Abstract
Matching blood flow to myocardial energy demand is vital for heart performance and recovery following ischemia. The molecular mechanisms responsible for transduction of myocardial energetic signals into reactive vasodilatation are, however, elusive. Adenylate kinase, associated with AMP signaling, is a sensitive reporter of the cellular energy state, yet the contribution of this phosphotransfer system in coupling myocardial metabolism with coronary flow has not been explored. Here, knock out of the major adenylate kinase isoform, AK1, disrupted the synchrony between inorganic phosphate Pi turnover at ATP-consuming sites and γ-ATP exchange at ATP synthesis sites, as revealed by 18O-assisted 31P NMR. This reduced energetic signal communication in the post-ischemic heart. AK1 gene deletion blunted vascular adenylate kinase phosphotransfer, compromised the contractility-coronary flow relationship, and precipitated inadequate coronary reflow following ischemia-reperfusion. Deficit in adenylate kinase activity abrogated AMP signal generation and reduced the vascular adenylate kinase/creatine kinase activity ratio essential for the response of metabolic sensors. The sarcolemma-associated splice variant AK1β facilitated adenosine production, a function lost in the absence of adenylate kinase activity. Adenosine treatment bypassed AK1 deficiency and restored post-ischemic flow to wild-type levels, achieving phenotype rescue. AK1 phosphotransfer thus transduces stress signals into adequate vascular response, providing linkage between cell bioenergetics and coronary flow.
Coronary blood flow is tightly linked to myocardial oxygen consumption securing adequate oxygenation and nutrient delivery and preventing underperfusion (1–4). Mismatch between myocardial blood supply and energy metabolism precipitates heart dysfunction, leading to aberrant force-frequency relationships in acute coronary and chronic metabolic syndromes (4–7). The no-reflow phenomenon, observed after vascular reperfusion, precipitates poor outcome (8). Despite advances made in elucidating components that regulate vascular tone, the intimate mechanisms integrating metabolic signals and securing adequate vasodilatation under stress remain partially understood (4, 9–13).
Regulation of vascular tone involves several effectors, including ATP-sensitive potassium (KATP) channels, adenosine and purinergic signaling, AMP-activated protein kinase (AMPK),3 nitric oxide (NO), and neurohormonal factors (1–4, 9–13). Intra- and extracellular nucleotide-mediated signaling pathways catalyzed by phosphotransfer enzymes may provide linkage between these otherwise distinct systems (14–16). In particular, adenylate kinase phosphotransfer couples with KATP channels, facilitating decoding of metabolic signals critical in adjusting excitability-dependent functions in response to demand (17, 19–21). Adenylate kinase, with a unique property to catalyze the reaction 2ADP ↔ ATP + AMP, is a sensitive reporter of the cellular energy state, translating small changes in the ATP/ADP balance into relative larger changes in AMP concentration, so that AMP-regulated enzymes and metabolic sensors can respond with high sensitivity and fidelity to stress signals (15–21). Adenylate kinase interacts with creatine kinase, a parallel phosphotransfer system, conveying positive and negative signals to stress response elements (7, 19–23). An imbalance between phosphotransfer enzymes compromises signal reception by metabolic sensors and disrupts maintenance of bioenergetic homeostasis (17, 20, 23–27). Although altered phosphotransfer enzyme activities are associated with abnormal vascular tone, such as hypertension (26, 27), evidence demonstrating the significance of phosphotransfer-mediated signaling in the vascular response is still lacking (11, 28).
In myocardial and vascular tissue, AK1 is the major adenylate kinase isoform. AK1 is localized in the cytosol and myofibrils where it serves as a metabolic hub connecting minor adenylate kinase isoforms, AK3 in the mitochondrial matrix, AK2 in the intermembrane space, AK6 in the nucleus, and ecto-AK1 in the extracellular space (16, 17, 25, 29–31). The AK1 splice variant, AK1β, is targeted to the plasma membrane and linked to KATP channel gating (32). Such strategic distribution of adenylate kinase isoforms creates a continuous phosphotransfer network, mediating energy transfer and metabolic signaling between cell compartments and along the interstitial space (14–16). Disruption in the network function, by deletion of the AK1 gene, lowers the muscle energetic efficiency, compromising relaxation kinetics and increasing vulnerability to stress (31, 33–35). AK1-deficient phenotype is characterized by a reduced maintenance of adenine nucleotide pools and inefficient signal communication to the nucleus and cytosolic metabolic sensors, including KATP channels and AMPK (17, 31, 33, 36). However, linkage between adenylate kinase-mediated AMP signaling and the regulation of vascular flow has so far not been established.
Here, analysis of the AK1-null mutant demonstrated that deficit in vascular and myocardial adenylate kinase catalysis blunts energetic communication, AMP, and adenosine metabolic signal transduction, compromising post-ischemic coronary reflow. These data provide the first direct evidence for the role of adenylate kinase as a metabolic monitor supporting regulation of the reactive vascular response in the stressed heart.
EXPERIMENTAL PROCEDURES
AK1 Knock-out Mice
AK1 knock-out mice were generated from embryonic stem cells carrying a replacement mutation in the AK1 gene (31, 33, 34). Inactivation of AK1 expression was achieved by homologous DNA recombination, with a HygroB cassette vector used to replace the entire exon 3–5 region in AK1 (33). Male homozygous AK1 null (AK1−/−) mice were compared with age- and sex-matched wild-type controls. The investigation conformed to the National Institutes of Health guidelines regulating the care and use of laboratory animals and was approved by the Institutional Animal Care and Use Committee.
Heart Perfusion
Hearts from heparinized (50 units, intraperitoneal) and anesthetized (75 mg/kg pentobarbital, intraperitoneal) mice were rapidly excised and perfused on a Langendorff apparatus with 95% O2/5% CO2-saturated Krebs-Henseleit (K-H) buffer (in mmol/liter: 118 NaCl, 5.3 KCl, 2.0 CaCl2, 19 NaHCO3, 1.2 MgSO4, 11.0 glucose, 0.5 EDTA; 37 °C) at a perfusion pressure of 70 mmHg (34, 37). Hearts were perfused for 45 min and subjected to 30 min of no-flow normothermic ischemia followed by a 30-min-long reperfusion. Left ventricular developed pressure (LVDP), left ventricular end diastolic pressure, rate-pressure product, and heart rate were derived from the left ventricular pressure signal monitored with a fluid-filled balloon-tipped pressure transducer (Harvard Apparatus). Through the protocol, coronary flow was recorded on-line using a small animal blood flow meter (Transonics System Inc.) interphased with a data acquisition system (MP150; Biopac Systems Inc.).
