Abstract
The associations between social support and depression, and between stress and depression have been the subject of considerable research, and although this has included valuable longitudinal designs, these have rarely controlled for the genetic effects that mediate these associations. The sample comprised 7,356 female and 4,882 male participants aged 18-95 from the Australian NHMRC Twin Registry (ATR). Of these, between 100 and 324 female pairs and between 41 and 169 male pairs, depending on the measure, were monozygotic (MZ) pairs discordant for depression. We use the co-twin control design in combination with prospective analyses to explore the association between a composite of predictors (perceived social support, stress, and support × stress) and depression. With familial effects included, both perceived support and stress were antecedents to, and sequelae of, depression, but no stress-buffering occurred. With familial effects controlled, stress was a sequela of a prior depressive episode, and neither lack of support nor stress were antecedents to depression, though their interaction approached significance for males. The male twin who later became depressed had previously reported lower perceived support in the face of multiple stressors compared to his co-twin who did not become depressed. We show that associations commonly observed with prospective designs are partly due to familial factors.
Major Depression (MD) has been projected to become the second-leading cause of disability worldwide by 2020 (discussed in Murray & Lopez, 1996). In this paper, we explore one attribute contributing to depression, a lack of social support in the face of stress or adversity (Brown & Harris, 1978; Brown et al., 1975). Two models of this association dominate the literature; (a) the main effects or social-cognitive model (Rhode & Lakey, 1999), where social support lessens the burden of pathology regardless of stress, and (b) the diathesis stress-buffering model (Cohen & Wills, 1985), where support lessens the burden of pathology only at higher levels of stress. We will compare these models using the co-twin control design (Cederlof et al. , 1977; Kendler et al., 1999; Kendler et al., 1993b) comprising, as described shortly, phenotypic analyses and the discordant MZ analyses that assess unique environment effects. In addition to this, we use a prospective design to assess the direction of association; does support, stress or support × stress influence subsequent levels of depression or is this direction reversed? Thus, by combining discordant MZ analyses and the prospective design, we assess whether effects of the unique environment are antecedents to or sequelae of risk for depression.
Overview of methodologies used in the literature
Several techniques have been used to assess the relationship between depression and both social support and stress, and we consider these before reviewing the literature. Cross-sectional research has been abundant but is limited. The associations between depression and both stress (K. S. Kendler et al., 1999) and social support (Wade & Kendler, 2000b) are mediated by genetic factors. These shared etiologies inflate the associations from any such cross-sectional studies. Consequently, more rigorous designs are required (Finch & Zautra, 1992). Longitudinal techniques, including the prospective design and the more comprehensive panel design, improve on designs that are cross-sectional because they can assess how variables are associated after controlling for shared etiologies.
Behavior-genetic studies have also made an important contribution to the literature addressing the association between depression and social support, stress and support × stress. One such technique is the co-twin control design (Cederlof et al., 1977; Kendler et al., 1999; Kendler et al., 1993b). It is conceptually similar to the more popular classical twin design, except that it considers discordance between twin pairs, not the degree of association or correlation between twin pairs.
In using the co-twin control design to assess the association between support and depression, we can compare the association observed for three samples: (a) a phenotypic sample, (i.e., of non-twins, or twins treated as individuals and not as pairs who are discordant) (b) a within-pairs test of MZ twins discordant for depression and (c) a within-pairs test of dizygotic (DZ) twins discordant for depression. Here we omit the analysis of DZ pairs, as detailed in the next paragraph. This aside, if social support and depression covary via unique environmental effects, then the association will be similar in MZ pairs, DZ pairs, and the phenotype. If they covary via the common environment, then the association will still exist for the phenotype, but will disappear for MZ pairs and DZ pairs. If support and depression covary via genetic influence, then the association will still be high for the phenotype, will not exist in MZ pairs, and will be about mid-way between the two for DZ pairs. Thus, the co-twin control design can elucidate how variables are associated; specifically, whether this association is due to (a) the unique environment, ‘causal’ factors, or (b) heredity or the common environment, ‘non-causal’ factors (Kendler et al., 1993b).
Here we only consider the discordant analyses for MZ and not DZ pairs. This is because our primary focus is to control all familial effects (both the common environment and genetic effects) and explore associations due solely to the unique environment. We include the phenotypic analyses to consider all associations without controlling for familial effects, in parallel with others using the co-twin design (Cederlof et al., 1977; Kendler et al., 1999; Kendler et al., 1993b), though some exclude the phenotypic analyses (Carr et al., 1981; Lynskey et al., 2006; Martin et al., 1982).
We review studies that employ either longitudinal designs or techniques from behavior genetics to assess the association between depression and social support, stress, and support × stress using adult samples. Further, we only consider studies of unselected samples and not those of selected samples (i.e., exposed to a particular stressful experience, such as breast cancer or combat), where studies are abundant.
The association between social support and depression
Longitudinal studies
Longitudinal research on the relationship between social support and depression has explored both the main effects of social support on subsequent levels of depression and the reverse, the effect of depression on subsequent levels of support. Research on the latter has found depression predicted lower social support in older (Cutrona et al., 1986) and middle aged adults (Johnson, 1991), but not in college students (Joiner & Metalsky, 1995). However, symptoms of depression did not predict a future increase in social rejection of male and female college students.
Most longitudinal research has explored the impact of social support on subsequent levels of depression. Lack of social support prospectively predicted symptoms of depression (Cohen et al., 1984; Fernandez et al., 1998; Finch & Zautra, 1992; Krause et al., 1989; Oxman et al., 1992; Russell & Cutrona, 1991; Wallace & O'Hara, 1992), onset of depression (Phifer & Murrell, 1986), the course of existing symptoms (Cutrona, 1984; Kivela & Pahkala, 1989; Lin & Ensel, 1984), and distinguished remitted depressives from controls (Billings & Moos, 1985). Conversely, support did not reduce symptoms of depression (Cranford, 2004; Fukukawa et al., 2000; Monroe, 1983; Monroe et al., 1983), and receiving adequate support from a single extramarital confidant did not protect against the onset of a depressive episode (O'Hara, 1986). Overall, the literature suggests social support is both an antecedent to, and a sequela of, depression, though the evidence for both is mixed.
Behavior genetic studies
Spotts et al. (2004) showed, in a sample of females, that the covariation between social support and depression comprised approximately two-thirds unique environment effects specific to social support and depression, and one-third genetic and unique environment effects shared with marital satisfaction. Hence, both genetic and unique environment factors explained the association between support and depression. Wade and Kendler (2000b) also explored the relationship between social support and depression in females, using a longitudinal sample. They found that the relationship between social support and depression depended on the source of social support. For example, they found no association at all when the social support was from for ‘friends’ and ‘confidants,’ but for support from relatives, the association was due primarily to shared genes. Interestingly, they found no evidence of support operating in just the traditional sense, with low levels causing depression.
Sex differences
Some longitudinal research has explored sex differences in the association between social support and depression. One prospective study found the quality of social contacts predicted sub-clinical depression in women but not men (Bildt & Michelsen, 2002). By contrast, another found that social attachments both predicted and were predicted by mental distress in men but not women (Johnson, 1991). Beyond these studies however, research is scarce.
A behavior genetic study exploring sex differences was performed by Kendler et al. (2005b). Overall, the relationship between lower support and the onset of MD was found to be stronger in women for a global measure of social support and for four of the seven sources of support: spouse, co-twin, parents and relatives. Thus, women with low social support appear more susceptible to depression than men with low social support.
The association between stress and depression
Several longitudinal studies explored the prospective association between stress and depression. Some showed stress was an antecedent to higher symptoms of depression (Cohen et al., 1984; Cranford, 2004; Fernandez et al., 1998; Monroe et al., 1983; Olstad et al., 2001), while others found no association (Monroe, 1983), even when considering this separately for sex (Bildt & Michelsen, 2002). Sex differences were also explored by Kendler et al. (2001) using DZ pairs discordant for sex. The result depended on the specific type of stressful event. Males were more susceptible to depression following either divorce/separation or work problems, while females were more susceptible following a problem getting on with someone in their close network. However, across all stressors, the male and female differences in the likelihood of depression were similar. A behavior-genetic study by Kendler et al. (1999) explored the association between stress and depression in females. They used the co-twin control design, and showed that about two-thirds of the association between stressful events and depression was due to the unique environment, with the balance due to heredity and the common environment. In summary, while the association between stress and depression is due, in part, to common genetic factors, unique environment factors clearly contribute to the association, and stress can be an antecedent to subsequent depression.
The association between social support, stress and depression
Longitudinal studies
Findings from longitudinal studies that explore stress-buffering (i.e., risk of depression increasing with each additional stressful event (SLE) for those with low but not high social support) are mixed. Some found evidence of stress-buffering (Cohen et al., 1984; Cohen et al., 1986; Dalgard et al., 1995; Fernandez et al., 1998; Frese, 1999; Monroe et al., 1983; Olstad et al., 2001; Ren et al., 1999; Stansfeld et al., 1997), while others found no such evidence (Cranford, 2004; Ingledew et al., 1997; Monroe, 1983; Wade & Kendler, 2000a). The studies differed in the measure of social support used, the time interval between occasions of measurement, whether support preceded or was preceded by the stressor, and the number of other predictors included in the analyses. Thus methodological differences may contribute to the inconsistent evidence for stress-buffering.
Behavior genetic studies
Kessler et al. (1992) showed that the nature of the relationship between MD, social support and stress in females depended on the source of social support. For example, for perceived friend support and frequency of club attendance the association with MD could be attributed to a third common process (e.g., locus of control), but for perceived spouse support, perceived relative support, confidant, frequency of interaction with relatives, frequency of interaction with friends, and frequency of church attendance it appears that levels of support affect depression in a causal mode.
Kendler et al. (2001), using the discordant MZ twin design with an all-female sample, showed that two of six dimensions of social support, problems with relatives and relative support (the other four dimensions being friend support, friend problems, confidants and social integration), helped explain the discordancy. So did exposure to stress; the pairs differed significantly such that the depressed twin had had higher exposure. Note, however, that compared to some other predictors, such as twin reports of maternal protectiveness, history of social phobia, and history of divorce, the associations between MD and stress and social support were very modest.
In possibly the most comprehensive study conducted to date on the causes of MD, Kendler and colleagues used longitudinal data and a behavior-genetic design to model the multitude of contributing pathways to MD in males (Kendler et al., 2006) and females (Kendler et al., 2002). Social support and stressful life events were components of adversity/interpersonal difficulties, one of the three major paths leading to MD (the other two major paths were internalzing problems and externalizing problems). This research confirms that low social support and stress are among the antecedents to the onset of depression.
Summary
Longitudinal and behavior-genetic techniques exploring the association between depression and support (as either a main effect or an interaction with stress) have varied; from panel and prospective designs, to the Cholesky and discordant twin-pair designs. The research on social support shows it is an antecedent to, and a sequela of, depression, though the findings are inconsistent. By contrast, the evidence for stress as an antecedent to depression appears more consistent, and the magnitude of the effect appears greater. Research on stress-buffering shows it does prospectively predict depression, but the findings are again inconsistent.
