | 21 | 26 525 | 0·8 (0·5–1·2) | 1·2% (0·5–1·9) | 20 | 15 | 6·4 (6·1–NE) |
| 81 | 42 (4%) | 50 | 25 404 | 2·0 (1·5–2·6) | 3·0% (1·9–4·0) | 46 | 31 | 6·4 (6·2–10·0) |
| 82 | 8 (<1%) | 16 | 26 827 | 0·6 (0·3–1·0) | 1·0% (0·4–1·7) | 12 | 9 | 7·4 (6·2–NE) |
| 83 | 34 (3%) | 30 | 26 065 | 1·2 (0·8–1·6) | 1·8% (1·0–2·7) | 24 | 21 | 6·3 (5·9–11·5) |
| 84 | 88 (8%) | 116 | 23 336 | 5·0 (4·1–6·0) | 6·7% (5·1–8·3) | 98 | 61 | 11·2 (6·6–16·6) |
|
| ||||||||
| CP6108 | 66 (6%) | 98 | 24 160 | 4·1 (3·3–4·9) | 5·3% (3·9–6·7) | 84 | 43 | 12·1 (9·0–NE) |
|
| ||||||||
| IS39 | 5 (<1%) | 9 | 27 057 | 0·3 (0·2–0·6) | 0·3% (0·0–0·6) | 8 | 4 | 19·8 (5·9–NE) |
Data are number or number (%), unless otherwise indicated. NE=not estimable.
Number of new infections was larger than incident infections because multiple infections were judged individually in the clearance analysis; new infections detected at a participant’s last visit were not analysed for clearance.
Median time to clearance of infection of any HPV type was significantly longer in men aged 18–30 years than in the other age groups (figure). For the oncogenic HPV types, median time to clearance was longest for HPV types 16, 35, and 51 (table 2). For the non-oncogenic HPV types, median time was longest for HPV types IS39, 40, and 62 (table 2). Median time to clearance of non-oncogenic and oncogenic infections was longest in men aged 18–30 years (figure). By contrast, median time to clearance of HPV 16 was not age dependent (figure). No differences in HPV clearance by country were noted (webappendix pp 5–8). At 12 months and 24 months, 885 (66%) of 1342 and 1023 (90%) of 1134 men, respectively, cleared newly acquired HPV infections. 31 (44%) of 71 newly acquired HPV 16 infections persisted at 12 months and seven (13%) of 56 persisted at 24 months. Five (22%) of 23 HPV 6 infections, neither of two HPV 11 infections, one (7%) of 14 HPV 16 infections, and none of six HPV 18 infections that initially cleared were detectable at a later study visit. In every case, reappearance of these HPV types was after a minimum of 12 months of non-detectable infection (data not shown).
In the multivariate analysis, acquisition of oncogenic HPV infection was significantly higher with ten to 49 or at least 50 lifetime female sexual partners compared with not more than one, and with at least three male anal-sex partners versus none in the previous 3 months (table 3). Acquisition of non-oncogenic HPV infection was significantly increased in men reporting two to nine and ten to 49 lifetime female sexual partners versus not more than one, current smokers, those with 13–15 years and more than 17 years of education compared with those with less than 12 years of education, and in men residing in Brazil versus those living in the USA (table 3).
Table 3.