18O-assisted 31P NMR Spectroscopy
The 18O labeling procedure is based on incorporation of one 18O atom, provided from [18O]H2O, into Pi with each ATP hydrolysis act and the subsequent distribution of 18O-labeled phosphoryls among other high energy phosphoryl-carrying molecules (37–39). This permits monitoring of energetic dynamics with determination of ATP consumption and synthesis rates, high energy phosphoryl transfer by creatine kinase and adenylate kinase reactions, and energetic communication between intracellular ATP hydrolysis and ATP synthesis sites (18, 33, 37–43). To follow the kinetics of 18O incorporation into phosphoryls, 18O-assisted 31P NMR spectroscopy, based on the 18O-induced shift in 31P NMR spectra, was applied (18, 37, 41). This method permits in a single run, without prior separation of phosphoryl-containing metabolites, the determination of concentrations and metabolic turnover rates of main components in the cellular energetic network and metabolic flux rates (37, 41–44). Accordingly, hearts were labeled with 18O, introduced for 30 s with the K-H buffer supplemented with 30% of 18O-labeled H2O (Isotec) at the end of reperfusion (18, 33, 37). Hearts were freeze-clamped, pulverized under liquid N2, and extracted in a solution containing 0.6 m HClO4 and 1 mm EDTA. Extracts were neutralized with 2 m KHCO3 and used to determine 18O incorporation. 18O labeling of Pi and γ-ATP was determined with 18O-assisted 31P NMR spectroscopy. High resolution 31P[16O] and 31P[18O] spectra were acquired at 202.5 MHz on a Bruker 11 T spectrometer (Avance) in 5-mm tubes at ambient temperature. For 31P spectra, signal accumulation (36,000 scans) was run without relaxation delay (acquisition time 1.61 s) with a pulse width of 10 µs (53° angle). Incorporation of each 18O induces isotope shifts of 0.0210 and 0.0228 ppm in the 31P NMR spectrum of Pi and γ-ATP, respectively. During signal acquisition, proton decoupling was applied with a 506-µs pulse width using the WALTZ-16 sequence at a 3-kHz radio frequency (37).
18O Labeling Rates and Metabolite Levels
18O labeling rates of Pi and γ-ATP were calculated as described (37–44). Percentages of 16O, 18O1, 18O2, and 18O3 phosphoryl species in γ-ATP and percentages of 16O, 18O1, 18O2, 18O3, and 18O4 phosphoryl species in Pi were proportional to the integrals of their respective peaks in the 31P NMR spectrum (37). Cumulative percentage of 18O labeling in Pi was calculated as [%18O1 + 2(% 18O2) + 3(% 18O3) + 4(% 18O4)]/[4(% 18O in H2O)], whereas the 18O labeling percentage values in γ-ATP was calculated as [%18O1 + 2(% 18O2) + 3(% 18O3)]/[3(% 18O in H2O)] (18, 37). The amount of 18O incorporated into Pi and γ-ATP expressed as nmol/min/mg protein was calculated by multiplying tissue levels of Pi and ATP by corresponding fractions of labeled phosphoryls. Tissue levels of Pi and ATP were determined by 31P NMR using methylene diphosphonic acid as a standard (18, 37). The Pi/γ-ATP 18O labeling ratio, an index of intracellular energetic communication (37, 42, 43), was calculated using the amount and percentage of 18O incorporated into Pi and γ-ATP. Such comparison is necessary for the proper interpretation of 18O labeling results (37–40).
Vascular Tissue Harvest
Thoracic aorta (arch and descending segments) was isolated from heparinized and anesthetized mice, washed in oxygenated heart perfusion medium, and cleared of connective tissue and fat (33). Tissue was frozen by immersion in liquid nitrogen and stored in liquid nitrogen until processing. To simulate ischemia in vitro, portions of aortic tissue samples were transferred into deoxygenated heart perfusion medium saturated with 95%N2 and 5% CO2 at 37 °C for 10 min and snap-frozen in liquid nitrogen.
Cardiomyocyte and Sarcolemma Isolation
Adult cardiomyocytes and sarcolemma fractions from mice hearts were isolated as described (17, 23). Hearts were homogenized (in mm: 10 HEPES, 1 EGTA, 1 dithiothreitol, 1 aprotinin, 0.2 phenylmethylsulfonyl fluoride, and 1 µg/ml leupeptin, pH 7.4) and were spun at 5,000 × g. Supernatant was centrifuged at 100,000 × g and membrane pellets suspended by sonication in (in mm) 20 HEPES, pH 7.4, 140 NaCl, 5 KCl, 2 MgCl2, 0.5 dithiothreitol, 1 aprotinin, 0.2 phenylmethylsulfonyl fluoride, and 2 µg/ml leupeptin. Sarcolemmal fraction was purified by sucrose density gradient centrifugation, and the degree of enrichment was determined based on sodium, K-ATPase activity (23). Adenylate kinase-mediated generation of AMP from added 0.2 mm ADP and subsequent adenosine production by 5′-nucleotidase present in cardiac sarcolemma was measured using high performance liquid chromatography (18, 33).
Immunocytochemistry
Localization of the membrane-associated AK1β isoform was detected using the anti-AK1β antibody (CIM Antibody Core, University of Texas-Southwestern), the Alexa Fluor 568 secondary antibody (Molecular Probes), and a standard immunostaining protocol (32).
Enzyme Activities and Metabolite Levels
Blood vessel tissue was ground in liquid nitrogen and extracted with buffer containing (in mm) 150 NaCl, 60 Tris-HCl, pH 7.5, 5 EDTA, 1 phenylmethylsulfonyl fluoride, 10 µg/ml leupeptin, 1 µg/ml aprotinin, and 0.2% Triton X-100 and centrifuged (10,000 × g, 4 °C). Adenylate kinase activity was measured with a Beckman DU 7400 spectrophotometer at 340 nm in medium containing (in mm) 100K+-acetate, 20 HEPES,pH7.5, 20 glucose, 4 MgCl2, 2 NADP+, 1 EDTA, 1 dithiothreitol, 2 ADP, 4.5 units/ml hexokinase, and 2 units/ml glucose-6-phosphate dehydrogenase (17, 33). Creatine kinase activity was measured using the CK-20 reagent kit (Sigma). Adenine nucleotide and nucleoside levels were determined in perchloric acid extracts using high performance liquid chromatography (33, 44). Freeze-clamped hearts or aortic tissues were pulverized in mortar with liquid nitrogen and extracted in a solution containing 0.6 m HClO4 and 1 mm EDTA (33, 37). Proteins were pelleted by centrifugation (15,000 × g, 10 min) and protein content determined with a DC Protein Assay kit (Bio-Rad). Extracts were neutralized with 2 m KHCO3 and analyzed using high performance liquid chromatography (33, 44).
Statistical Analysis
Data are expressed as mean ± S.E. Student’s t test was used for statistical analysis and p < 0.05 predetermined.