Some of the reviewed literature has controlled for heredity and the common environment when exploring the association between support and depression (Wade & Kendler, 2000b), or support and depression and stress and depression (Kendler & Gardner, 2001), but not support × stress and depression. Further, none of the research so far has explored these associations in males using a prospective design. Our research addresses these issues.
Method
Participants
The participants comprised an older and younger cohort (detailed in Table 1) from the Australian NHMRC Twin Register. All provided written informed consent under study protocols approved by the Queensland Institute of Medical Research Human Research Ethics Committee. During the period 1988-1990 study participants were mailed an extensive Health and Lifestyle Questionnaire (HLQ) containing items addressing social support and stressful life events (N =16,154, response rate 82%). The symptoms of anxiety and depression in the older cohort are typical of the Australian population (Kendler et al., 1986), although the level of education completed is higher, particularly for males (Baker et al., 1996). Over the period 1992-2000, participants were interviewed by telephone using a version of the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) modified for use in Australia (N = 16,302 response rate 83%), a comprehensive psychiatric interview assessing the physical, psychological and social manifestations of alcoholism and psychiatric disorders in adults (Bucholz et al., 1994) according to DSM-IV criteria (American-Psychiatric-Association., 1987). Summary statistics characterizing the cohorts are provided in Table 1.
Table 1.
Timing of the Assessments and Response Rates for the Two Cohorts Used in the Current Study; The Health and Lifestyle Questionnaire (HLQ) Provided Data on Personal (PLE) and network (NLE) Stressful Life Events (SLE), The SSAGA Interview Instrument Assessed Depression
| Older cohort Born <1964 | Younger cohort Born 1964-1971 | Combined | |
|---|---|---|---|
| HLQ | |||
| Mailed in Years | 1988-1989 | 1989-1990 | |
| N mailed (all these individuals were complete pairs) | 7616 | 8538 | 16154 |
| N of eligible participants | 7338a | 6502b | 13840 |
| N received (% responders, % female) | 6329 (86d, 65) | 5060 (78d, 57) | 11389 (82, 61) |
| Mean age (SD; range) | 42.3 (14.1; 24-95) | 23.4 (2.3; 18-29) | 32.3 (13.6; 18-95) |
| % with 1+ PLEs | 54 M, 53 F | 67 M, 67 F | 59 M, 58 F |
| % with 1+ NLEs | 63 M, 68 F | 61 M, 64 F | 62 M, 67 F |
| SSAGA | |||
| Telephoned in Years | 1992-1994 | 1996-2000 | |
| Mean Interval, HLQ to SSAGA (SD; range) | 3.9 (0.5; 1.1-5.0) | 6.7 (1.4; 3.8-10.0) | 4.2 (1.0; 1.1-10.0) |
| N telephoned (all these individuals were complete pairs) | 7764e | 8538 | 16302 |
| N of eligible participants | 7321f | 7325g | 14646 |
| N participating in depression section (% responders, % F) | 5992 (82, 65) | 6226 (85, 55) | 12190 (83, 60) |
| Mean age (SD; range) | 46.1 (14.2; 28-99) | 30.3 (2.4; 24-37) | 37.8 (12.7; 24-99) |
| % with Lifetime Major Depression (MD)h, i | 16 M, 18 F | 15 M, 22 F | 15 M, 20 F |
Note:
139 twin pairs were excluded, as one or both twins had died since participation in 1981.
18 pairs were in the older cohort so were excluded from the younger cohort, attempts were made to locate all non-responders but despite extensive effort 1000 pairs could not be recontacted - understandable, as we had recruited the majority of this cohort some 10 years earlier when they were at school.
c Includes participants who had failed to return a completed questionnaire but were followed up by telephone (up to five times), and asked to then complete an abbreviated telephone interview to obtain basic demographic information.
Response rate was higher for the older cohort as they were a sample of known responders; each had responded to a mail survey in 1981.
Includes the 3808 twin pairs from the HLQ plus 74 additional pairs.
443 individuals were ineligible for the study as they either; could not be located (87 individuals), were overseas (59 individuals), were deceased (295 individuals), or were not assigned for interview by the end of the study (2 individuals).
1213 were ineligible for the study as 268 pairs were lost and 677 individuals either; could not be located, were deceased, were incapacitated, were otherwise unable to complete the interview, or were not assigned for interview by the end of the study.
For a conservative diagnosis of DSM-IV MD, cases with postpartum onset are removed. Hence, the MD rate for females in the older cohort is lower than that in previous reports of this cohort (Bierut et al, 1999; Gillespie et al, 2005).
The MD diagnoses reported in this table includes cases with first onsets occurring before (as well as after) the reporting of SLE.
Measures
Kessler Perceived Social Support (KPSS)
The Kessler Perceived Social Support (KPSS) measure (Kessler et al., 1992; Kessler et al., 1994) contained 18 items. They comprised three questions assessing respondents’ belief that members of their social network would be willing to (a) listen to their problems, (b) understand the way they felt about things, and (c) help if help were needed. The three questions were asked for six sources of support (spouse, twin, children, parents, relatives and friends) on a four-point response scale; ‘not at all’ (0), ‘a little’ (1), ‘quite a bit’ (2), ‘a great deal’ (3).
A factor analysis (Coventry et al., 2004) showed a single factor solution explained 30% of the variability in both males and females. Further, Coventry (2008) showed that, of the unique environment variance in social support, a single factor explained 51% and 46% of the variance in males and females respectively. Hence, the discordant MZ analyses will capture approximately half the variance by using this single factor. The Cronbach's alpha for the single phenotypic factor was .87 (Coventry et al., 2004), and the test-retest reliability was .65, based on a sub-sample of 879 twins who completed the questionnaire twice at a mean interval of 2.1 years (Coventry et al., 2004).
To ensure that responses referred to current members of the support network, we removed the responses for participants who reported on (a) spouse support without apparently being married (585), (b) support from a deceased twin (17), (c) support from children without apparently having any children (163) and (d) support from parents even though both parents were reported deceased (667). We also deleted a support source if a participant had incomplete responses on any of the three items (help, listen and understand) that comprised each source. We then computed the single factor as the mean of all non-missing responses, so responses potentially range from 0 to 4.
Stressful Life Events (SLE)
The HLQ included a total of 40 items in three SLE inventories (personal, social problems, and network) which were adapted from the List of Threatening Experiences (LTE) proposed by Brugha et al. (1985). These inventories and the two factors (personal, [PLE] and network [NLE] life events), created from the 40 items using a preliminary factor analysis (Coventry, 2008), are presented in Table 2. We recoded eight or more stressful life events (the highest 1.3% of cases for both personal and network SLE) to seven.
Table 2.
The Stressful Life Events (SLE) Items and the Two Factors, Personal and Network SLE, Derived From These Items
| Stressful Life Events (SLE) experienced in the past 12 months | |
| Personal SLE (PLE) factor | |
| 12-item inventory of personal life events | Divorce; marital separation; broken engagement or steady relationship; separation from other loved one or close friend; serious illness or injury; serious accident (not involving personal injury); being burgled or robbed; laid off or sacked from job; other serious difficulties at work; major financial problems; legal troubles or involvement with police; and living in unpleasant surroundings. |
| 7-item social problem inventory | Serious problems in relationships with a spouse, other family member, close friend, neighbour, someone living with them (e.g., child or elderly parent), their twin, or a workmate or co-worker. |
| Network SLE (NLE) factor | |
| 21-item network life events (NLE) inventory | Had participant's close relative or friend, died, suffered a serious illness / injury, or suffered a serious personal crisis. |
The internal reliability for our personal SLE factor (which comprised the personal and social problem items), was .68, and for our network factor, was .65. These are low, but this is expected since the scale comprises events that are largely independent. These reliability coefficients would in fact be bolstered by genetic effects that are consistently observed to be significant for SLE (Foley et al., 1996; Kendler et al., 1993a; Plomin et al., 1990; Thapar & McGuffin, 1996; Wang et al., 2005; Wierzbicki, 1989).
We considered treating the social problem items (see Table 2) as a measure of social support rather than a measure of SLE, because the measure of social support used by Wade et al. (2000b) contained similar social problem items. To resolve this, we ran a factor analysis that included items from the KPSS (18 items) and all SLE items (12 personal, 21 network, and 7 social problem items). A two-factor solution clearly delineated a social support factor and a SLE factor, and, interestingly, the seven social problem items clearly loaded on the SLE factor. This suggested the social problem items were more closely associated with the SLE items, so we treated them as SLE, not social support.
Depression
We derived two diagnoses of depression from the telephone interview; (a) DSM-IV diagnosis of MD, (b) a less severe depression diagnosis of sub-clinical depression (SCD). Diagnoses with SCD comprised individuals who had experienced two or more weeks of feeling depressed/down or a lot less interested in most things or unable to enjoy the things usually enjoyed. The more severe diagnosis of MD also required, first, least four of the following symptoms; (a) significant weight loss, (b) insomnia, (c) psychomotor agitation/retardation, (d) fatigue or loss of energy, (e) feelings of worthlessness or excessive/inappropriate guilt, (f) diminished ability to think/concentrate or indecisiveness and, (g) recurrent thoughts of death or suicide ideation or attempts. And second, impaired functioning, requiring that (a) help be sought or received from a doctor or other health professional, or (b) functioning in major responsibilities (e.g., job, home duties, study) or other areas of life be affected.
Participants with MD and SCD diagnoses were removed if (1) they met the criteria for mixed episode (i.e., manic depression), (2) their symptoms not due to childbirth, substance use (prescription medication, drugs or alcohol), serious illness, or events that were later found to be untrue, or (3) the symptoms were not better accounted for by bereavement.
Many studies on depression simply consider MD. We were keen to use a less severe criterion for two reasons: First, to maximise the sample size, particularly with male MZ twins discordant for depression only occurring during specific timeframes (discussed shortly); second, because the literature generally shows depression is not comprised of distinct sub-categories, but is a continuous dimension of liability (Akiskal et al., 1997; Cox et al., 2001; Judd et al., 1998; Kendell, 1982; Kendler & Gardner, 1998; Kessler et al., & Swartz, 1997; Maes et al., 1987; Zimmerman et al., 1986).
Participants with the MD or SCD diagnoses were not mutually exclusive. The MD and SCD variables comprised the same distribution, but the placement of the threshold for affected/unaffected was higher for MD. Therefore, the participants with MD (15% in males and 20% in females; see Table 1 for further details) were a subset of the participants with SCD (34% in males and 38% in females).