Factors independently associated with infection with human papillomavirus (HPV)
| Any HPV
|
Oncogenic HPV
|
Non-oncogenic HPV
|
||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate* | Univariate | Multivariate* | Univariate | Multivariate† | |
| Country | ||||||
| USA | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 |
| Brazil | 1·07 (0·82–1·40) | 0·93 (0·69–1·27) | 0·89 (0·69–1·15) | 0·81 (0·61–1·09) | 1·56 (1·2–2·03) | 2·04 (1·47–2·83) |
| Mexico | 0·82 (0·63–1·08) | 0·83 (0·63–1·10) | 0·65 (0·49–0·86) | 0·75 (0·56–1·00) | 0·99 (0·75–1·31) | 1·27 (0·90–1·78) |
|
| ||||||
| Age | 1·00 (0·99–1·00) | 0·99 (0·98–1·00) | 0·99 (0·98–1·00) | 0·99 (0·98–1·00) | 1·00 (0·99–1·01) | 0·99 (0·98–1·00) |
|
| ||||||
| Current smoker | ||||||
| No | NA | NA | NA | NA | 1·00 | 1·00 |
| Yes | NA | NA | NA | NA | 1·25 (0·97–1·62) | 1·33 (1·01–1·74) |
|
| ||||||
| Education (years) | ||||||
| <12 | NA | NA | NA | NA | 1·00 | 1·00 |
| 12 | NA | NA | NA | NA | 1·25 (0·89–1·77) | 1·25 (0·87–1·80) |
| 13–15 | NA | NA | NA | NA | 1·33 (0·96–1·85) | 1·77 (1·19–2·61) |
| 16 | NA | NA | NA | NA | 1·22 (0·85–1·75) | 1·35 (0·92–1·98) |
| ≥17 | NA | NA | NA | NA | 1·64 (0·97–2·75) | 2·17 (1·23–3·81) |
|
| ||||||
| Lifetime female sexual partners | ||||||
| 0–1 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 |
| 2–9 | 1·24 (0·92–1·66) | 1·42 (1·04–1·94) | 1·00 (0·73–1·36) | 1·18 (0·85–1·64) | 1·30 (0·96–1·78) | 1·39 (1·01–1·90) |
| 10–49 | 2·18 (1·57–3·04) | 2·74 (1·92–3·92) | 1·65 (1·19–2·29) | 2·18 (1·53–3·12) | 2·15 (1·55–2·97) | 2·12 (1·51–2·97) |
| ≥50 | 1·25 (0·62–2·52) | 1·72 (0·81–3·65) | 1·83 (1·09–3·06) | 2·40 (1·38–4·18) | 1·52 (0·78–2·99) | 1·53 (0·74–3·15) |
| Refused to answer | 1·50 (0·84–2·68) | 1·89 (0·99–3·62) | 1·43 (0·84–2·42) | 2·08 (1·17–3·72) | 1·69 (0·94–3·03) | 1·59 (0·81–3·15) |
|
| ||||||
| Male anal-sex partners in past 3 months | ||||||
| None | 1·00 | 1·00 | 1·00 | 1·00 | NA | NA |
| 1 | 1·16 (0·63–2·12) | 1·34 (0·72–2·49) | 1·51 (0·85–2·70) | 1·70 (0·94–3·07) | NA | NA |
| 2 | 2·47 (0·61–9·95) | 3·17 (0·76–13·15) | 1·27 (0·41–3·95) | 1·52 (0·48–4·86) | NA | NA |
| ≥3 | 1·66 (0·93–2·97) | 2·33 (1·23–4·41) | 1·85 (1·12–3·06) | 2·57 (1·46–4·49) | NA | NA |
Data are hazard ratios (95% CI). NA=not applicable.
Adjusted for country, age (continuous), lifetime number of female sexual partners, and recent number of male anal-sex partners.
Adjusted for country, age (continuous), current smoking status, years of education, and lifetime number of female sexual partners.
Men residing in Brazil and Mexico versus those living in the USA, and those reporting two to nine, ten to 49, and at least 50 lifetime female partners compared with no more than one were less likely to clear an oncogenic infection (table 4). The probability of clearing an oncogenic HPV infection increased with increasing age (table 4). Factors that reduced the likelihood of clearing a non-oncogenic infection were testing positive for HPV at enrolment, reporting a high number of female partners in the past 3 months (three partners compared with none), and residing in Brazil versus USA (table 4).
Table 4.