RESULTS
AK1 Knock Out Mitigates Energetic Signal Communication
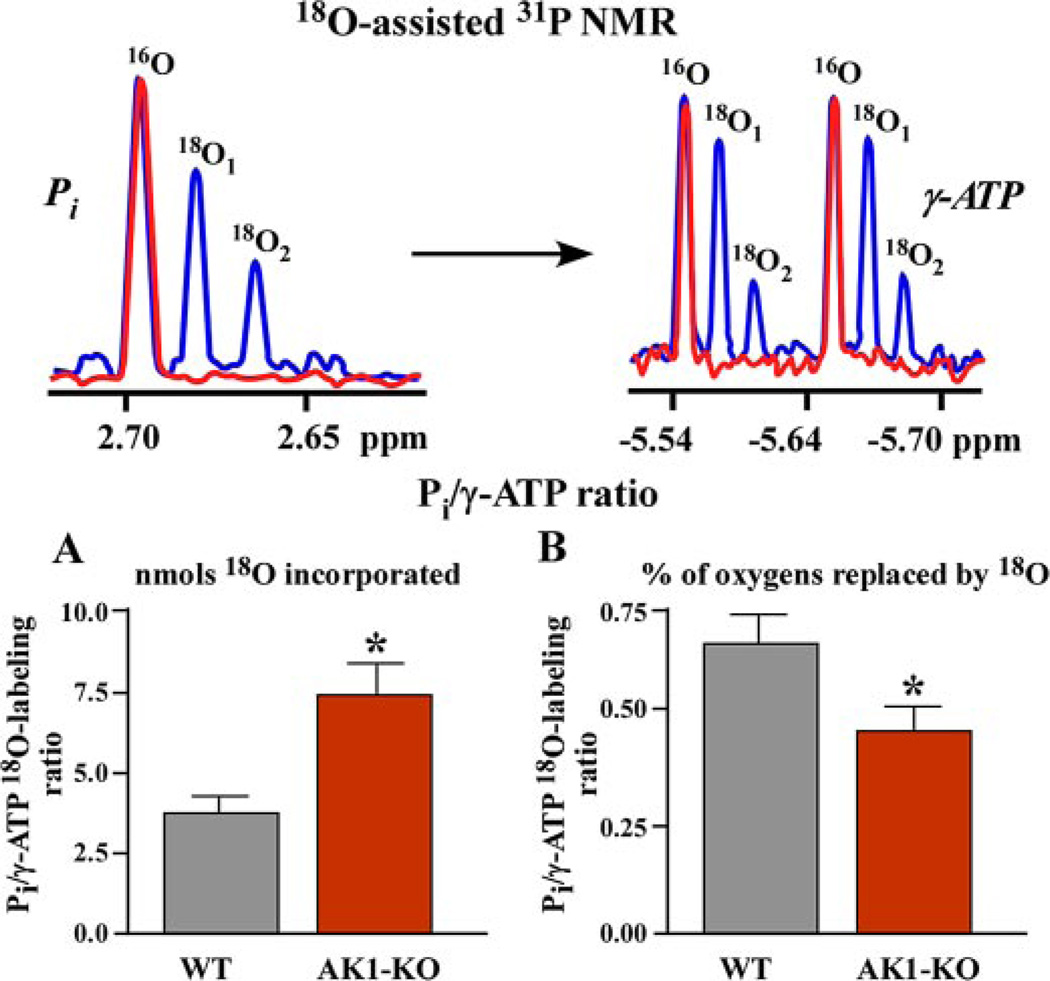
The difference in the kinetics of Pi and γ-ATP 18O labeling, detected by 18O-assisted 31P NMR spectroscopy (Fig. 1), indicates the degree of synchronization between ATP consumption and ATP production sites. Control hearts, not subjected to ischemia-reperfusion, demonstrate a Pi/γ-ATP labeling ratio of 1.2–1.5 expressed in mass units of incorporated 18O and 0.90–0.95 expressed as percentage of phosphoryl oxygens replaced by 18O (37, 42, 43), indicating efficient energetic communication between ATP consumption and ATP production sites. In ischemia-reperfusion-challenged wild-type hearts, perfused with medium containing H2[18O], the Pi/γ-ATP labeling ratio was 3.83 ± 0.50, expressed in mass units of 18O-incorporated in Pi, and γ-ATP versus 0.65 ± 0.08, expressed in percentage of phosphoryl oxygens replaced by 18O (n = 5; Fig. 1A). In the AK1 null mutant, with an adenylate kinase-catalyzed phosphotransfer rate blunted by 60% (34), the Pi/γ-ATP labeling ratio expressed in mass units increased to 7.44 ± 0.80 while the percentage ratio decreased to 0.46 ± 0.06 (n = 4) compared with wild type (p < 0.05; Fig. 1B). This indicates that AK1 knock out causes accumulation of 18O-labeled Pi species at ATPases and ineffective delivery to ATP synthesis or γ-ATP labeling sites, indicating disturbed energetic signal communication.
FIGURE 1. Disturbed intracellular energetic communication in AK1-KO hearts after ischemia-reperfusion assessed by 18O-assisted 31P NMR.
Upper panel, 18O-assisted 31P NMR recordings of 18O-induced shift (blue) in 31P NMR spectra of Pi (red) and γ-ATP (blue and red, respectively) indicating incorporation of one and two atoms of 18O and metabolic activity of these energetic intermediates. Lower panels, A and B, changes in Pi/γ-ATP labeling ratios (by 18O mass (A) and by percentage of 18O (B) incorporated into corresponding phosphoryls) in wild-type (WT) and adenylate kinase knock-out (AK1-KO) hearts after ischemia-reperfusion. Increased 18O mass in Pi compared with γ-ATP (A) along with diminished percentage labeling ratio (B) indicates disrupted communication between ATP consumption and ATP synthesis sites in AK1-KO hearts.
Myocardial Energetics Versus Coronary Flow
In wild-type hearts, correlation coefficients of energetic parameters reflecting ATPase and adenylate kinase velocities, Pi, and β-ADP and β-ATP turnover rates (34) versus basal coronary flow were 0.58, 0.58 and 0.53. A significant negative correlation, r = −0.97 (p < 0.05, n = 5), was observed between myocardial GTP levels (34) and basal coronary flow, indicating distribution of control among energetic signaling systems. AK1 knock-out hearts had a higher correlation coefficient for ATPase velocity or Pi 18O labeling and basal coronary flow, r = 0.80, and a negative correlation between creatine kinase velocity or creatine phosphate 18O labeling, creatine phosphate levels (34), and basal coronary flow, r = −0.85 and r = −0.95 (p < 0.05, n = 4), indicating imbalanced phosphotransfer signaling. Post-ischemic coronary flow recovery of AK1 knock-out hearts correlated with β-ADP and β-ATP turnover rates (34), reflecting the remaining adenylate kinase isoform velocity, r = 0.98 and r = 0.99 (p < 0.05, n = 4), respectively. This tight relationship suggests that adenylate kinase phosphotransfer becomes rate-limiting in the myocardial-vasculature energetic communication in ischemia-injured hearts.