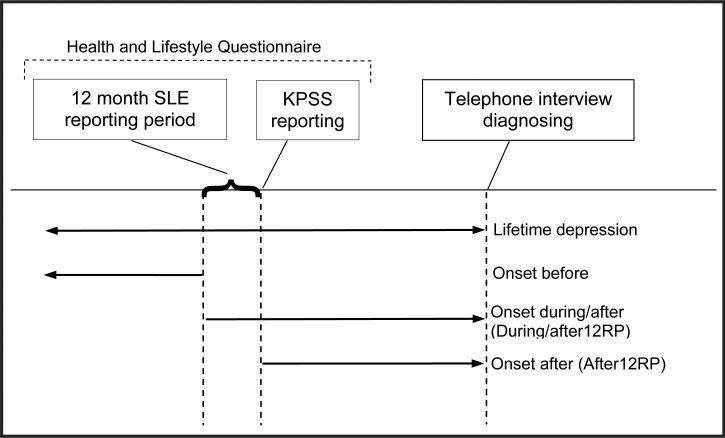
We defined four different time periods for the onset of depression (Gillespie et al., 2005), based on the onset of the first depressive episode, and present these in Figure 1. This was somewhat complicated as the questionnaire assessed perceived social support at the time of the questionnaire, but assessed SLE over the 12-month period before the questionnaire. To accommodate this, we defined four time-periods that comprised onset occurring:
Before, during, or after the 12-month reporting period (RP) for SLE, called Lifetime depression.
Before the 12 month reporting period for SLE, called Before12RP. As a rough guide, participants meeting this criterion represented between 46 and 52% of those with a lifetime diagnoses of depression.
Both (i) during or after the 12 month reporting period for SLE and (ii) in the 12 months before and after KPSS reporting, called During/after12RP. Participants meeting this criterion represented between 48 and 54% of those with a lifetime diagnoses of depression.
After SLE and KPSS reporting, called After12RP.
Figure 1.
The four different timeframes defined according to the onset of the first episode of depression. The Health and Lifestyle Questionnaire (HLQ) asked about participant's current level of perceived social support (KPSS) and their SLEs experienced over the last 12 months. The depression timeframes were relative to the 12-month Stressful Life Event reporting period (12RP).
Participants who had onsets of depression occurring outside the stipulated timeframes were treated as missing (discussed in Coventry, 2008).
Analysis
Polychoric correlations
We used polychoric correlations, computed in PRELIS (Jöreskog & Sörbom, 2005), to measure the association between each of our variables, since some were categorical (depression) and some were skewed (depression and SLE).
Phenotypic analyses
The phenotypic analyses were run using logistic regressions that predicted dichotomous measures of depression (MD and SCD) from sex, age, KPSS, SLE, and the KPSS by SLE interaction.
Discordant analyses
Our analyses of twin pairs discordant for depression were concerned with differences within twin pairs, where one but not both from the pair had depression. For these analyses, we ran a conditional logistic regression using the Cox regression procedure in SPSS. Time to onset was constant and stratification was by family so the twins were treated as a pair. The predictor variables included KPSS, SLE and the SLE by KPSS interaction. All predictor variables were standardized, thus making the odds ratios (OR) comparable for each predictor (Aiken & West, 1991). In the current analyses, the unit of analysis was the difference score between twin pairs, which were normally distributed for each predictor.
In line with previous research (Kendler et al., 1999), in the discordant analyses we only considered effects of personal, not network, SLE. This is because members of a twin pair will share many of their network SLE. For the phenotypic analyses however, we do consider the effects of network SLE.
Prospective design
In the prospective analyses, we explored KPSS and SLE as antecedents to depression by entering depression occurring either During/after12RP or After12RP as our dependent variable (DV). This was equivalent to a regression predicting depression at Time 2 from perceived support and SLE at Time 1, controlling for depression at Time 1 (which was constant since no participants had depression at Time 1). We also explored the reverse, KPSS and SLE as sequelae of depression, by entering depression occurring before SLE reporting (Before12RP) as our dependent variable. We applied this prospective design to both the phenotypic and discordant analyses.
Statistics used
We initially interpret significance using the Wald test. However, in small samples, the Wald is less reliable than the likelihood ratio test. Therefore, we also report significance using minus twice the difference in the log likelihood (-2ΔLL) for predictors significant at .01 on the Wald test. This -2ΔLL statistic has the distribution of a chi-squared statistic with degrees of freedom equal to the difference in number of parameters for successively nested models. Accordingly, we fitted stepwise regressions, which assessed the change in model fit with the addition of each predictor. Hence, the -2ΔLL estimates significance after controlling for predictors previously entered in the model. By contrast, the Wald test reports significance after controlling for all other predictors. Likewise, the OR (strength of effect) does the same. Hereafter, we consider significance according to -2ΔLL, and the strength of effect according to the OR.
Corrections for multiple testing
A Bonferroni correction was performed for the separate male and female analyses since they represented two sub-samples, with different subjects in each (Bland & Altman, 1995). Hence, with an α = .050, only p < .025 (.050/2) were significant. We did not apply a correction for the different severities of depression (MD or SCD), the different timeframes of depression (Lifetime, Before12RP, During/after12RP and After12RP), nor the different SLE measures (personal or network) but, instead, interpret the results with caution.
Comparing the phenotypic and discordant analyses
To compare the fit of the phenotypic analyses against those that used discordant twin pairs, we, like (Kendler et al., 1999), do not present a formal test to compare these models, but simply compare the ORs for each predictor. We treated our phenotypic and discordant analyses slightly differently in that we included age and sex as covariates in the phenotypic but not the discordant analyses. To consider the effects of this, we ran the phenotypic analyses with age and sex excluded as predictors. The differences in the estimates of the three predictors (KPSS, SLE and KPSS × SLE) were negligible, suggesting our different treatment might be inconsequential.
Results
Polychoric correlations
We present the polychoric correlations between all depression, KPSS, SLE and age variables in Table 3. Generally, the correlations for SCD were similar to the correlations for MD, so we only discuss correlations with MD here. Overall, the correlations with depression were stronger for personal life events than for both network life events and the KPSS.
Table 3.
Polychoric Correlations Between Major Depression (MD), Sub-Clinical Depression (SCD), Kessler Perceived Social Support (KPSS), Stressful Life Events (SLE), and Age for Males (Below Diagonal) and Females (Above Diagonal)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | ||||||||||||||
| 1 | MD Lifetime | r | 1.00 | 1.00 | 1.00 | .98 | .99 | .99 | .99 | -.11 | .27 | .08 | -.04 | |
| N | 6343 | 6329 | 6294 | 6215 | 5118 | 4961 | 4827 | 5065 | 5082 | 5018 | 6736 | |||
| 2 | MD Before12RP1 | r | 1.00 | 1.00 | 1.00 | .98 | .99 | .91 | .90 | -.13 | .26 | .12 | .07 | |
| N | 4426 | 5968 | 5967 | 5872 | 5039 | 4695 | 4589 | 4787 | 4802 | 4741 | 6311 | |||
| 3 | MD During/after12RP | r | 1.00 | 1.00 | 1.00 | .98 | .97 | .99 | .99 | -.07 | .29 | .05 | -.15 | |
| N | 4449 | 4277 | 6302 | 5849 | 4748 | 4965 | 4830 | 4720 | 4732 | 4674 | 6287 | |||
| 4 | MD After12RP | r | 1.00 | 1.00 | 1.00 | .97 | .97 | .99 | .99 | -.09 | .28 | .04 | -.15 | |
| N | 4432 | 4275 | 4435 | 5812 | 4733 | 4941 | 4830 | 4695 | 4707 | 4650 | 6250 | |||
| 5 | SCD Lifetime | r | .98 | .97 | .97 | .97 | 1.00 | 1.00 | 1.00 | -.11 | .32 | .10 | -.09 | |
| N | 4303 | 4140 | 4158 | 4140 | 5139 | 4969 | 4833 | 4663 | 4682 | 4620 | 6194 | |||
| 6 | SCD Before12RP | r | .98 | .98 | .96 | .96 | 1.00 | 1.00 | 1.00 | -.12 | .32 | .12 | .01 | |
| N | 3643 | 3615 | 3497 | 3493 | 3654 | 3865 | 3857 | 3881 | 3898 | 3840 | 5101 | |||
| 7 | SCD During/after12RP | r | .98 | .91 | .99 | .98 | 1.00 | 1.00 | 1.00 | -.07 | .28 | .06 | -.20 | |
| N | 3531 | 3394 | 3531 | 3516 | 3533 | 2871 | 4833 | 3743 | 3754 | 3701 | 4927 | |||
| 8 | SCD After12RP | r | .98 | .88 | .99 | .99 | 1.00 | 1.00 | 1.00 | -.08 | .26 | .04 | -.21 | |
| N | 3441 | 3328 | 3441 | 3440 | 3442 | 2866 | 3442 | 3643 | 3655 | 3602 | 4792 | |||
| 9 | KPSS | r | -.10 | -.08 | -.11 | -.11 | -.09 | -.09 | -.08 | -.10 | -.18 | -.04 | -.02 | |
| N | 2841 | 2748 | 2731 | 2722 | 2688 | 2286 | 2223 | 2171 | 6011 | 5938 | 6041 | |||
| 10 | Personal SLE | r | .22 | .19 | .23 | .23 | .27 | .26 | .24 | .22 | -.15 | .28 | -.22 | |
| N | 2873 | 2779 | 2760 | 2751 | 2722 | 2318 | 2246 | 2194 | 3402 | 5970 | 6076 | |||
| 11 | Network SLE | r | .16 | .18 | .14 | .14 | .16 | .18 | .10 | .10 | .00 | .29 | .03 | |
| N | 2834 | 2744 | 2723 | 2714 | 2684 | 2288 | 2219 | 2167 | 3343 | 3386 | 5995 | |||
| 12 | Age | r | .03 | .14 | -.06 | -.07 | -.03 | .04 | -.10 | -.10 | .01 | -.23 | .01 | |
| N | 4557 | 4373 | 4391 | 4373 | 4259 | 3603 | 3482 | 3392 | 3411 | 3456 | 3390 | |||
| Males | ||||||||||||||
NOTE:
12RP= the 12-month reporting period of SLE. See Figure 1 for details.
Phenotypic analyses
We present the results of the phenotypic analyses for Lifetime depression and onset Before12RP in tables 4 and 5 for personal and network SLE respectively, and for onset During/after12RP and After12RP in Tables 6 and 7 for personal and network SLE respectively. For age and sex, we only present the Wald statistic, not the -2ΔLL, since the two were virtually identical. The overall model fits (i.e., with all predictors entered) for all analyses were significant (p < .001), suggesting that all the predictors as a composite significantly predicted depression (SCD and MD).
Table 4.