Factors independently associated with clearance of human papillomavirus (HPV) infections
| Any HPV | Oncogenic HPV | Non-oncogenic HPV | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate* | Univariate | Multivariate† | Univariate | Multivariate‡ | |
| Country | ||||||
| USA | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 |
| Brazil | 0·85 (0·75–0·96) | 0·69 (0·58–0·83) | 0·86 (0·72–1·02) | 0·71 (0·56–0·91) | 0·86 (0·73–1·00) | 0·81 (0·69–0·96) |
| Mexico | 0·86 (0·74–0·99) | 0·86 (0·67–1·11) | 0·81 (0·68–0·98) | 0·73 (0·57–0·94) | 0·89 (0·74–1·07) | 0·87 (0·73–1·04) |
|
| ||||||
| Age | 1·00 (1·00–1·01) | 1·01 (1·00–1·02) | 1·01 (1·00–1·02) | 1·02 (1·01–1·03) | 1·00 (1·00–1·01) | 1·00 (1·00–1·01) |
|
| ||||||
| Race | ||||||
| White | 1·00 | 1·00 | NA | NA | NA | NA |
| American Indian | 1·03 (0·73–1·46) | 1·19 (0·81–1·73) | NA | NA | NA | NA |
| Asian | 1·18 (0·64–2·21) | 1·24 (0·74–2·10) | NA | NA | NA | NA |
| Black | 1·15 (0·99–1·35) | 1·26 (1·08–1·48) | NA | NA | NA | NA |
| Mixed | 0·99 (0·86–1·13) | 0·98 (0·78–1·23) | NA | NA | NA | NA |
|
| ||||||
| Education (years) | ||||||
| <12 | 1·00 | 1·00 | 1·00 | 1·00 | NA | NA |
| 12 | 1·04 (0·87–1·23) | 1·08 (0·90–1·30) | 1·06 (0·85–1·33) | 1·20 (0·95–1·52) | NA | NA |
| 13–15 | 1·09 (0·92–1·28) | 0·98 (0·81–1·19) | 1·07 (0·86–1·33) | 1·02 (0·76–1·37) | NA | NA |
| 16 | 0·91 (0·76–1·08) | 0·80 (0·66–0·96) | 0·87 (0·68–1·10) | 0·83 (0·63–1·09) | NA | NA |
| ≥17 | 1·16 (0·88–1·53) | 1·08 (0·83–1·42) | 1·18 (0·83–1·67) | 1·10 (0·76–1·60) | NA | NA |
|
| ||||||
| Lifetime female sexual partners | ||||||
| 0–1 | NA | NA | 1·00 | 1·00 | NA | NA |
| 2–9 | NA | NA | 0·74 (0·58–0·94) | 0·70 (0·55–0·90) | NA | NA |
| 10–49 | NA | NA | 0·70 (0·55–0·89) | 0·64 (0·51–0·82) | NA | NA |
| ≥50 | NA | NA | 0·62 (0·40–0·96) | 0·49 (0·31–0·76) | NA | NA |
| Refused to answer | NA | NA | 0·86 (0·62–1·19) | 0·78 (0·55–1·10) | NA | NA |
|
| ||||||
| Female partners in past 3 months | ||||||
| None | 1·00 | 1·00 | NA | NA | 1·00 | 1·00 |
| 1 | 0·77 (0·66–0·90) | 0·78 (0·67–0·91) | NA | NA | 0·77 (0·63–0·93) | 0·76 (0·63–0·91) |
| 2 | 0·75 (0·61–0·91) | 0·77 (0·63–0·94) | NA | NA | 0·72 (0·56–0·93) | 0·72 (0·56–0·93) |
| ≥3 | 0·71 (0·59–0·86) | 0·72 (0·60–0·88) | NA | NA | 0·69 (0·54–0·87) | 0·65 (0·51–0·82) |
| Refused to answer | 0·90 (0·71–1·13) | 0·95 (0·75–1·20) | NA | NA | 0·86 (0·65–1·14) | 0·91 (0·69–1·21) |
|
| ||||||
| Male anal-sex partners in past 3 months | ||||||
| None | 1·00 | 1·00 | NA | NA | NA | NA |
| 1 | 1·20 (0·82–1·77) | 1·10 (0·75–1·62) | NA | NA | NA | NA |
| 2 | 1·00 (0·55–1·80) | 1·03 (0·57–1·83) | NA | NA | NA | NA |
| ≥3 | 1·31(1·01–1·68) | 1·44 (1·10–1·88) | NA | NA | NA | NA |
|
| ||||||
| HPV status at baseline | ||||||
| Negative | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 |
| Positive | 0·83 (0·74–0·93) | 0·85 (0·76–0·96) | 0·88 (0·76–1·04) | 0·95 (0·80–1·12) | 0·80 (0·69–0·92) | 0·81 (0·70–0·93) |
Data are hazard ratios (95% CI).