AK1 Deficiency Alters the Heart Contractility-Flow Relationship and Abrogates Coronary Reflow after Ischemia-Reperfusion
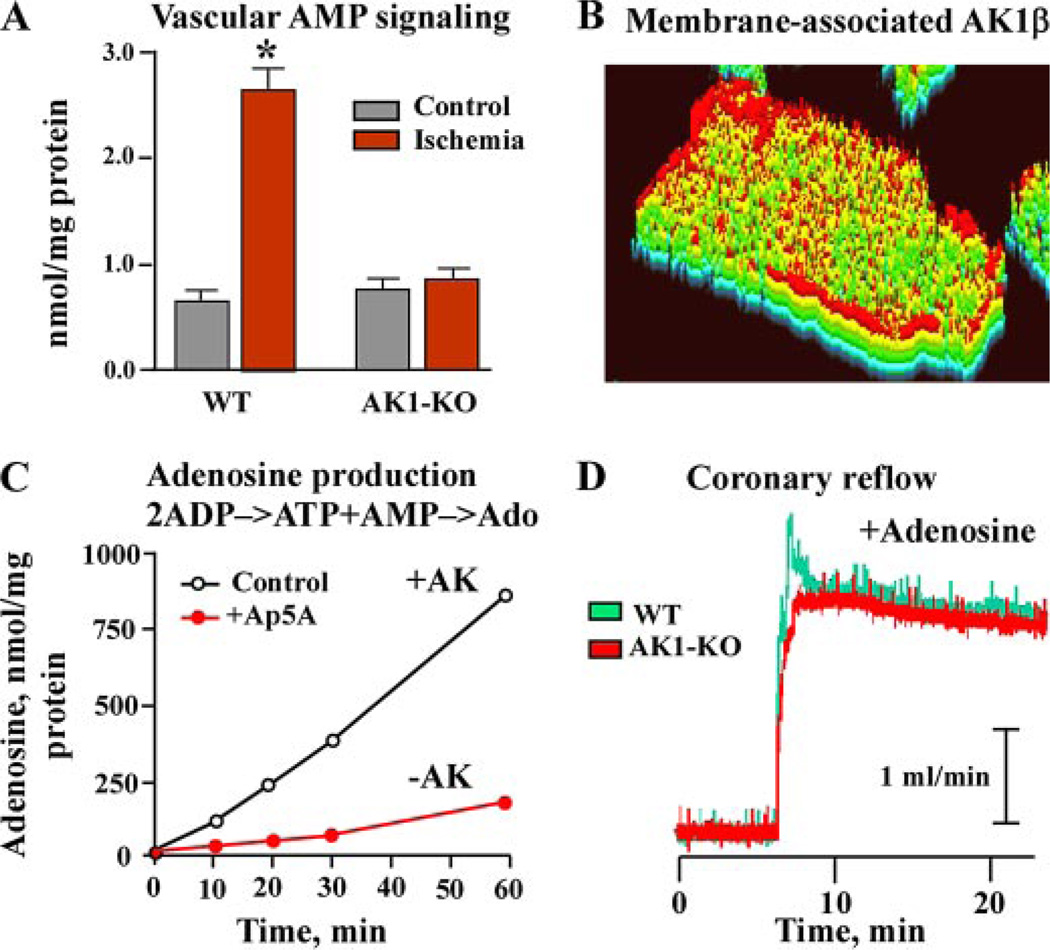
The AK1 null mutant displayed reduced vascular adenylate kinase activity, at 0.08 ± 0.02 µmol/min/mg protein (n = 5) compared with 0.25 ± 0.04 µmol/min/mg (n = 4) in wild type (p < 0.001; Fig. 2A). Vascular creatine kinase activity, the parallel phosphotransfer pathway, was essentially unchanged at 0.17 ± 0.04 µmol/min/mg in wild type (n = 4) and 0.19 ± 0.05 µmol/min/mg protein in AK1 knockouts (n = 5). However, the adenylate kinase/creatine kinase activity ratio was significantly diminished, from 1.45 ± 0.12 in wild type to 0.42 ± 0.13 in AK1 knockouts (p < 0.01; Fig. 2B). AK1 knock-out hearts demonstrated a dampened oscillation of contractility and coronary flow, which occurs in the wild type and has been related to alternations of myocardial metabolic cycles (11, 45). While the relationship between changes in heart contractility above base line and corresponding increments in coronary flow was steep in wild-type hearts (Fig. 2C), the slope was significantly shallower in AK1-knock-out hearts, indicating altered signal communication from the myocardium to the vasculature. The slope of the left ventricular developed pressure-coronary flow relation was halved, from 0.040 ± 0.007 in wild-type to 0.021 ± 0.004 in AK1 knock-out hearts (Fig. 2C). Despite this difference in the heart contractility-flow relationship, basal coronary flow under well oxygenated conditions was on average similar among groups. However, recovery of flow after ischemia-reperfusion was markedly compromised in AK1 knock-out hearts (Fig. 2D). Basal coronary flow was 2.83 ± 0.31 ml/min (n = 5) and 2.68 ± 0.24 ml/min (n = 4) in weight-matched wild-type and AK1 knock-out hearts, respectively. Following ischemia-reperfusion, recovery of flow essentially reached pre-ischemic levels, i.e. 98 ± 4.0%, in the wild type but remained at 67 ± 5.0% of pre-ischemic levels in AK1-deficient hearts (p < 0.05). Thus, a reduced adenylate kinase activity and an altered adenylate kinase/creatine kinase activity ratio in the setting of AK1 deficiency contribute to inadequate coronary reflow after ischemia-reperfusion.
FIGURE 2. Adenylate kinase AK1 gene knock out reduces vascular AK activity, shallows the relationship between coronary flow and heart contractility, and compromises coronary reflow after ischemia-reperfusion.
A and B, compared with wild-type (WT), adenylate kinase knock-out (AK1-KO) hearts display markedly diminished AK activity in vascular (aorta) tissue along with a decreased AK/creatine kinase (CK) activity ratio, indicating cellular phosphotransfer imbalance. C, the relationship between natural fluctuations in heart contractility (left ventricle developed pressure, ΔLVDP) and increments in coronary flow (ΔCF) is dampened in AK1-KO hearts. D, coronary reflow after ischemia-reperfusion is blunted in AK1-KO hearts (red) compared with WT counterparts (green).
Defective Vascular AMP and Adenosine Signaling in AK1 Knock-out Mice
Deletion of the AK1 gene reduced the generation of vascular AMP signaling in response to ischemia (Fig. 3A). In isolated wild-type aorta, AMP levels increased from 0.65 ± 0.08 nmol/mg protein in well oxygenated conditions to 2.56 ± 0.24 nmol/mg protein (n = 5, p < 0.01) after 10 min of simulated ischemia. However, AK1 knockouts lacked this response as AMP levels remained essentially unchanged, 0.78 ± 0.09 nmol/mg protein in normoxic conditions and 0.93 ± 0.11 nmol/mg protein (n = 4) following 10 min of ischemia. The AK1 gene produces two transcripts, the cytosolic AK1 isoform and the membrane-associated AK1β splice variant (32). Immunocytochemistry, using a specific AK1β antibody, demonstrated a predominant sarcolemmal distribution of the AK1β isoform (Fig. 3B). The membrane-associated adenylate kinase-generated AMP is efficiently transformed by 5′-nucleotidase into adenosine, a mediator implicated in the vascular response to metabolic stress (Fig. 3C). Inhibition of sarcolemmal adenylate kinase activity with the specific inhibitor diadenosine pentaphosphate (Ap5A) abrogated adenosine production. The rate of adenosine production was 18.3 ± 1.2 nmol/min/mg protein in the presence of adenylate kinase activity and 4.1 ± 0.3 nmol/min/mg protein in its absence (n = 3, p < 0.01). Thus, adenylate kinase deficiency compromises vascular AMP signal generation and adenosine efflux in the stressed myocardium.