Logistic Regressions Predicting Depression (Lifetime and Before12RP) From Kessler Perceived Social Support (KPSS), Personal Stressful Life Events (SLE) and the Interaction Between SLE and KPSS
| N | Full model | Sex | Age | KPSS |
Personal SLE |
KPSS × ⍰SLE |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| -2ΔLL | Wald | Wald | Wald | -2ΔLL | Wald | -2ΔLL | Wald | -2ΔLL | |||||||||||
| Δ χ 25 | p | OR | p | OR | p | OR | p | Δ χ21 | p | OR | p | Δ χ21 | p | OR | p | Δ | p | ||
| Major Depression (MD) | |||||||||||||||||||
| Lifetime depression | |||||||||||||||||||
| M | 7523 | 263.10 | .000** | 1.49 | .000** | 0.94 | .053 | 0.88 | .000 | 32.68 | .000** | 1.53 | .000 | 185.18 | .000** | 1.05 | .109 | 2.58 | .108 |
| M | 2750 | 54.86 | .000** | 1.03 | .670 | 0.87 | .012 | 10.09 | .001** | 1.41 | .000 | 42.66 | .000** | 1.06 | .246 | 1.35 | .245 | ||
| F | 4773 | 193.88 | .000** | 0.90 | .012* | 0.89 | .004 | 23.35 | .000** | 1.61 | .000 | 149.96 | .000** | 1.03 | .491 | 0.47 | .491 | ||
| Onset before 12mth SLE reporting period (Before12RP) | |||||||||||||||||||
| M | 6850 | 177.98 | .000** | 1.45 | .000** | 1.23 | .000** | 0.87 | .001 | 23.93 | .000** | 1.60 | .000 | 130.09 | .000** | 1.06 | .102 | 2.68 | .102 |
| M | 2548 | 54.46 | .000** | 1.40 | .000** | 0.85 | .035 | 6.75 | .009* | 1.52 | .000 | 35.04 | .000** | 1.10 | .132 | 2.30 | .129 | ||
| F | 4302 | 118.90 | .000** | 1.16 | .003** | 0.88 | .010 | 17.86 | .000** | 1.66 | .000 | 98.82 | .000** | 1.03 | .468 | 0.53 | .468 | ||
| Sub-Clinical Depression (SCD) | |||||||||||||||||||
| Lifetime depression | |||||||||||||||||||
| M | 7322 | 401.62 | .000** | 1.31 | .000** | 0.88 | .000** | 0.89 | .000 | 47.05 | .000** | 1.60 | .000 | 284.20 | .000** | 1.02 | .404 | 0.69 | .405 |
| M | 2679 | 108.32 | .000** | 0.89 | .015* | 0.91 | .020 | 11.36 | .001** | 1.47 | .000 | 79.51 | .000** | 1.00 | .914 | 0.01 | .914 | ||
| F | 4643 | 285.85 | .000** | 0.88 | .000** | 0.89 | .000 | 36.62 | .000** | 1.69 | .000 | 210.50 | .000** | 1.02 | .565 | 0.33 | .566 | ||
| Onset before 12mth SLE reporting period (Before12RP) | |||||||||||||||||||
| M | 6142 | 298.99 | .000** | 1.29 | .000** | 1.14 | .000** | 0.88 | .000 | 38.66 | .000** | 1.71 | .000 | 246.99 | .000** | 1.05 | .118 | 2.43 | .119 |
| M | 2278 | 74.48 | .000** | 1.12 | .046 | 0.89 | .039 | 8.33 | .004** | 1.55 | .000 | 65.43 | .000** | 1.04 | .451 | 0.57 | .451 | ||
| F | 3864 | 221.02 | .000** | 1.15 | .001** | 0.87 | .001 | 31.22 | .000** | 1.83 | .000 | 187.75 | .000** | 1.04 | .318 | 0.99 | .321 | ||
Note. p: ‘.000’ = ‘<.001’; For males M females (M & F), p < .050 (*) were significant at α = .050 and p < .010 (**) were significant at α = .010; For M or F, we used a Bonferonni correction (p/2), so p < .025 (*) were significant at α = .050 and p < .005 (**) were significant at α = .010.
Table 5.
Logistic Regressions Predicting Depression (Lifetime and Before12RP) From Kessler Perceived Social Support (KPSS), Network Stressful Life Events (SLE) and the Interaction Between SLE and KPSS
| N | Full model | Sex | Age | KPSS |
Network SLE |
KPSS × ⍰SLE |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| -2ΔLL | Wald | Wald | Wald | -2ΔLL | Wald | -2ΔLL | Wald | -2ΔLL | |||||||||||
| Δ χ 25 | p | OR | p | OR | p | OR | p | Δ χ21 | p | OR | p | Δ χ21 | p | OR | p | Δ χ21 | p | ||
| Major Depression (MD) | |||||||||||||||||||
| Lifetime depression | |||||||||||||||||||
| M & F | 7428 | 127.44 | .000** | 1.47 | .000** | 0.85 | .000** | 0.84 | .000 | 33.23 | .000** | 1.23 | .000 | 47.02 | .000** | 0.94 | .053 | 3.76 | .053 |
| M | 2716 | 30.65 | .000** | 0.95 | .424 | 0.82 | .000 | 11.20 | .001** | 1.23 | .000 | 16.85 | .000** | 0.92 | .148 | 2.12 | .145 | ||
| F | 4712 | 73.88 | .000** | 0.81 | .000** | 0.84 | .000 | 22.80 | .000** | 1.23 | .000 | 30.73 | .000** | 0.95 | .169 | 1.90 | .168 | ||
| Onset before 12mth SLE reporting period (Before12RP) | |||||||||||||||||||
| M & F | 6765 | 88.57 | .000** | 1.38 | .000** | 1.11 | .014* | 0.83 | .000 | 24.13 | .000** | 1.26 | .000 | 38.80 | .000** | 0.93 | .061 | 3.53 | .060 |
| M | 2522 | 33.41 | .000** | 1.28 | .001** | 0.82 | .007 | 6.69 | .010* | 1.29 | .000 | 14.80 | .000** | 0.96 | .515 | 0.43 | .514 | ||
| F | 4243 | 47.87 | .000** | 1.04 | .453 | 0.83 | .000 | 18.22 | .000** | 1.25 | .000 | 24.50 | .000** | 0.92 | .067 | 3.37 | .067 | ||
| Sub-Clinical Depression (SCD) | |||||||||||||||||||
| Lifetime depression | |||||||||||||||||||
| M & F | 7227 | 175.63 | .000** | 1.31 | .000** | 0.80 | .000** | 0.84 | .000 | 47.73 | .000** | 1.22 | .000 | 59.67 | .000** | 1.00 | .856 | 0.03 | .856 |
| M | 2645 | 57.68 | .000** | 0.81 | .000** | 0.86 | .000 | 11.48 | .001** | 1.26 | .000 | 28.18 | .000** | 0.97 | .562 | 0.34 | .562 | ||
| F | 4582 | 103.10 | .000** | 0.80 | .000** | 0.83 | .000 | 37.10 | .000** | 1.20 | .000 | 32.14 | .000** | 1.01 | .735 | 0.11 | .735 | ||
| Onset before 12mth SLE reporting period (Before12RP) | |||||||||||||||||||
| M & F | 6059 | 105.31 | .000** | 1.27 | .000** | 1.02 | .507 | 0.83 | .000 | 39.23 | .000** | 1.25 | .000 | 51.76 | .000** | 0.97 | .279 | 1.17 | .278 |
| M | 2252 | 32.13 | .000** | 1.01 | .877 | 0.85 | .003 | 8.05 | .005* | 1.29 | .000 | 23.90 | .000** | 0.99 | .799 | 0.06 | .799 | ||
| F | 3807 | 63.04 | .000** | 1.03 | .505 | 0.81 | .000 | 32.17 | .000** | 1.22 | .000 | 28.11 | .000** | 0.96 | .297 | 1.09 | .296 | ||
Note. p: ‘.000’ = ‘<.001’; For males & females (M & F), p < .050 (*) were significant at α = .050 and p < .010 (**) were significant at α = .010; For M or F, we used a Bonferonni correction (p/2), so p < .025 (*) were significant at α = .050 and p < .005 (**) were significant at α = .010.
Table 6.
Logistic Regressions Predicting Depression (During/after12RP and After12RP) From Kessler Perceived Social Support (KPSS), Personal Stressful Life Events (SLE) and the Interaction Between SLE and KPSS
| N | Full model | Sex | Age | KPSS | Personal SLE | KPSS × ⍰SLE | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| -2ΔLL | Wald | Wald | Wald | -2ΔLL | Wald | -2ΔLL | Wald | -2ΔLL | |||||||||||
| Δ χ25 | p | OR | p | OR | p | OR | p | Δ χ21 | p | OR | p | Δ χ21 | p | OR | p | Δ χ21 | p | ||
| Major Depression (MD) | |||||||||||||||||||
| Onset during/after 12mth SLE reporting period (During/after12RP) | |||||||||||||||||||
| M | 6829 | 225.70 | .000** | 1.57 | .000** | 0.62 | .000** | 0.89 | .010 | 15.10 | .000** | 1.46 | .000 | 83.81 | .000** | 1.03 | .474 | 0.52 | .473 |
| M | 2541 | 44.86 | .000** | 0.63 | .000** | 0.88 | .082 | 4.99 | .026 | 1.26 | .001 | 11.63 | .001** | 1.00 | .974 | 0.00 | .974 | ||
| F | 4288 | 175.89 | .000** | 0.61 | .000** | 0.91 | .078 | 10.24 | .001** | 1.59 | .000 | 80.49 | .000** | 1.02 | .714 | 0.13 | .714 | ||
| Onset after 12mth SLE reporting period (After12RP) | |||||||||||||||||||
| M | 6755 | 202.14 | .000** | 1.58 | .000** | 0.59 | .000** | 0.87 | .004 | 16.10 | .000** | 1.42 | .000 | 63.62 | .000** | 1.03 | .442 | 0.59 | .441 |
| M | 2518 | 46.94 | .000** | 0.57 | .000** | 0.87 | .079 | 4.45 | .035 | 1.25 | .003 | 8.92 | .003** | 1.02 | .749 | 0.10 | .749 | ||
| F | 4237 | 148.89 | .000** | 0.60 | .000** | 0.88 | .032 | 11.57 | .001** | 1.52 | .000 | 60.55 | .000** | 1.01 | .786 | 0.07 | .785 | ||
| Sub-Clinical Depression (SCD) | |||||||||||||||||||
| Onset during/after 12mth SLE reporting period (During/after12RP) | |||||||||||||||||||
| M | 5942 | 350.46 | .000** | 1.36 | .000** | 0.59 | .000** | 0.90 | .002 | 20.66 | .000** | 1.46 | .000 | 112.14 | .000** | 0.99 | .815 | 0.05 | .815 |
| M | 2215 | 97.61 | .000** | 0.62 | .000** | 0.91 | .096 | 5.77 | .016* | 1.36 | .000 | 33.13 | .000** | 0.97 | .589 | 0.29 | .588 | ||
| F | 3727 | 248.68 | .000** | 0.58 | .000** | 0.90 | .012 | 15.35 | .000** | 1.52 | .000 | 81.23 | .000** | 0.99 | .911 | 0.01 | .911 | ||
| Onset after 12mth SLE reporting period (After12RP) | |||||||||||||||||||
| M | 5791 | 314.58 | .000** | 1.38 | .000** | 0.57 | .000** | 0.88 | .000 | 24.13 | .000** | 1.40 | .000 | 82.83 | .000** | 0.97 | .480 | 0.50 | .479 |
| M | 2163 | 88.97 | .000** | 0.60 | .000** | 0.88 | .034 | 8.10 | .004** | 1.31 | .000 | 24.99 | .000** | 0.95 | .388 | 0.75 | .386 | ||
| F | 3628 | 221.27 | .000** | 0.56 | .000** | 0.88 | .005 | 16.31 | .000** | 1.45 | .000 | 59.35 | .000** | 0.98 | .677 | 0.17 | .677 | ||
Note. p: ‘.000’ = ‘<.001’; For males & females (M & F), p < .050 (*) were significant at α = .050 and p < .010 (**) were significant at α = .010; For M or F, we used a Bonferonni correction (p/2), so p < .025 (*) were significant at α = .050 and p < .005 (**) were significant at α = .010.