Adjusted for country, age (continuous), race, education, number of female partners in past 3 months, number of male anal-sex partners in the past 3 months, and HPV status at baseline.
Adjusted for country, age (continuous), education, lifetime number of female partners, and HPV status at baseline.
Adjusted for country, age (continuous), number of female partners in the past 3 months, and HPV status at baseline.
Discussion
The incidence of genital HPV infection in men was high and relatively constant across age groups in Brazil, Mexico, and the USA. Acquisition of a new HPV infection was strongly associated with sexual behaviour with female and male sexual partners. Similarly, the probability of clearing an HPV infection was strongly associated with sexual behaviour. We noted no association with age and incidence of any, oncogenic, or non-oncogenic HPV types, although the probability of clearing these infections increased with age.
Although HPV prevalence in men has been reported in several cross-sectional studies,26 comprehensive sampling of the external genitalia or type-specific HPV incidence and clearance rates have been reported in few studies. Similar incidences to the overall HPV incidence reported in our study have been reported in studies of US students15 and community men.14 However, lower incidences were reported among Mexican13 and Danish military men.12 Because of differences in populations studied, age distribution, sampling protocols, follow-up intervals, and length of follow-up, a direct comparison of the studies is not possible. However, irrespective of the population studied, the results of all studies showed high rates of acquisition of HPV infection in men.
Whereas risk of HPV decreases with increasing age in women,27,28 men seem to have a stable risk for acquiring new HPV infections throughout their life. In a previous study of men in the USA, we noted that the incidence of HPV infection was constant over the age range 18–44 years.14 In this study, the incidence was constant in men aged 18–70 years and residing in Brazil, Mexico, and USA. HPV infections acquired late in life, and those acquired at young ages and persisting, might affect the risk of disease in men and affect transmission to sexual partners at older ages. This finding might partly account for the bimodal HPV prevalence with age noted among women in certain Latin American countries.29 Why there is a lack of an age association with HPV incidence in men is not known. In the HIM cohort, number of partners and new partners in the past 3 months was similar for the age groups, hence potentially providing continued exposure to HPV throughout life. Another possible explanation is sex differences in the HPV immune response. HPV infection of keratinised epithelium, such as the penile skin, might generate lower and weaker immune responses than infection of mucosal epithelium such as the cervix or anal canal. Men have a lower prevalence of antibodies to HPV than do women despite higher genital HPV DNA prevalence.30 In men who have circulating HPV antibodies, titres are much lower than in women. Higher exposures (eg, large number of sexual partners) might be required in men to produce an immune response that confers protection against subsequent infections. If indeed men remain at high risk of acquiring new HPV infections through out their lives, then vaccination of older men might be warranted.
We here report faster clearance of oncogenic HPV infections in men with increasing age. The more rapid clearance noted in older men might be related to a higher prevalence of HPV antibodies in older men.30 Caution should be taken in the interpretation of these results because we noted substantial variability in the clearance rates according to the HPV types in the group of viruses known to be carcinogenic, including HPV 16. Similar to what has been reported in women, the median time to clearance of HPV 16 is nearly two times longer (about 12 months) than with other oncogenic HPV types (eg, 6·3 months for HPV 18). Clearance of specific HPV types by age group needs to be further assessed in large studies.
Limitations need to be considered in the interpretion of the results of this epidemiological study. Because of the methods of recruitment and rigors of study participation, the cohort presented here is likely to be a select population. Therefore, the estimates of HPV incidence cannot be generalised to the men in the three countries. However, the assessment of factors associated with HPV acquisition and clearance are less prone to bias. HPV incidence might have been underestimated because we used the date of HPV detection as the date of occurrence. However, comparisons of incidence within the study population are probably less affected by this underestimation. HPV transmission to sexual partners was not directly studied.
The results from this study provide much needed data about the incidence and clearance of HPV infection in men; these data are essential for the development of realistic cost-effectiveness models for male HPV vaccination internationally.
Figure. Kaplan Meier estimates of the cumulative incidence and time to clearance of any, type 16, oncogenic, and non-oncogenic human papillomavirus (HPV) infections by age.