FIGURE 3. Adenylate kinase deficiency reduces vascular AMP signaling and cardiac membrane adenosine generation, while supplementation of adenosine rescues disrupted coronary reflow in AK1-KO hearts.
A, generation of vascular (aorta) AMP signal is markedly diminished in AK1-KO mice in response to ischemia. B, isolated myocytes stained with anti-AK1β antibody. Color-transformed image indicates predominantly sarcolemmal localization of the AK1β splice isoform (red). C, isolated sarcolemma demonstrates bound AK activity (AK1β splice isoform) that facilitates adenosine production from added 0.2 mm ADP. Inhibition of AK activity with a specific inhibitor, diadenosine pentaphosphate (Ap5A, 25 µm), abrogated adenosine production. D, compromised reflow in AK1-KO hearts after ischemia-reperfusion can be rescued by perfusion with adenosine (50 µm).
Rescue of Defective Phenotype in AK1 Knock-out Hearts
To test whether addition of adenosine could rescue disrupted coronary reflow in AK1 knock-out hearts, perfusion medium was supplemented with adenosine. Addition of adenosine (50 µm) nullified the difference in recovery of coronary flow between wild-type and AK1-deficient hearts following ischemia-reperfusion (Fig. 3C). Coronary flow, in the presence of adenosine, was 3.65 ± 0.31 ml/min in wild-type (n = 4) and 3.60 ± 0.28 ml/min in AK1 knock-out (n = 4) hearts. Adenosine supplementation thus rescues defective coronary flow in AK1 knockout hearts, bypassing the deficit in adenylate kinase-mediated generation of AMP/adenosine and the malfunction in linkage between cell bioenergetics and coronary flow.
DISCUSSION
The molecular components supporting metabolic linkage between the ischemic myocardium, experiencing oxygen and nutrient deficit, and the vascular response, adjusting flow to meet energy demand, are only partially understood. Here, we identify adenylate kinase as a necessary element in facilitating energy communication and transduction of metabolic signals from the myocardium to the vasculature, regulating the contractility-flow relationship and coronary reflow in the post-ischemic heart.
Efficient intracellular and intercellular communication of energetic and metabolic signals is critical for maintenance of cell homeostasis and induction of adequate functional response (5–7, 14–25). Using the 18O-assisted 31P NMR technique, we demonstrate that energetic communication between ATPases and ATP generation sites is compromised in AK1-deficient hearts, as indicated from altered Pi/γ-ATP 18O labeling ratios (37). These findings are in accord with the function of adenylate kinase-catalyzed phosphorelay to support communication between mitochondria and myofibrils, nucleus or membrane metabolic sensors (17, 19, 22, 43, 44, 46). Thus, deletion of AK1 mitigates intracellular metabolic signaling disrupting integration and synchronization of ATP-consuming and ATP-producing processes in response to stress.
This study also demonstrates that along with defective myocardial intracellular energetic communication, AK1 deficiency blunts vascular AMP signal generation. Moreover, deficiency in AK1 and the membrane-associated AK1β splice variant reduced adenosine generation, critical in the vascular response. Deletion of AK1 also diminishes myocardial AMP and adenosine generation (31, 34). Thus, metabolic signaling is compromised in both tissues, preventing cross-talk in response to stress. Poor coronary reflow in AK1 knock-out hearts after ischemia-reperfusion was rescued with adenosine treatment, which bypassed AK1 deficiency and restored post-ischemic flow to wild-type levels. Adenosine protects against vascular dysfunction via A1 receptors and determines coronary flow via A2A and A2B receptors (1, 47). Also, adenosine present in the extracellular space can be transported back into myocytes where it is phosphorylated to AMP and ADP by adenosine kinase and adenylate kinase, respectively (48). Such changes in nucleotide distribution would alter intracellular metabolic dynamics that govern ion channels and contractile behavior as well as the activity of energy-sensing protein kinases (7, 11, 25, 48–52) and, thus, coronary vasoreactivity. Although we cannot exclude altered nitric oxide signaling and/or other mechanisms, that adenosine supplementation rescued deficient reflow in AK1 knock-out hearts suggests that defective AMP/adenosine signaling directly contributes to the abnormal phenotype.
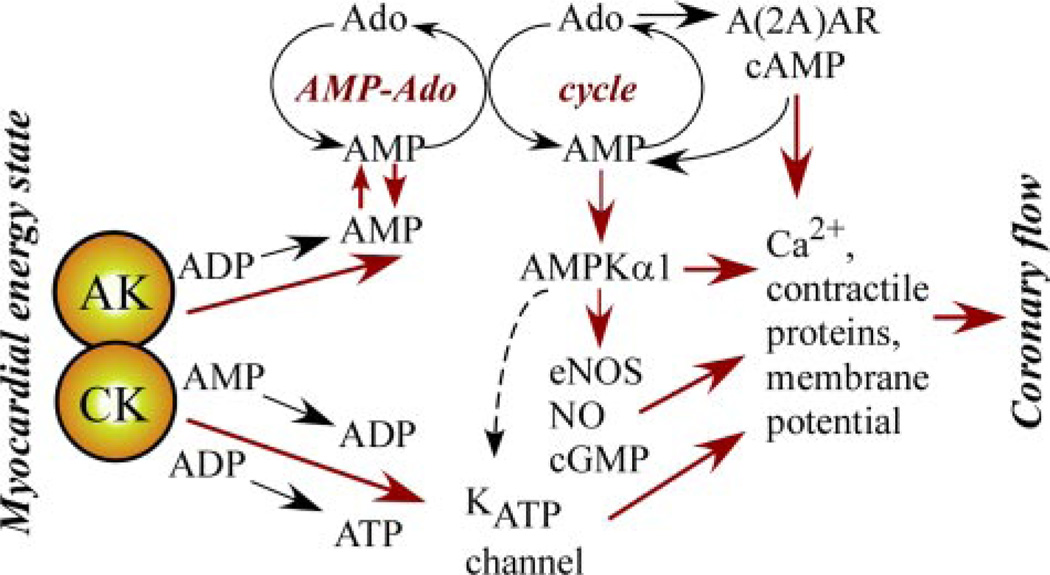
In the heart, under normoxia creatine kinase suppresses adenylate kinase phosphotransfer by scavenging cellular ADP and maintainingKATP channels closed with low signaling through the AK → AMP → AMPK and AK → AMP → adenosine systems (Fig. 4) (7, 19 –21, 25). Hypoxia or metabolic stress diminishes creatine kinase and increases adenylate kinase flux, inducing AMP generation and downstream AMP/adenosine signaling (18, 23). Deletion of AK1 shifts this balance toward creatine kinase, compromising metabolic signaling and coronary reflow after ischemia-reperfusion. This is supported by the observed shift in the negative correlation of coronary flow toward the creatine kinase system in AK1 knock-out hearts. AK1 deficiency compromises metabolic signal reception by metabolic sensors such as KATP channels and AMPK (17–21, 36), and altered adenylate and creatine kinase phosphotransfer activities are associated with abnormal vascular tone (26, 27). The significance of adenylate kinase-mediated myocardial to vascular metabolic signaling is underscored by the tight association between ATP and ADP β-phosphoryl turnover and recovery of coronary flow following ischemia, as well as by a compromised contractility-flow relationship in AK1-deficient hearts.