Table 7.
Logistic Regressions Predicting Depression (During/after12RP and After12RP) From Kessler Perceived Social Support (KPSS), Network Stressful Life Events (SLE) and the Interaction Between SLE and KPSS
| N | Full model | Sex | Age | KPSS |
Network SLE |
KPSS × ⍰SLE |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| -2ΔLL | Wald | Wald | Wald | -2ΔLL | Wald | -2ΔLL | Wald | -2ΔLL | |||||||||||
| Δ χ25 | p | OR | p | OR | p | OR | p | Δ χ21 | p | OR | p | Δ χ21 | p | OR | p | Δ χ21 | p | ||
| Major Depression (MD) | |||||||||||||||||||
| Onset during/after 12mth SLE reporting period (During/after12RP) | |||||||||||||||||||
| M | 6744 | 155.45 | .000** | 1.58 | .000** | 0.56 | .000** | 0.85 | .000 | 15.20 | .000** | 1.19 | .000 | 16.83 | .000** | 0.95 | .253 | 1.30 | .253 |
| M | 2510 | 41.82 | .000** | 0.59 | .000** | 0.82 | .011 | 5.96 | .015* | 1.19 | .028 | 6.95 | .008* | 0.89 | .122 | 2.43 | .119 | ||
| F | 4234 | 99.09 | .000** | 0.55 | .000** | 0.85 | .003 | 9.41 | .002** | 1.18 | .001 | 10.14 | .001** | 0.98 | .753 | 0.10 | .754 | ||
| Onset after 12mth SLE reporting period (After12RP) | |||||||||||||||||||
| M | 6670 | 149.36 | .000** | 1.60 | .000** | 0.55 | .000** | 0.83 | .000 | 16.95 | .000** | 1.18 | .000 | 13.57 | .000** | 0.95 | .304 | 1.05 | .304 |
| M | 2487 | 46.63 | .000** | 0.52 | .000** | 0.82 | .017 | 5.44 | .020* | 1.25 | .005 | 8.84 | .003** | 0.94 | .422 | 0.65 | .420 | ||
| F | 4183 | 88.93 | .000** | 0.55 | .000** | 0.83 | .001 | 11.35 | .001** | 1.14 | .014 | 5.90 | .015* | 0.97 | .562 | 0.34 | .562 | ||
| Sub-Clinical Depression (SCD) | |||||||||||||||||||
| Onset during/after 12mth SLE reporting period (During/after12RP) | |||||||||||||||||||
| M | 5866 | 252.04 | .000** | 1.35 | .000** | 0.55 | .000** | 0.86 | .000 | 21.00 | .000** | 1.17 | .000 | 20.58 | .000** | 1.04 | .309 | 1.04 | .309 |
| M | 2191 | 73.89 | .000** | 0.57 | .000** | 0.86 | .009 | 6.26 | .012* | 1.20 | .002 | 9.71 | .002** | 0.97 | .587 | 0.30 | .587 | ||
| F | 3675 | 172.07 | .000** | 0.54 | .000** | 0.85 | .000 | 15.04 | .000** | 1.15 | .001 | 11.20 | .001** | 1.08 | .082 | 3.04 | .081 | ||
| Onset after 12mth SLE reporting period (After12RP) | |||||||||||||||||||
| M | 5715 | 240.50 | .000** | 1.37 | .000** | 0.53 | .000** | 0.84 | .000 | 25.11 | .000** | 1.16 | .000 | 15.11 | .000** | 1.03 | .426 | 0.63 | .426 |
| M | 2139 | 72.11 | .000** | 0.56 | .000** | 0.83 | .002 | 8.74 | .003** | 1.20 | .004 | 8.61 | .003** | 0.96 | .548 | 0.36 | .547 | ||
| F | 3576 | 162.88 | .000** | 0.52 | .000** | 0.83 | .000 | 16.57 | .000** | 1.12 | .011 | 7.20 | .007* | 1.08 | .126 | 2.36 | .125 | ||
Note. p: ‘.000’ = ‘<.001’; For males & females (M & F), p < .050 (*) were significant at α = .050 and p < .010 (**) were significant at α = .010; For M or F, we used a Bonferonni correction (p/2), so p < .025 (*) were significant at α = .050 and p < .005 (**) were significant at α = .010.
Sex
Depression was significantly higher in females across all analyses, as expected (p = .01). The OR for sex when averaged across all analyses was 1.42 (SD = 0.11; range = 1.27 to 1.60). This suggests that for every depressed male, there are 1.42 depressed females.
Age
Age varied in its association with depression. For all the analyses of onset occurring During/after12RP or After12RP, age was significant (p < .01), with depression declining with age (mean OR = 0.57; SD = 0.03; range = 0.52 to 0.63). For Lifetime depression (SCD and MD), the direction was the same, though the effect was not as strong, and was not significant for MD in males. However, for onset Before12RP, the direction of the association reversed and depression increased with age. However, this was not consistently significant across the analyses of SCD and MD with network and personal SLE. These findings suggest a curvilinear association, with depression increasing with age in early adulthood but decreasing with age in middle and older adulthood.
KPSS
All associations between perceived social support and depression were in the expected direction; non-depressed individuals had higher perceived support than the depressed participants (mean OR = 0.86; SD = 0.03; range = 0.81 to 0.91). This strength of effect (i.e., the OR) was also consistent and was similar in males and females.
SLE
Personal and network SLE were a significant predictor in all analyses. For SLE, the strength of effect was greater than that observed for the KPSS. For personal life events, the average OR of 1.51 (SD = 0.14; range = 1.25 to 1.83) showed individuals experiencing SLE were more likely to be depressed. For network events, the direction of this effect was the same, but the average OR was smaller, at 1.21 (SD = 0.05; range = 1.12 to 1.29). For personal life events but not network life events, the strength of effect was marginally higher in females than males. Across the different timeframes, there was a slight difference in the strength of the effect, with smaller effects for During/after12RP and After12RP than for Lifetime and Before12RP.
The SLE × KPSS interaction
We observed few interactions between KPSS and SLE in predicting SCD and MD. Across the 48 separate analyses, the ORs were close to 1.00 (mean OR = 0.98; range = 0.89 to 1.08). Further, we observed only two interactions and these only approached significance; p = .053 when network SLE × KPSS predicted Lifetime MD in males and females, and p = .060 when network SLE × KPSS predicted onset Before12RP in males and females. By chance alone, we would expect two at α = .05. Therefore, across the analyses, there is minimal support for an interaction.
A limitation with these phenotypic analyses is that the interdependence between twin pairs might inflate the p-values, but not the odds ratios. However, elsewhere, (Coventry, 2008), we show with additional analyses that this did little to inflate the p-values. Hence, it is unlikely the effects we observe are simply an artefact of non-independent data though we interpret our results with some caution.
Summary
Depression was higher in females, while the effects of age depended on whether depression occurred before or after SLE. Lower support was associated with higher depression, with the strength of effect similar across sex, depression severity and depression timeframe. Both personal and network SLE were associated with depression, with no pronounced differences across sex, depression severity and depression timeframe. There was minimal support for stress-buffering.
Analyses of MZ Pairs Discordant for Depression
Fit of the full regression models
For the analyses of MZ pairs discordant for depression, we present results for Lifetime depression and Before12RP in Table 8, and the results for During/after12RP and After12RP in Table 9. Across all analyses, the full regression models (i.e. with all predictors entered) were only significant in one of the 24 regressions with another two approaching significance. This suggests the prediction provided by the combination of independent variables was not significantly better than a null model with these independent variables fixed at zero. These results are in contrast to those from the phenotypic analyses, where the fits were clearly significant. While the full regressions were not significant, this does not negate interpreting individual predictors, some of which were significant, which we report next.
Table 8.
Cox Regressions Predicting Monozygotic (MZ) Twin Pairs Discordant for Depression (Lifetime and Before12RP) From Kessler Perceived Social Support (KPSS), Personal Stressful Life Events (SLE), and the KPSS × SLE interaction
| N | Full model | KPSS | Personal SLE |
KPSS ⍰⍰SLE |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| -2ΔLL | Wald | Wald | -2ΔLL | Wald | -2ΔLL | ||||||||
| Δ χ23 | p | OR | p | OR | p | Δ χ21 | p | OR | p | Δ χ21 | p | ||
| Major Depression (MD) | |||||||||||||
| Lifetime depression | |||||||||||||
| M & F | 327 | 8.92 | .030* | 1.06 | .550 | 1.23 | .009 | 8.53 | .003** | 0.95 | .470 | 0.53 | .467 |
| M | 105 | 4.33 | .228 | 0.89 | .521 | 1.27 | .096 | 3.47 | .063 | 0.94 | .697 | 0.15 | .696 |
| F | 222 | 6.06 | .109 | 1.13 | .270 | 1.20 | .046 | 5.04 | .025 | 0.94 | .464 | 0.54 | .461 |
| Onset before 12mth SLE reporting period (Before12RP) | |||||||||||||
| M & F | 157 | 5.67 | .129 | 1.08 | .595 | 1.36 | .022 | 5.45 | .020* | 1.07 | .593 | 0.29 | .593 |
| M | 53 | 5.37 | .146 | 0.75 | .310 | 1.53 | .052 | 3.55 | .059 | 1.26 | .336 | 0.94 | .332 |
| F | 104 | 3.05 | .384 | 1.23 | .228 | 1.27 | .187 | 2.00 | .157 | 1.01 | .941 | 0.01 | .941 |
| Sub-Clinical Depression (SCD) | |||||||||||||
| Lifetime depression | |||||||||||||
| M & F | 493 | 7.68 | .053 | 1.06 | .417 | 1.16 | .014 | 7.11 | .008** | 0.96 | .520 | 0.42 | .519 |
| M | 169 | 2.06 | .561 | 1.06 | .625 | 1.14 | .226 | 1.80 | .180 | 0.96 | .711 | 0.14 | .710 |
| F | 324 | 5.68 | .128 | 1.05 | .514 | 1.17 | .032 | 5.35 | .021* | 0.96 | .597 | 0.28 | .596 |
| Onset before 12mth SLE reporting period (Before12RP) | |||||||||||||
| M & F | 259 | 7.36 | .061 | 1.05 | .639 | 1.29 | .008 | 6.58 | .010* | 1.08 | .351 | 0.87 | .350 |
| M | 94 | 2.62 | .453 | 0.99 | .969 | 1.28 | .150 | 1.19 | .275 | 1.21 | .228 | 1.51 | .220 |
| F | 165 | 5.70 | .127 | 1.07 | .573 | 1.31 | .020 | 5.66 | .017* | 1.03 | .777 | 0.08 | .777 |
Note: p: For males & females (M & F), p < .050 (*) were significant at α = .050 and p < .010 (**) were significant at α = .010; For M or F, we used a Bonferroni correction (p/2), so p < .025 (*) were significant at α = .050 and p < .005 (**) were significant at α = .010.