(A) Incidence of any HPV. (B) Incidence of HPV 16. (C) Incidence of oncogenic HPV. (D) Incidence of non-oncogenic HPV. (E) Clearance of any HPV. (F) Clearance of HPV 16. (G) Clearance of oncogenic HPV. (H) Clearance of non-oncogenic HPV. NE=non estimable. *For overall difference in either HPV incidence or clearance by age group.
Acknowledgments
This project was supported through a grant from the National Cancer Institute, National Institutes of Health (CA RO1CA098803). We thank the Tissue Core staffof the Moffitt Cancer Center for their help in managing biological samples from the US site.
Footnotes
Contributors
ARG was responsible for the study design and for obtaining funding. ARG, EL, LLV, JS, and MA supervised data collection. DS oversaw the testing and genotyping of HPV samples. MRP, WF, and JL were responsible for data analysis and interpretation. ARG, MA, MRP, GA, JL, and DR drafted the report. All authors critically revised the report.
HPV in Men Study Team Members
USA Kathy Eyring, Christine Gage, Nadia Lambermont, Kim Isaacs, Andrea M Leto, Emily Jolles, Kayoko Kay, Amanda Sivia, Pauline Schwalm-Andel, Rana Zaki, Sireesha Banduvula, Kyle Wolf, Steven McAnany, Shannon McCarthy. Brazil B Fietzek, E Brito, F Cernicchiaro, F Silva, G Ribeiro, J Antunes, L Galan, R Bocalon, R Hessel, R Matsuo, R Otero, R Terreri, S Araujo, V Relvas, V Souza. Mexico A Cruz, P Hernández, A Rodríguez-Cid, G Alvarez, O Rojas, D A Salazar, N Herrera, A Rodríguez, P Román.
Conflicts of interest
ARG receives support from Merck, the manufacturer of the HPV vaccine, for the phase 3 Quadrivalent HPV Vaccine Trial among Men (Tampa, FL, USA); and is also a consultant to Merck, a member of the Merck Male HPV Advisory Board, and is on the speaker’s bureau for Merck. LLV is a consultant for Merck. The other authors declare that they have no potential conflicts of interest.
Contributor Information
Prof Anna R Giuliano, H Lee Moffitt Cancer Center, Tampa, FL, USA.
Ji-Hyun Lee, H Lee Moffitt Cancer Center, Tampa, FL, USA.
William Fulp, H Lee Moffitt Cancer Center, Tampa, FL, USA.
Prof Luisa L Villa, Ludwig Institute for Research on Cancer, São Paulo, Brazil.
Prof Eduardo Lazcano, Instituto Nacional de Salud Pública, Cuernavaca, México.
Mary R Papenfuss, H Lee Moffitt Cancer Center, Tampa, FL, USA.
Martha Abrahamsen, H Lee Moffitt Cancer Center, Tampa, FL, USA.
Jorge Salmeron, Instituto Nacional de Salud Pública, Cuernavaca, México.
Gabriella M Anic, H Lee Moffitt Cancer Center, Tampa, FL, USA.
Dana E Rollison, H Lee Moffitt Cancer Center, Tampa, FL, USA.
Danelle Smith, H Lee Moffitt Cancer Center, Tampa, FL, USA.