FIGURE 4. Summary of the myocardial-vascular metabolic signal transduction cascades initiated by phosphotransfer redistribution between adenylate kinase (AK) and creatine kinase (CK) governing the AMP/adenosine (Ado) cycle and the response of metabolic sensors (ATP-sensitive potassium channel, KATP, and AMP-activated protein kinase, AMPK).
Hypoxia or metabolic stress diminishes CK and increases AK flux, inducing AMP generation and subsequent AMP/adenosine signaling events. Adenosine/AMP signals delivered to vascular tissue through intercellular and para-cellular pathways induce signaling through adenosine receptors A(2A)AR, AMPKα1, and KATP channels. AMPKα1 activates eNOS, inducing NO/cGMP signaling, and could regulate KATP channels. Collectively, A(2A)AR, AMPKα1, eNOS, and KATP signaling converge on contractile protein, Ca2+, and membrane potential regulation, critical determinants of vascular tone.
Muscles from AK1-deficient mice display increased glycolytic enzyme activity and mitochondrial volume along with specific variations in transcripts involved in glycolysis and mitochondrial metabolism and in gene products defining structural and myogenic events (31, 33, 34, 53). Such wide scale remodeling in the AK1 knock-out muscles suggests a compensatory response capable of reducing, and not exuberating, the energetic burden caused by AK1 gene deficiency (17, 43, 53). Electron microscopy and gene array analysis, as well as energetic and functional ischemia-reperfusion studies, do not indicate increase in tissue damage, changes in apoptotic signaling, or deficit in contractile recovery in the AK1 knockout compared with wild type (33, 53). The fact that adenosine addition rescued coronary flow defects caused by AK1 deletion suggests that vascular viability is not compromised (54, 55). Thus, changes in AMP/adenosine signaling in the AK1-deficient environment are critical in defining the outcome of metabolic regulation on vascular tone.
Intrinsic regulation of vascular tone depends on the arrangement of the cellular energetic system and on the distinct contribution of ATP-producing and phosphotransfer pathways (1, 4–7, 26–28, 48, 50, 56). These pathways regulate energy-dependent steps responsible for coupling vascular metabolism with contraction, including myosin ATPase, myosin light chain kinase, and ATP-dependent Ca2+ pump (4, 11, 28, 50, 56, 57). Our data demonstrate that vascular tissue displays a comparable activity of adenylate and creatine kinase phosphotransfer systems that maintain a balance of positive and negative signals to metabolic sensors and glycolytic enzymes (17, 20, 23). Glycolytic metabolism, which supports membrane ATPases and ion channel activity, has a privileged role in vascular contractile reactivity and vasorelaxation (56, 57). In this regard, the vascular metabolic signal transduction cascade would be initiated by phosphotransfer redistribution between adenylate kinase and creatine kinase, governing AMP/adenosine signaling and the response of metabolic sensors, such as KATP channels and AMPK (Fig. 4) (7, 17–26, 51, 52). Subsequently, regulation of vascular tone would proceed through modulation of the resting membrane potential and Ca2+ influx, which is partially governed by KATP channels (2, 3, 7, 9, 58, 59). Recent studies indicate that knock out of vascular KATP channels disrupts coupling of coronary vasoreactivity with myocardial energy demand (58, 59). KATP channel or adenosine receptor blockade does not, however, abolish coronary vasodilatation (3, 56), indicating the existence of parallel signaling pathways (12, 48).
Signals originating in the myocardium must be transmitted to the vasculature to adjust coronary flow (1–4). Coupled intracellular and extracellular (ecto-AK) adenylate kinases regulate nucleotide equilibrium and propagate nucleotide-based signals at cellular and intercellular distances (14, 16, 19, 46). The heart also possesses a potent AMP-adenosine cycle/relay operating through the myocardium and vascular tissue (Fig. 4) (60, 61). Adenosine signals are tightly regulated in cardiomyocytes and vascular endothelial cells. As such, adenosine is constantly released and taken up through adenosine transporters (61). Inside the cell, adenosine is converted to AMP by the adenosine kinase while intracellular AMP levels are regulated by adenylate kinase (19, 61). Normally this cycle operates without, or with little, output of adenosine to vascular tissue. Metabolic imbalances trigger AK-mediated AMP generation and release of adenosine (61). This signal is modulated by transporters and by the adenylate kinase reaction to prevent excess of adenosine signaling, regimenting the coronary response. Disruption in tuning of adenosine signals by adenosine transporter inhibitors results in excessive adenosine signaling unleashing maximal vascular response, i.e. the coronary flow reserve (4, 62). Delivered to the vasculature, adenosine can act through endothelial and smooth muscle adenosine receptors to generate cyclic AMP, which signals to the Ca2+ transport machinery (Fig. 4). Consequently cyclic AMP could be converted to AMP by phosphodiesterase, thus contributing to metabolic signaling through AMPK and endothelial NO synthase (eNOS) pathways (63, 64). In this regard, coronary flow in AK1-deficient hearts remains responsive to exogenous adenosine signals, indicating an intact intrinsic vascular endothelium and smooth muscle signal reception system.
Recent data indicate that extracellular adenosine can activate AMPK through a mechanism that requires uptake of adenosine and conversion to AMP (65). Vasorelaxation induced by activation of AMPK is abolished in AMPKα1−/− (major vascular isoform), but not in AMPKα2−/−-deficient, mice (66). It is also known that AMPK can phosphorylate and activate eNOS (10), which contributes to elevated eNOS activity and subsequent NO production (64). However, inhibition of eNOS does not prevent AMPK activation-induced aortic relaxation (66), suggesting direct effects of AMP signaling on smooth muscle cells. In this regard, inhibition of NO synthesis attenuates coronary dilation during adenosine infusion or pacing-induced increase in metabolic demand (67). Thus, AK1 deficiency and changes in adenine nucleotide metabolism could impair eNOS activation and NO generation. Collectively with the present data, emerging evidence suggests the existence of an integrated metabolic signaling system securing transmission and tuning of energetic signals from the myocardium to vasculature (Fig. 4).
In summary, myocardial and vascular adenylate kinase phosphotransfer is necessary in facilitating intracellular and intercellular energetic communication and AMP metabolic signal transduction, regulating myocardial-vasculature cross-talk and coronary reflow after ischemia-reperfusion. In this way, adenylate kinase is a pivotal monitor of cellular energetic imbalances, generating and transmitting a feedback reply to metabolic sensors to adjust vascular blood flow.
Acknowledgment
We thank Dr. Slobodan Macura, Mayo Clinic Analytical Nuclear Magnetic Resonance Facility, for the use of the spectrometer.
Footnotes
This work was supported by grants from the National Institutes of Health, Marriott Heart Disease Research Program, Marriott Foundation, Ted Nash Long Life Foundation, Netherlands Organization for Scientific Research, Council for Medical Research (NOW-GMW) Program (901-01-095) and the Dutch Cancer Society (KWF) (KUN 98– 1808).