Table 9.
Cox Regressions Predicting Monozygotic (MZ) Twin Pairs Discordant for Depression (During/after12RP and After12RP) From Kessler Perceived Social Support (KPSS), Personal Stressful Life Events (SLE) and the KPSS × SLE Interaction
| N | Full model | KPSS | Personal SLE |
KPSS ⍰⍰SLE |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| -2ΔLL | Wald | Wald | -2ΔLL | Wald | -2ΔLL | ||||||||
| Δ χ23 | p | OR | p | OR | p | Δ χ21 | p | OR | p | Δ χ21 | p | ||
| Major Depression (MD) | |||||||||||||
| Onset during/after 12mth SLE reporting period (During/after12RP) | |||||||||||||
| M & F | 164 | 5.64 | .130 | 1.06 | .641 | 1.24 | .085 | 3.12 | .077 | 0.81 | .105 | 2.97 | .085 |
| M | 51 | 3.85 | .278 | 0.93 | .752 | 1.22 | .406 | 0.46 | .499 | 0.58 | .073 | 3.62 | .057 |
| F | 113 | 3.65 | .302 | 1.10 | .574 | 1.25 | .119 | 2.73 | .098 | 0.87 | .330 | 1.04 | .308 |
| Onset after 12mth SLE reporting period (After12RP) | |||||||||||||
| M & F | 147 | 3.62 | .306 | 1.09 | .557 | 1.11 | .437 | 0.58 | .448 | 0.79 | .097 | 3.19 | .074 |
| M | 44 | 3.98 | .264 | 0.96 | .881 | 1.17 | .560 | 0.12 | .727 | 0.53 | .062 | 4.11 | .043 |
| F | 103 | 1.65 | .648 | 1.11 | .536 | 1.10 | .536 | 0.45 | .504 | 0.87 | .322 | 1.08 | .298 |
| Sub-Clinical Depression (SCD) | |||||||||||||
| Onset during/after 12mth SLE reporting period (During/after12RP) | |||||||||||||
| M & F | 238 | 5.53 | .137 | 1.07 | .532 | 1.14 | .185 | 2.32 | .128 | 0.84 | .077 | 3.40 | .065 |
| M | 77 | 6.52 | .089 | 1.12 | .534 | 1.27 | .215 | 1.19 | .276 | 0.62 | .024 | 5.70 | .017* |
| F | 161 | 1.95 | .583 | 1.05 | .709 | 1.11 | .336 | 1.26 | .262 | 0.92 | .413 | 0.70 | .404 |
| Onset after 12mth SLE reporting period (After12RP) | |||||||||||||
| M & F | 210 | 4.46 | .216 | 1.05 | .672 | 1.11 | .348 | 1.42 | .234 | 0.84 | .084 | 3.27 | .071 |
| M | 70 | 6.02 | .111 | 1.10 | .628 | 1.32 | .242 | 1.67 | .196 | 0.61 | .038 | 4.86 | .027 |
| F | 140 | 1.29 | .732 | 1.03 | .833 | 1.06 | .626 | 0.43 | .511 | 0.91 | .359 | 0.88 | .349 |
Note: p: For males & females (M & F), p < .050 (*) were significant at α = .050 and p < .010 (**) were significant at α = .010; For M or F, we used a Bonferroni correction (p/2), so p < .025 (*) were significant at α = .050 and p < .005 (**) were significant at α = .010.
SLE
For personal SLEs (since network events were not analyzed here, as previously discussed), the significant effects were for Lifetime depression and onset Before12RP, not onset During/after12RP or After12RP. They were significant (p < .05) in the joint male and female analyses of MD and SCD and in the female analyses of SCD but not MD. The ORs for personal SLE suggested the effects were similar across the different timeframes of depression, or marginally higher for earlier than later timeframes. The average OR for personal SLE was 1.22 (SD = 0.10). As expected, this was lower than the average OR from the phenotypic analyses of 1.51 (SD = 0.14). This suggests about half the association (.22/.51) between support and depression is due to effects of the unique environment.
The SLE × KPSS interaction
Despite seeing no main effects at all for KPSS, personal SLE interacted with KPSS in the joint male/female analyses and in the male analyses. However, this was only for onset During/after12RP and After12RP (not Lifetime or Before12RP), where the main effects for personal SLE had generally not been significant. In males/females combined, this interaction approached significance; for MD (OR = 0.81, p = .085 and OR = 0.79, p = .074 for During/after12RP and After12RP respectively) and SCD (OR = 0.84, p = .065 and OR = 0.84, p = .071 for During/after12RP and After12RP respectively).
From the separate analyses of males and females, it appears that the males largely drove the interaction. In males, it either approached significance, for MD (OR = 0.58, p = .057 and OR = 0.53, p = .043 for During/after12RP and After12RP respectively) and SCD (OR = 0.61, p = .027 for After12RP respectively), or was significant, for SCD (OR = 0.62, p = .017 for During/after12RP). The ORs also suggested this interaction was more pronounced for males (mean OR = 0.59), than females (mean OR = 0.89), and for onset During/after12RP and After12RP, than for Lifetime depression or onset Before12RP. The sample sizes for these interactions were small, particularly for MD (51 and 44 pairs for During/after12RP and After12RP respectively). The sample was slightly higher for SCD.
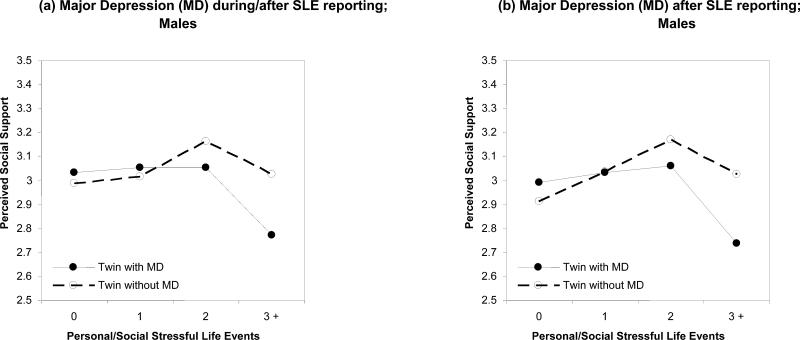
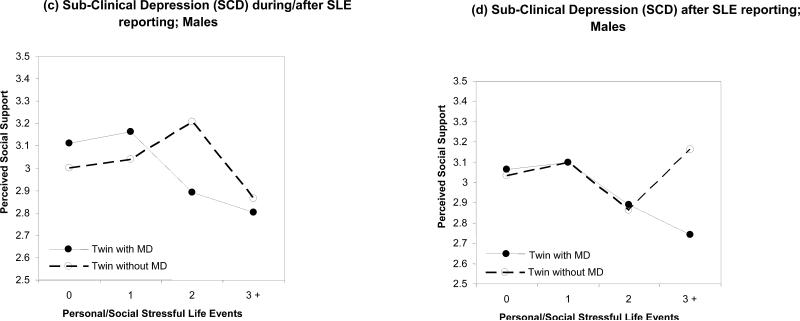
The interactions were generally in a stress-buffering direction (i.e., high but not low support is increasingly protective with increasing SLE), as seen in Figure 2, (a) through to (d). With little exposure to SLEs, perceived support was similar in the depressed and non-depressed twins. However, with two or more SLEs, support was generally lower in the twins who later became depressed than in the co-twins who did not become depressed (even though they also experienced two or more SLEs). However, there were some departures from this general trend. For SCD, for the During/after12RP but not the After12RP timeframes, the difference in KPSS between the depressed and non-depressed twins was primarily for two SLE and dissipated with three or more SLEs.
Figure 2.
Interactions between Kessler Perceived Social Support (KPSS) and personal Stressful Life Events (SLE) in predicting male MZ twin pairs discordant for depression (Major Depression (MD) and Sub-Clinical Depression (SCD)) using the Cox regression. None of the shown interactions were significant after correcting for multiple testing.
There are two possible explanations for this finding. First, it might possibly reflect chance variation, with a decline in the prevalence of discordant pairs who experience multiple SLEs. Second, it is possible that multiple SLE reached a threshold whereby the individuals were so stressed it made little difference whether they had high support or not, even though high support was effective in preventing SCD at stress levels immediately below this threshold, as has been discussed elsewhere (Kendler et al., 2005a).
Discussion
The prospective design assessed whether perceived social support, SLE and support × SLE were antecedents to, or sequelae of, depression by using different timeframes for depression onset (Gillespie et al., 2005); Lifetime depression and onsets Before12RP, During/after12RP and After12RP. Further, by using MZ twin pairs discordant for depression, we explored whether there was an association when considering only effects of the unique environment by controlling all familial influence.
The ORs showed the main effects for personal SLE from the discordant analyses were approximately half those from the phenotypic analyses. Hence, a combination of unique environment and familial effects account for the association between these SLE measures and depression. Further, these unique environment effects were significant. Although though this was primarily for Lifetime depression and Before12RP in females, the ORs suggested this association was equally strong in males and females, but declined slightly with subsequent timeframes. Hence, while the unique environment effects of personal SLE are an antecedent to, and a sequela of, depression, there was more support for the latter.
For perceived support, the contrast between the phenotypic and discordant analyses showed that, generally, the OR from the phenotypic analyses were stronger than those from the discordant analyses (which were not significant), suggesting again a combination of unique environment and familial effects explained the association between support and depression. The discordant analyses showed the main effects of perceived support were neither a significant antecedent to, nor sequela of, depression. This is contrary to a body of literature observing these effects. However, in contrast to these previous researchers, we only considered effects of the unique environment. Very few of these previous researchers controlled for familial effects. That our prospective associations were clearly stronger when we combined the familial effects with the effects of the unique environment suggests that the positive findings of previous researchers are, in part, due to the contributions of these familial effects.
Across the different timeframes, for both the phenotypic and discordant analyses, there were few differences in the effect sizes for perceived support. However, for SLE, we observed a slight decline in the effect size with later timeframes. Generally, across all analyses, the effects were similar between Lifetime and Before12RP and between During/after12RP and After12RP. It is understandable that effects for Lifetime and Before12RP are similar. If we take, for example, participants aged 30 years at the time of the questionnaire, for both Lifetime and Before12RP, they will be reporting on depression occurring in the 30 years beforehand. While this is a long lead-time, we have taken the view that any depressive episode, no matter what age, will still represent a precursor, in one twin, for subsequent KPSS or SLE levels. We were hesitant to refine these timeframes further (i.e., to be within closer proximity to the SLE reporting window) because of the resulting reduction in sample size.