References
- 1.Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:16–36. [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer facts and figures. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 3.Koshiol JE, Laurent SA, Pimenta JM. Rate and predictors of new genital warts claims and genital warts–related healthcare utilization among privately insured patients in the United States. Sex Transm Dis Dec. 2004;31:748–52. doi: 10.1097/01.olq.0000145851.76025.ad. [DOI] [PubMed] [Google Scholar]
- 4.Buckley JD, Doll R, Harris RW, Vessey MP, Williams PT. Case-control study of the husbands of women with dysplasia or carcinoma of the cervix uteri. Lancet. 1981;2:1010–15. doi: 10.1016/s0140-6736(81)91215-0. [DOI] [PubMed] [Google Scholar]
- 5.Zunzunegui MV, King MC, Coria CF, Charlet J. Male influences on cervical cancer risk. Am J Epidemiol. 1986;123:302–07. doi: 10.1093/oxfordjournals.aje.a114238. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal SS, Sehgal A, Sardana S, Kumar A, Luthra UK. Role of male behavior in cervical carcinogenesis among women with one lifetime sexual partner. Cancer. 1993;72:1666–69. doi: 10.1002/1097-0142(19930901)72:5<1666::aid-cncr2820720528>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Bosch FX, Castellsague X, Munoz N, et al. Male sexual behavior and human papillomavirus DNA: key risk factors for cervical cancer in Spain. J Natl Cancer Inst. 1996;88:1060–67. doi: 10.1093/jnci/88.15.1060. [DOI] [PubMed] [Google Scholar]
- 8.Castellsague X, Bosch FX, Munoz N, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346:1105–12. doi: 10.1056/NEJMoa011688. [DOI] [PubMed] [Google Scholar]
- 9.Kjaer SK, Sigurdsson K, Iversen O, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) vaccine against high-grade cervical and external genital lesions. Cancer Prev. 2009;2:868–78. doi: 10.1158/1940-6207.CAPR-09-0031. [DOI] [PubMed] [Google Scholar]
- 10.Van Doornum GJ, Prins M, Juffermans LH, et al. Regional distribution and incidence of human papillomavirus infections among heterosexual men and women with multiple sexual partners: a prospective study. Genitourin Med. 1994;70:240–46. doi: 10.1136/sti.70.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wikstrom A, Popescu C, Forslund O. Asymptomatic penile HPV infection: a prospective study. Int J STD AIDS. 2000;11:80–84. doi: 10.1177/095646240001100203. [DOI] [PubMed] [Google Scholar]
- 12.Kjaer SK, Munk C, Winther JF, Jorgensen HO, Meijer CJ, van den Brule AJ. Acquisition and persistence of human papillomavirus infection in younger men: a prospective follow-up study among Danish soldiers. Cancer Epidemiol Biomarkers Prev. 2005;14:1528–33. doi: 10.1158/1055-9965.EPI-04-0754. [DOI] [PubMed] [Google Scholar]
- 13.Lajous M, Mueller N, Cruz-Valdez A, et al. Determinants of prevalence, acquisition, and persistence of human papillomavirus in healthy Mexican military men. Cancer Epidemiol Biomarkers Prev. 2005;14:1710–16. doi: 10.1158/1055-9965.EPI-04-0926. [DOI] [PubMed] [Google Scholar]
- 14.Giuliano AR, Lu B, Nielson CM, et al. Age-specific prevalence, incidence, and duration of human papillomavirus infections in a cohort of 290 US men. J Infect Dis. 2008;198:827–35. doi: 10.1086/591095. [DOI] [PubMed] [Google Scholar]
- 15.Partridge JM, Hughes JP, Feng Q, et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis. 2007;196:1128–36. doi: 10.1086/521192. [DOI] [PubMed] [Google Scholar]
- 16.Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2036–43. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliano AR, Lazcano E, Villa LL, et al. Circumcision and sexual behavior: factors independently associated with human papillomavirus detection among men in the HIM study. Int J Cancer. 2009;124:1251–57. doi: 10.1002/ijc.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyitray AG, Kim J, Hsu CH, et al. Test-retest reliability of a sexual behavior interview for men residing in Brazil, Mexico, and the United States: the HPV in Men (HIM) Study. Am J Epidemiol. 2009;170:965–74. doi: 10.1093/aje/kwp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores R, Abalos AT, Nielson CM, Abrahamsen M, Harris RB, Giuliano AR. Reliability of sample collection and laboratory testing for HPV detection in men. J Virol Methods. 2008;149:136–43. doi: 10.1016/j.jviromet.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single–hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–27. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 23.Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR) Am J Epidemiol. 1990;131:373–75. doi: 10.1093/oxfordjournals.aje.a115507. [DOI] [PubMed] [Google Scholar]
- 24.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–78. [Google Scholar]
- 25.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martin-gale-based residuals. Biometrika. 1993;80:557–72. [Google Scholar]
- 26.Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: a systematic review of the literature. J Infect Dis. 2006;194:1044–57. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- 27.Castle PE, Schiffman M, Herrero R, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1808–16. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- 28.Munoz N, Mendez F, Posso H, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190:2077–87. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 29.de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–59. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 30.Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: national health and nutrition examination survey 2003–2004. J Infect Dis. 2009;200:1059–67. doi: 10.1086/604729. [DOI] [PubMed] [Google Scholar]