The abbreviations used are: AMPK, AMP-activated protein kinase; NO, nitric oxide; eNOS, endothelial NO synthase; AK1, adenylate kinase 1; KO, knockout.
REFERENCES
- 1.Berne RM. Circ. Res. 1980;47:807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- 2.Daut J, Klieber HG, Cyrys S, Noack T. Cardiovasc. Res. 1994;28:811–817. doi: 10.1093/cvr/28.6.811. [DOI] [PubMed] [Google Scholar]
- 3.Ishibashi Y, Duncker DJ, Zhang J, Bache RJ. Circ. Res. 1998;82:346–359. doi: 10.1161/01.res.82.3.346. [DOI] [PubMed] [Google Scholar]
- 4.Tune JD, Gorman MW, Feigl EO. J. Appl. Physiol. 2004;97:404–415. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- 5.Dzeja PP, Redfield MM, Burnet JC, Terzic A. Curr. Cardiol. Rep. 2000;2:212–217. doi: 10.1007/s11886-000-0071-9. [DOI] [PubMed] [Google Scholar]
- 6.Ingwall JS. J. Mol. Cell. Cardiol. 2004;37:613–623. doi: 10.1016/j.yjmcc.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A, Wallimann T. J. Physiol. 2006;571:253–273. doi: 10.1113/jphysiol.2005.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito H. J. Cardiol. 2001;37:39–42. [PubMed] [Google Scholar]
- 9.Melchert PJ, Duncker DJ, Traverse JH, Bache RJ. Am. J. Physiol. 1999;277:H617–H625. doi: 10.1152/ajpheart.1999.277.2.H617. [DOI] [PubMed] [Google Scholar]
- 10.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. J. Biol. Chem. 2003;278:31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 11.Rubin LJ, Magliola L, Feng X, Jones AW, Hale CC. J. Appl. Physiol. 2005;98:296–306. doi: 10.1152/japplphysiol.00075.2004. [DOI] [PubMed] [Google Scholar]
- 12.Kakkar R, Ye B, Stoller DA, Smelley M, Shi NQ, Galles K, Hadhazy M, Makielski JC, McNally EM. Circ. Res. 2006;98:682–689. doi: 10.1161/01.RES.0000207498.40005.e7. [DOI] [PubMed] [Google Scholar]
- 13.Guns PJ, Korda A, Crauwels HM, Van Assche T, Robaye B, Boeynaems JM, Bult H. Br. J. Pharmacol. 2005;146:288–295. doi: 10.1038/sj.bjp.0706326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yegutkin GG, Henttinen T, Jalkanen S. FASEB J. 2001;15:251–260. doi: 10.1096/fj.00-0268com. [DOI] [PubMed] [Google Scholar]
- 15.Dzeja PP, Terzic A. J. Exp. Biol. 2003;206:2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- 16.Picher M, Boucher RC. J. Biol. Chem. 2003;278:11256–11264. doi: 10.1074/jbc.M208071200. [DOI] [PubMed] [Google Scholar]
- 17.Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, Hodgson D, Bienengraeber M, Puceat M, Janssen E, Wieringa B, Terzic A. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7623–7628. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pucar D, Dzeja PP, Bast P, Gumina RJ, Drahl C, Lim L, Juranic N, Macura S, Terzic A. Mol. Cell. Biochem. 2004;256–257:281–289. doi: 10.1023/b:mcbi.0000009875.30308.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzeja PP, Zeleznikar RJ, Goldberg ND. Mol. Cell. Biochem. 1998;184:169–182. [PubMed] [Google Scholar]
- 20.Dzeja PP, Terzic A. FASEB J. 1998;12:523–529. doi: 10.1096/fasebj.12.7.523. [DOI] [PubMed] [Google Scholar]
- 21.Selivanov VA, Alekseev AE, Hodgson DM, Dzeja PP, Terzic A. Mol. Cell. Biochem. 2004;256–257:243–256. doi: 10.1023/b:mcbi.0000009872.35940.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dzeja PP, Bortolon R, Perez-Terzic C, Holmuhamedov EL, Terzic A. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10156–10161. doi: 10.1073/pnas.152259999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abraham MR, Selivanov VA, Hodgson DM, Pucar D, Zingman LV, Wieringa B, Dzeja PP, Alekseev AE, Terzic A. J. Biol. Chem. 2002;277:24427–24434. doi: 10.1074/jbc.M201777200. [DOI] [PubMed] [Google Scholar]
- 24.Hodgson DM, Zingman LV, Kane GC, Perez-Terzic C, Bienengraeber M, Ozcan C, Gumina RJ, Pucar D, O’Coclain F, Mann DL, Alekseev AE, Terzic A. EMBO J. 2003;22:1732–1742. doi: 10.1093/emboj/cdg192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dzeja PP, Terzic A. In: Creatine Kinase. Vial C, editor. New York: Nova Science; 2006. pp. 195–221. [Google Scholar]
- 26.Brewster LM, Mairuhu G, Bindraban NR, Koopmans RP, Clark JF, van Montfrans GA. Circulation. 2006;114:2034–2039. doi: 10.1161/CIRCULATIONAHA.105.584490. [DOI] [PubMed] [Google Scholar]
- 27.Seccia TM, Atlante A, Vulpis V, Marra E, Passarella S, Pirrelli A. Clin. Exp. Hypertens. 1998;20:345–358. doi: 10.3109/10641969809052126. [DOI] [PubMed] [Google Scholar]
- 28.Ishida Y, Riesinger I, Wallimann T, Paul RJ. Mol. Cell. Biochem. 1994;133–134:39–50. doi: 10.1007/BF01267946. [DOI] [PubMed] [Google Scholar]
- 29.Kurokawa Y, Takenaka H, Sumida M, Oka K, Hamada M, Kuby SA. Enzyme. 1990;43:57–71. doi: 10.1159/000468708. [DOI] [PubMed] [Google Scholar]
- 30.Tanabe T, Yamada M, Noma T, Kajii T, Nakazawa A. J. Biochem. 1993;113:200–207. doi: 10.1093/oxfordjournals.jbchem.a124026. [DOI] [PubMed] [Google Scholar]
- 31.Pucar D, Janssen E, Dzeja PP, Juranic N, Macura S, Wieringa B, Terzic A. J. Biol. Chem. 2000;275:41424–41429. doi: 10.1074/jbc.M007903200. [DOI] [PubMed] [Google Scholar]
- 32.Janssen E, Kuiper J, Hodgson D, Zingman LV, Alekseev AE, Terzic A, Wieringa B. Mol. Cell. Biochem. 2004;256–257:59–72. doi: 10.1023/b:mcbi.0000009859.15267.db. [DOI] [PubMed] [Google Scholar]