The one effect that was unique to some timeframes and not others was the trend of a male interaction between personal SLE and KPSS, observed for only the later timeframes (During/after12RP and After12RP). The interaction in males was evident for different severities of depression, MD and SCD. This interaction suggests that the male twin who experiences many SLE but who also has lower perceived support at the end of this reporting period for SLE will be more likely than the co-twin to have an onset of depression in the years that follow. Thus, for males at least, stress-buffering effects of support might reduce depression after controlling for all familial effects.
We observed few differences across two severities of depression; SCD and MD. Mostly, the effect sizes for the associations were similar for each, or greater for MD. This is consistent with the notion of depression as a liability with a continuous distribution.
Limitations
Our sample comprises participants who had resided in Australia for at least a decade prior to interview, as the Australian NHMRC Twin Register (ATR) recruited them some 11 to 17 years earlier (for the older and younger cohorts respectively). At least for the younger cohort, that the ATR was able to contact them means they completed their schooling in Australia. This suggests they will have an established family and social network. By contrast, people working abroad, or immigrants, might not have this same family or social network established. Some interesting research has explored social support in migrant populations (Carta et al., 2001). The associations we observe may differ for individuals that have had less opportunity to establish themselves socially.
Second, the window between when participants reported their perceived social support and SLE and when they completed the interview for depression was 4.2 years on average, which is quite long when compared with other research. Further, the effects of stressful events are, generally, more immediate than our window would suggest (Kendler et al., 1998; Surtees & Wainwright, 1999). In attending to this limitation, we hoped to select only participants who experienced onsets of depression in the years immediately following the SLE reporting period, but, in the interests of retaining a sufficiently large sample, we could not. Therefore, our research considers medium term (three to five year) implications of the stressful event. Strictly speaking, it is not comparable to research considering only the immediate term.
Third, our analyses were not truly prospective in that, rather than assessing depression at the time of or soon after each depressive episode, we relied on participant's recall of when in their lives each depressive episode occurred. Hence, our measure is limited to the extent that participants recall was reliable.
Fourth, the current interpretations have assumed that differences between twin pairs are a consequence of ‘real’ unique environmental variance. It is also possible however, that these differences result from error variance, random developmental mutations or epigenetic effects including DNA methylation.
Fifth, an important avenue for future research will be to extend our research to the individual KPSS subscales; support from spouse, co-twin, children, parents, relatives, friends, and confidant, and helping support. We can then make comparisons with other studies which also considered some (Wade & Kendler, 2000b) or most (Kendler et al., 2005b) of these sub-scales.
Sixth, as previously mentioned, we did not fully account for multiple testing. While we corrected for the separate male and female analyses, we made no correction for the different timeframes (Lifetime, Before12RP, During/after12RP and After12RP), since the precise correction required was indeterminate. Further, in the phenotypic analyses (not necessary for discordant analyses), we made no correction for the different SLE measures (personal and network). Hence, it is appropriate to interpret our results with some caution.
Conclusion
In our phenotypic analyses, we observed associations of depression with low perceived social support and high stress, and, like Wade et al. (2000a), we found no evidence for stress buffering in these phenotypic analyses. We then controlled the familial effects. Intriguingly, neither support nor stress on their own were antecedents to depression, though stress was a sequela of depression. However, in males, the combination of support and stress interacted to provide a trend of stress-buffering against depression. Hence, perceived support in the face of multiple stressors was an antecedent mitigating subsequent depression.
While this finding provides promise for intervention, we must be cognisant of the role of support and stress when alongside the multitude of other important antecedents for depression. No individual risk factor alone causes depression. Rather, a combination of risk factors is required. To name but a few: genetic vulnerability, early-onset anxiety, substance abuse, low parental warmth and marital problems (Kendler & Prescott, 2006). Social support merely represents one risk factor for depression, and relative to these other factors, its influence is moderate at best.
Acknowledgments
This research was support by grants to N.G.M. from the National Health and Medical Research Council (NHMRC; 941177 & 971232) and to A.C.H. from the US Public Health Service (AA07535, AA07728 & AA10249). An Australian Postgraduate Award and Postdoctoral Fellowship from the University of New England supported W.L.C. We would like to thank the twins (drawn from the Australian NH&MRC Twin Registry) for their cooperation.
References
- Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Sage; Newbury Park, CA: 1991. [Google Scholar]
- Akiskal HS, Judd LL, Gillin JC, Lemmi H. Sub-threshold depressions: clinical and polysomnographic validation of dysthymic residual and masked forms. Journal of Affective Disorders. 1997;45:53–63. doi: 10.1016/s0165-0327(97)00059-1. [DOI] [PubMed] [Google Scholar]
- American-Psychiatric-Association . Diagnostic and statistical manual of mental disorders. revised 3 ed. Author; Washington, DC: 1987. [Google Scholar]
- Baker LA, Treloar SA, Reynolds CA, Heath AC, Martin NG. Genetics of educational attainment in Australian twins: Sex differences and secular changes. Behavior Genetics. 1996;26:89–102. doi: 10.1007/BF02359887. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Heath AC, Bucholz KK, Dinwiddie SH, Madden PAF, Statham DJ, Dunne MP, Martin NG. Major depressive disorder in a community-based twin sample; are there difference genetic and environmental contributions for men and women? Archives of General Psychiatry. 1999;56:557–563. doi: 10.1001/archpsyc.56.6.557. [DOI] [PubMed] [Google Scholar]
- Bildt C, Michelsen H. Gender differences in the effects from working conditions on mental health: a 4-year follow-up. International Archives of Occupational and Environmental Health. 2002;75:252–258. doi: 10.1007/s00420-001-0299-8. [DOI] [PubMed] [Google Scholar]
- Billings AG, Moos RH. Psychosocial processes of remission in unipolar depression: comparing depressed patients with matched community controls. Journal of Consulting and Clinical Psychology. 1985;53:314–325. doi: 10.1037//0022-006x.53.3.314. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. British Medical Journal. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Social origins of depression: A study of psychiatric disorders in women. Tavistock; London: 1978. [Google Scholar]
- Brown GW, Ni Bhrolchain MN, Harris TO. Social class and psychiatric disturbance among women in an urban population. Sociology. 1975;9:225–254. [Google Scholar]
- Brugha T, Bebbington P, Tennant C, Hurry J. The List of Threatening Experiences: a subset of 12 life event categories with considerable long-term contextual threat. Psychol Medicine. 1985;15:189–194. doi: 10.1017/s003329170002105x. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie DH, Hesselbrock VM, Nurnberger JL, Reich T, Schmidt I, Schukit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report of the reliability of the SSAGA. Journal of Studies on Alcoholism. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Carr AB, Martin NG, Whitfield JB. Usefulness of the co-twin control design in investigations as exemplified in a study of effects of ascorbic acid on laboratory test results. Clinical Chemistry. 1981;27:1469–1470. [PubMed] [Google Scholar]
- Carta MG, Coppo P, Reda MA, Hardoy MC, Carpiniello B. Depression and social change. From transcultural psychiatry to a constructivist model. Epidemiologia e Psichiatria Sociale. 2001;10:46–58. doi: 10.1017/s1121189x00008538. [DOI] [PubMed] [Google Scholar]
- Cederlof R, Friberg L, Lundman T. The interactions of smoking, environment and heredity and their implications for disease etiology. A report of epidemiological studies on the Swedish twin registries. Acta Medica Scandinavica Supplement. 1977;612:1–128. [PubMed] [Google Scholar]
- Cohen LH, McGowan J, Fooskas S, Rose S. Positive life events and social support and the relationship between life stress and psychological disorder. American Journal of Community Psychology. 1984;12:567–587. doi: 10.1007/BF00897213. [DOI] [PubMed] [Google Scholar]
- Cohen S, Sherrod DR, Clark MS. Social Skills and the Stress-Protective Role of Social Support. Journal of Personality and Social Psychology. 1986;50:963–973. doi: 10.1037//0022-3514.50.5.963. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98:310–357. [PubMed] [Google Scholar]
- Coventry WL. Perceived Social Support: Its Genetic and Environmental Etiology and Association with Depression. University of New England; Armidale: 2008. [Google Scholar]
- Coventry WL, Gillespie NA, Heath Az. C., Martin NG. Perceived social support in a large community sample: Age and sex differences. Social Psychiatry & Psychiatric Epidemiology. 2004;39:625–636. doi: 10.1007/s00127-004-0795-8. [DOI] [PubMed] [Google Scholar]
- Cox BJ, Enns MW, Larsen DK. The continuity of depression symptoms: use of cluster analysis for profile identification in patient and student samples. Journal of Affective Disorders. 2001;65:67–73. doi: 10.1016/s0165-0327(00)00253-6. [DOI] [PubMed] [Google Scholar]
- Cranford JA. Stress-buffering or stress-exacerbation? Social support and social undermining as moderators of the relationship between perceived stress and depressive symptoms among married people. Personal Relationships. 2004;11:23–40. doi: 10.1111/j.1475-6811.2004.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrona CE. Social support and stress in the transition to parenthood. Journal of Abnormal Psychology. 1984;93:378–390. doi: 10.1037//0021-843x.93.4.378. [DOI] [PubMed] [Google Scholar]
- Cutrona CE, Russell D, Rose J. Social support and adaptation to stress by the elderly. Journal of Psychology and Aging. 1986;1:47–54. doi: 10.1037//0882-7974.1.1.47. [DOI] [PubMed] [Google Scholar]
- Dalgard OS, Bjorck S, Tambs K. Social support, negative life events and mental health. British Journal of Psychiatry. 1995;166:29–34. doi: 10.1192/bjp.166.1.29. [DOI] [PubMed] [Google Scholar]
- Fernandez ME, Mutran EJ, Reitzes DC. Moderating the effects of stress on depressive symptoms. Research on Aging. 1998;20:163–183. [Google Scholar]
- Finch JF, Zautra AJ. Testing latent longitudinal models of social ties and depression among the elderly: a comparison of distribution-free and maximum likelihood estimates with non-normal data. Psychology and Aging. 1992;7:107–118. doi: 10.1037/0882-7974.7.1.107. [DOI] [PubMed] [Google Scholar]
- Foley DL, Neale MC, Kendler KS. A longitudinal study of stressful life events assessed at interview with an epidemiological sample of adult twins: the basis of individual variation in event exposure. Psychological Medicine. 1996;26:1239–1252. doi: 10.1017/s0033291700035960. [DOI] [PubMed] [Google Scholar]