- 33.Janssen E, Dzeja PP, Oerlemans F, Simonetti AW, Heerschap A, de Haan A, Rush PS, Terjung RR, Wieringa B, Terzic A. EMBO J. 2000;19:6371–6381. doi: 10.1093/emboj/19.23.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pucar D, Bast P, Gumina RJ, Lim L, Drahl C, Juranic N, Macura S, Janssen E, Wieringa B, Terzic A, Dzeja PP. Am. J. Physiol. 2002;283:H776–H782. doi: 10.1152/ajpheart.00116.2002. [DOI] [PubMed] [Google Scholar]
- 35.Hancock CR, Janssen E, Terjung RL. Am. J. Physiol. 2005;288:C1287–C1297. doi: 10.1152/ajpcell.00567.2004. [DOI] [PubMed] [Google Scholar]
- 36.Hancock CR, Janssen E, Terjung RL. J. Appl. Physiol. 2006;100:406–413. doi: 10.1152/japplphysiol.00885.2005. [DOI] [PubMed] [Google Scholar]
- 37.Pucar D, Dzeja PP, Bast P, Juranic N, Macura S, Terzic A. J. Biol. Chem. 2001;276:44812–44819. doi: 10.1074/jbc.M104425200. [DOI] [PubMed] [Google Scholar]
- 38.Dawis SM, Walseth TF, Deeg MA, Heyman RA, Graeff RM, Goldberg ND. Biophys. J. 1989;55:79–99. doi: 10.1016/S0006-3495(89)82782-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeleznikar RJ, Goldberg ND. J. Biol. Chem. 1991;266:15110–15119. [PubMed] [Google Scholar]
- 40.Dzeja PP, Zeleznikar RJ, Goldberg ND. J. Biol. Chem. 2002;271:12847–12851. doi: 10.1074/jbc.271.22.12847. [DOI] [PubMed] [Google Scholar]
- 41.Dzeja PP, Pucar D, Bast P, Juranic N, Macura S, Terzic A. MAGMA. 2002;14:180–181. [Google Scholar]
- 42.Gumina RJ, Pucar D, Bast P, Hodgson DM, Kurtz CE, Dzeja PP, Miki T, Seino S, Terzic A. Am. J. Physiol. 2003;284:H2106–H2113. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- 43.Janssen E, Terzic A, Wieringa B, Dzeja PP. J. Biol. Chem. 2003;278:30441–30449. doi: 10.1074/jbc.M303150200. [DOI] [PubMed] [Google Scholar]
- 44.Dzeja PP, Terzic A, Wieringa B. Mol. Cell. Biochem. 2004;256–257:13–27. doi: 10.1023/b:mcbi.0000009856.23646.38. [DOI] [PubMed] [Google Scholar]
- 45.Sutherland FJ, Baker KE, Shattock MJ, Hearse DJ. Clin. Exp. Pharmacol. Physiol. 2003;30:879–884. doi: 10.1046/j.1440-1681.2003.03926.x. [DOI] [PubMed] [Google Scholar]
- 46.Dzeja PP, Terzic A. In: Handbook of Neurochemistry and Molecular Neurobiology: Brain Energetics. Integration of Molecular and Cellular Processes. Gibson G, Dienel G, editors. New York: Springer; 2006. pp. 641–666. [Google Scholar]
- 47.Flood AJ, Willems L, Headrick JP. Cardiovasc. Res. 2002;55:161–170. doi: 10.1016/s0008-6363(02)00329-2. [DOI] [PubMed] [Google Scholar]
- 48.Evans AM, Mustard KJ, Wyatt CN, Peers C, Dipp M, Kumar P, Kinnear NP, Hardie DG. J. Biol. Chem. 2005;280:41504–41511. doi: 10.1074/jbc.M510040200. [DOI] [PubMed] [Google Scholar]
- 49.Dzeja PP, Vitkevicius KT, Redfield MM, Burnett JC, Terzic A. Circ. Res. 1999;84:1137–1143. doi: 10.1161/01.res.84.10.1137. [DOI] [PubMed] [Google Scholar]
- 50.Savabi F, Geiger PJ, Bessman SP. Biochem. Med. Metab. Biol. 1986;35:227–238. doi: 10.1016/0885-4505(86)90078-2. [DOI] [PubMed] [Google Scholar]
- 51.Frederich M, Balschi JA. J. Biol. Chem. 2002;277:1928–1932. doi: 10.1074/jbc.M107128200. [DOI] [PubMed] [Google Scholar]
- 52.Hardie DG. J. Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 53.Janssen E, de Groof A, Wijers M, Fransen J, Dzeja PP, Terzic A, Wieringa B. J. Biol. Chem. 2003;278:12937–12945. doi: 10.1074/jbc.M211465200. [DOI] [PubMed] [Google Scholar]
- 54.Hayashi M, Kim SW, Imanaka-Yoshida K, Yoshida T, Abel ED, Eliceiri B, Yang Y, Ulevitch RJ, Lee JD. J. Clin. Investig. 2004;113:1138–1148. doi: 10.1172/JCI19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikeno F, Inagaki K, Rezaee M, Mochly-Rosen D. Cardiovasc. Res. 2007;73:699–709. doi: 10.1016/j.cardiores.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paul RJ. Annu. Rev. Physiol. 1989;51:331–349. doi: 10.1146/annurev.ph.51.030189.001555. [DOI] [PubMed] [Google Scholar]
- 57.Pastorino JG, Hoek JB. Curr. Med. Chem. 2003;10:1535–1551. doi: 10.2174/0929867033457269. [DOI] [PubMed] [Google Scholar]
- 58.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Nat. Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 59.Kane GC, Lam CF, O’Cochlain F, Hodgson DM, Reyes S, Liu XK, Miki T, Seino S, Katusic ZS, Terzic A. FASEB J. 2006;20:2271–2280. doi: 10.1096/fj.06-6349com. [DOI] [PubMed] [Google Scholar]
- 60.Kroll K, Decking UK, Dreikorn K, Schrader J. Circ. Res. 1993;73:846–856. doi: 10.1161/01.res.73.5.846. [DOI] [PubMed] [Google Scholar]
- 61.Decking UK, Schlieper G, Kroll K, Schrader J. Circ. Res. 1997;81:154–164. doi: 10.1161/01.res.81.2.154. [DOI] [PubMed] [Google Scholar]
- 62.Nitenberg A, Antony I. Eur. Heart J. 1995;16:7–21. doi: 10.1093/eurheartj/16.suppl_i.7. [DOI] [PubMed] [Google Scholar]
- 63.Morrison RR, Talukder MA, Ledent C, Mustafa SJ. Am. J. Physiol. 2002;282:H437–H444. doi: 10.1152/ajpheart.00723.2001. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Kassab GS, Garland T, Jr, Shyy JY. Arterioscler. Thromb. Vasc. Biol. 2006;26:1281–1287. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- 65.da Silva CG, Jarzyna R, Specht A, Kaczmarek E. Circ. Res. 2006;98:e39–47. doi: 10.1161/01.RES.0000215436.92414.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goirand F, Solar M, Athea Y, Viollet B, Mateo P, Fortín D, Leclerc J, Hoerter J, Ventura-Clapier R, Garnier A. J. Physiol. 2007;581:1163–1171. doi: 10.1113/jphysiol.2007.132589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones CJ, Kuo L, Davis MJ, DeFily DV, Chilian WM. Circulation. 1995;91:1807–1813. doi: 10.1161/01.cir.91.6.1807. [DOI] [PubMed] [Google Scholar]