- Frese M. Social support as a moderator of the relationship between work stressors and psychological dysfunctioning: a longitudinal study with objective measures. Journal of Occupational Health Psychology. 1999;4:179–192. doi: 10.1037//1076-8998.4.3.179. [DOI] [PubMed] [Google Scholar]
- Fukukawa Y, Tsuboi S, Niino N, Ando F, Kosugi S, Shimokata H. Effects of social support and self-esteem on depressive symptoms in Japanese middle-aged and elderly people. Journal of epidemiology / Japan Epidemiological Association. 2000;10:S63–69. doi: 10.2188/jea.10.1sup_63. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychological Medicine. 2005;35:101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- Ingledew DK, Hardy L, Cooper CL. Do resources bolster coping and does coping buffer stress? An organizational study with longitudinal aspect and control for negative affectivity. Jouranl of Occupational Health Psychology. 1997;2:118–133. doi: 10.1037//1076-8998.2.2.118. [DOI] [PubMed] [Google Scholar]
- Johnson TP. Mental health, social relations, and social selection: a longitudinal analysis. Journal of Health and Social Behavior. 1991;32:408–423. [PubMed] [Google Scholar]
- Joiner TE, Metalsky GI. A prospective test of an integrative interpersonal theory of depression: a naturalistic study of college room-mates. Journal of Personality and Social Psychology. 1995;69:778–788. doi: 10.1037//0022-3514.69.4.778. [DOI] [PubMed] [Google Scholar]
- Jöreskog K, Sörbom D. LISREL (Version 8.72) Scientific Software International, Inc.; 2005. [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB. A prospective 12-year study of subsyndromal and syndromal major depression symptoms in unipolar major depressive disorders. Archives of General Psychiatry. 1998;55:694–700. doi: 10.1001/archpsyc.55.8.694. [DOI] [PubMed] [Google Scholar]
- Kendell RE. The choice of diagnostic criteria for biological research. Archives of General Psychiatry. 1982;39:1334–1339. doi: 10.1001/archpsyc.1982.04290110084014. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Boundaries of major depression: an evaluation of DSM-VI criteria. American Journal of Psychiatry. 1998;155:172–177. doi: 10.1176/ajp.155.2.172. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Monozygotic twins discordant for major depression: a preliminary exploration of the role of environmental experiences in the aetiology and course of illness. Psychological Medicine. 2001;31:411–423. doi: 10.1017/s0033291701003622. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in women. The American Journal of Psychiatry. 2002;159:1133–1145. doi: 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in men. The American Journal of Psychiatry. 2006;163:115–124. doi: 10.1176/appi.ajp.163.1.115. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Martin NG, Eaves LJ. Symptoms of anxiety and depression in a volunteer twin population: The etiologic role of genetic and environmental factors. Archives of General Psychiatry. 1986;43:213–221. doi: 10.1001/archpsyc.1986.01800030023002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Stressful life events and major depression: risk period, long- term contextual threat, and diagnostic specificity. Journal of Nervous and Mental Disorders. 1998;186:661–669. doi: 10.1097/00005053-199811000-00001. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The Interaction of Stressful Life Events and a Serotonin Transporter Polymorphism in the Prediction of Episodes of Major Depression. Archives of General Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Prescott CA. Sex differences in the relationship between social support and risk for major depression: a longitudinal study of opposite-sex twin pairs. The American Journal of Psychiatry. 2005;162:250–256. doi: 10.1176/appi.ajp.162.2.250. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A twin study of recent life events and difficulties. Archives of General Psychiatry. 1993;50:789–796. doi: 10.1001/archpsyc.1993.01820220041005. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression: a causal analysis. Archives of General Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, environment, and psychopathology: understanding the causes of psychiatric and substance use disorders. The Guilford Press; New York and London: 2006. [Google Scholar]
- Kendler KS, Thornton LM, Prescott CA. Gender differences in the rates of exposure to stressful life events and sensitivity to their depressogenic effects. American Journal of Psychiatry. 2001;158:587–593. doi: 10.1176/appi.ajp.158.4.587. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Kendler KS, Heath AC, Neale MC, Eaves LJ. Social support, depressed mood, and adjustment to stress: A genetic epidemiologic investigation. Journal of Personality and Social Psychology. 1992;62:257–272. doi: 10.1037//0022-3514.62.2.257. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Kendler KS, Heath AC, Neale MC, Eaves LJ. Perceived support and adjustment to stress in a general population sample of female twins. Psychological Medicine. 1994;24:317–334. doi: 10.1017/s0033291700027306. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Zhao S, Blazer DG, Swartz M. Prevalence, correlates, and course of major depression and major depression in the national comorbidity survey. Journal of Affective Disorders. 1997;45:19–30. doi: 10.1016/s0165-0327(97)00056-6. [DOI] [PubMed] [Google Scholar]
- Kivela SL, Pahkala K. The prognosis of depression in old age. International Journal of Geriatric Psychiatry. 1989;1:119–133. doi: 10.1017/s104161028900013x. [DOI] [PubMed] [Google Scholar]
- Krause N, Liang J, Yatomi N. Satisfaction with social support and depression symptoms: a panel analysis. Psychology and Aging. 1989;4:88–97. doi: 10.1037//0882-7974.4.1.88. [DOI] [PubMed] [Google Scholar]
- Lin N, Ensel WM. Depression mobility and its social etiology: the role of life events and social support. Journal of Health and Social Behaviour. 1984;25:176–188. [PubMed] [Google Scholar]
- Lynskey MT, Vink JM, Boomsma DI. Early onset cannabis use and progression to other drug use in a sample of Dutch twins. Behavior Genetics. 2006;36:195–200. doi: 10.1007/s10519-005-9023-x. [DOI] [PubMed] [Google Scholar]
- Maes M, De Ruyter M, Suy E. Prediction of subtype and severity of depression by means of dexamethasone suppression test, L-tryptophan: completing amino acid ratio, and MHPG flow. Biological Psychiatry. 1987;22:177–188. doi: 10.1016/0006-3223(87)90228-9. [DOI] [PubMed] [Google Scholar]
- Martin NG, Carr AB, Oakeshott JG, Clark P. Human Genetics, Part A: The Unfolding Genome. Alan R. Liss, Inc.; New York: 1982. Co-twin control studies: Vitamin C and the common cold. pp. 365–373. [PubMed] [Google Scholar]
- Monroe SM. Social support and disorder: Towards an untangling of cause and effect. American Journal of Community Psychology. 1983;11:81–97. doi: 10.1007/BF00898420. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Imhoff DF, Wise BD, Harris EJ. Prediction of psychological symptoms under high-risk psychosocial circumstances: life events, social support, and symptom specificity. Journal of Abnormal Psychology. 1983;92:338–350. doi: 10.1037//0021-843x.92.3.338. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Evidence-based health policy--lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- O'Hara MW. Social support, life events, and depression during pregnancy and puerperium. Archives of General Psychiatry. 1986;43:569–573. doi: 10.1001/archpsyc.1986.01800060063008. [DOI] [PubMed] [Google Scholar]
- Olstad R, Sexton H, Sogaard AJ. The Finnmark study. A prospective population study of the social support buffer hypothesis, specific stressors and mental distress. Social Psychiatry and Psychiatric Epidemiology. 2001;36:582–589. doi: 10.1007/s127-001-8197-0. [DOI] [PubMed] [Google Scholar]
- Oxman TE, Berkman LF, Kasl S, Freeman DH, Barrett J. Social support and depressive symptoms in the elderly. American Journal of Epidemiology. 1992;135:356–368. doi: 10.1093/oxfordjournals.aje.a116297. [DOI] [PubMed] [Google Scholar]
- Phifer JF, Murrell SA. Etiologic factors in the onset of depressive symptoms in older adults. Journal of Consulting and Clinical Psychology. 1986;95:282–291. doi: 10.1037//0021-843x.95.3.282. [DOI] [PubMed] [Google Scholar]
- Plomin R, Lichtenstein P, Pedersen NL, McClearn GE, Nesselroade JR. Genetic influences on life events during the last half of the life span. Psychological Ageing. 1990;5:25–30. doi: 10.1037//0882-7974.5.1.25. [DOI] [PubMed] [Google Scholar]
- Ren XS, Skinner K, Lee A, Kazis L. Social support, social selection and self-assessed health status: results from the veterans health study in the United States. Social Science and Medicine. 1999;48:1721–1734. doi: 10.1016/s0277-9536(99)00069-6. [DOI] [PubMed] [Google Scholar]
- Rhode GL, Lakey B. Social support and psychological disorder: insight from social psychology. In: Kowalski RM, Leary R, editors. The social psychology of emotional and behavioral problems: interfaces of social and clinical psychology. American Psychological Association; Washington, DC: 1999. [Google Scholar]
- Russell DW, Cutrona CE. Social support, stress, and depressive symptoms among the elderly: test of process model. Psychology and Aging. 1991;6:190–201. doi: 10.1037//0882-7974.6.2.190. [DOI] [PubMed] [Google Scholar]
- Spotts EL, Neiderhiser JM, Ganiban J, Reiss D, Lichtenstein P, Hansson K, Cederblad M, Pedersen NL. Accounting for depressive symptoms in women: a twin study of associations with interpersonal relationships. Journal of Affective Disorders. 2004;82:101–111. doi: 10.1016/j.jad.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Stansfeld SA, Rael EGS, Head J, Shipley M, Marmot M. Social support and psychiatric sickness absence: a prospective study of British civil servants. Psychological Medicine. 1997;27:35–48. doi: 10.1017/s0033291796004254. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW. Surviving adversity: event decay, vulnerability and the onset of anxiety and depressive disorder. Eur Arch Psychiatry Clin Neurosci. 1999;249:86–95. doi: 10.1007/s004060050071. [DOI] [PubMed] [Google Scholar]
- Thapar A, McGuffin P. Genetic influences on life events in childhood. Psychlogical Medicine. 1996;26:813–820. doi: 10.1017/s0033291700037831. [DOI] [PubMed] [Google Scholar]
- Wade TD, Kendler KS. Absence of interactions between social support and stressful life events in the prediction of major depression and depressive symptomatology in women. Psychological Medicine. 2000a;30:965–974. doi: 10.1017/s0033291799002251. [DOI] [PubMed] [Google Scholar]
- Wade TD, Kendler KS. The relationship between social support and major depression: cross-sectional, longitudinal, and genetic perspectives. The Journal of Nervous and Mental Disease. 2000b;188:251–258. doi: 10.1097/00005053-200005000-00001. [DOI] [PubMed] [Google Scholar]
- Wallace J, O'Hara MW. Increases in depressive symptomatology in the rural elderly: results from a cross-sectional and longitudinal study. Journal of Abnormal Psychology. 1992;101:398–404. doi: 10.1037//0021-843x.101.3.398. [DOI] [PubMed] [Google Scholar]
- Wang X, Trivedi R, Treiber F, Snieder H. Genetic and Environmental Influences on Anger Expression, John Henryism, and Stressful Life Events: The Georgia Cardiovascular Twin Study. Psychosomatic Medicine. 2005;67:16–23. doi: 10.1097/01.psy.0000146331.10104.d4. [DOI] [PubMed] [Google Scholar]
- Wierzbicki M. Twins’ responses to pleasant, unpleasant, and life events. Journal of Genetic Psychology. 1989;150:135–145. doi: 10.1080/00221325.1989.9914585. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Coryell W, Pfohl B. Melancholic subtyping: a qualitative or quantitative distinction? American Journal of Psychiatry. 1986;143:98–100. doi: 10.1176/ajp.143.1.98. [DOI] [PubMed] [Google Scholar]