Summary
Anergic B cells are characterized by impaired signaling and activation following aggregation of their antigen receptors (BCR). The molecular basis of this impairment is not understood. In studies reported here Src homology-2 (SH2)-containing inositol 5-phosphatase SHIP-1 and its adaptor Dok-1 were found to be constitutively phosphorylated in anergic B cells, and activation of this inhibitory circuit was dependent on Src-family kinase activity and consequent to biased BCR immunoreceptor tyrosine-based activation motif (ITAM) monophosphorylation. B cell-targeted deletion of SHIP-1 caused severe lupus-like disease. Moreover, absence of SHIP-1 in B cells led to loss of anergy as indicated by restoration of BCR signaling, loss of anergic surface phenotype and production of autoantibodies. Thus chronic BCR signals maintain anergy in part via ITAM monophosphorylation-directed activation of an inhibitory signaling circuit involving SHIP-1 and Dok-1.
Introduction
Remarkably, nearly 70% of newly produced immature B cells are autoreactive and must be silenced to prevent autoimmunity (Wardemann et al., 2003). We have recently shown that many of these cells are silenced by anergy (Merrell et al., 2006). Anergy is a condition of antigen unresponsiveness that is induced by chronic autoantigen occupancy of as few as ~20% of antigen receptors (BCR), and can be rapidly reversed by removal of autoantigen from the BCR (Gauld et al., 2005; Goodnow et al., 1991). Curiously, chronically occupied receptors transduce both positive and negative signals. Reflecting the former, anergic B cells display a slight elevation (~50nM) in intracellular free calcium concentration ([Ca2+]i), as well as activation of the transcription factor NFAT and the tyrosine kinase ERK, that are lost within a few minutes of autoantigen dissociation from receptors (Gauld et al., 2005). Other BCR-linked pathways that are associated with cell activation, including those involving the signaling intermediaries Card11, NFκB and JNK, are not activated by chronic BCR occupancy, nor are these triggered by stimulation of unoccupied BCR on anergic cells (Benschop et al., 2001; Cambier et al., 2007; Cooke et al., 1994; Erikson et al., 1991; Gauld et al., 2005; Healy et al., 1998; Healy et al., 1997; Jun and Goodnow, 2003). Furthermore, some responses, e.g. antigen induced upregulation of CD86 expression, are restored following autoantigen dissociation from receptors (Gauld et al., 2005). These findings demonstrate that chronically occupied antigen receptors simultaneously transduce non-durable signals that stimulate some pathways that normally emanate from the BCR, while inhibiting others.
Little is known regarding the biochemical nature of inhibitory signals that are transduced by chronically occupied BCR and prevent activation of NFκB, CARD11 and JNK, while allowing activation of NFAT and ERK. It is possible that the former responses are limited by the inability to stimulate increased [Ca2+]i beyond the elevated basal concentrations in anergic cells. In support of this possibility are findings of Dolmetsch et al (Dolmetsch et al., 1997), which demonstrate that activation of NFκB requires greater [Ca2+]i than activation of NFAT. Regulatory mechanisms that govern calcium mobilization in anergic cells must target upstream signaling events since calcium store depletion-induced extracellular influx is intact in anergic cells (Yarkoni and Cambier, 2011). Consistent with this possibility, a recent study showed in a transgenic model that in anergic B cells BCR stimulation fails to induce normal accumulation of PtdIns3,4,5P3, a second messenger required for phospholipase C (PLCγ) signaling and, in turn, activation of calcium mobilization as well as Card 11, NFκB, JNK and Erk pathways (Browne et al., 2009). These findings suggest that failed generation or increased consumption of PtdIns3,4,5P3 may mediate the unresponsiveness of anergic B cells. Indeed, Browne and colleagues have reported that anergic anti-hen eggwhite lysoszyme (HEL) (MD4.ML5) B cells express increased amounts of the inositol lipid phosphatase PTEN that may consume PtdIns3,4,5P3 (Browne et al., 2009). However, this mechanism would seem to have durability inconsistent with minute time-scale reversibilty of anergy seen in the Ars/A1 immunoglobulin transgenic model (Gauld et al., 2005).
Here we report studies that address the basis of regulatory signaling by chronically occupied antigen receptors by analysis of anomalies in protein tyrosine phosphorylation in anergic cells. Results indicate that chronic BCR occupancy leads to biased monophosphorylation of the BCR CD79 ITAM motifs, leading in turn to Src-family kinase-dependent phosphorylation of the inositol 5-phosphatase SHIP-1 and its adaptor protein Downstream of kinase (Dok-1). Furthermore we show that SHIP-1 is critical for maintenance of anergy. B cell-targeted SHIP-1 gene ablation restores BCR signaling in anergic cells and leads to severe, early onset lupus-like autoimmunity. These findings indicate that two mechanisms that reduce the amount of PtdIns3,4,5P3 in cells are operative in maintaining B cell anergy. These include activation of the SHIP-1-Dok-1 circuit, which is seen in MD4.ML5 anti-hen egg lysozyme-hen egg lysozyme and Ars/A1 anti-DNA transgenic models of anergy, as well as in naturally occurring An1 (Anergic-1) B cells, and upregulation of PTEN, which seems to occur uniquely in cells with very high autoantigen avidity, e.g. MD4.ML5 B cells.
Results
Increased tyrosine phosphorylation of SHIP-1 and its adaptor Dok-1 in anergic B cells
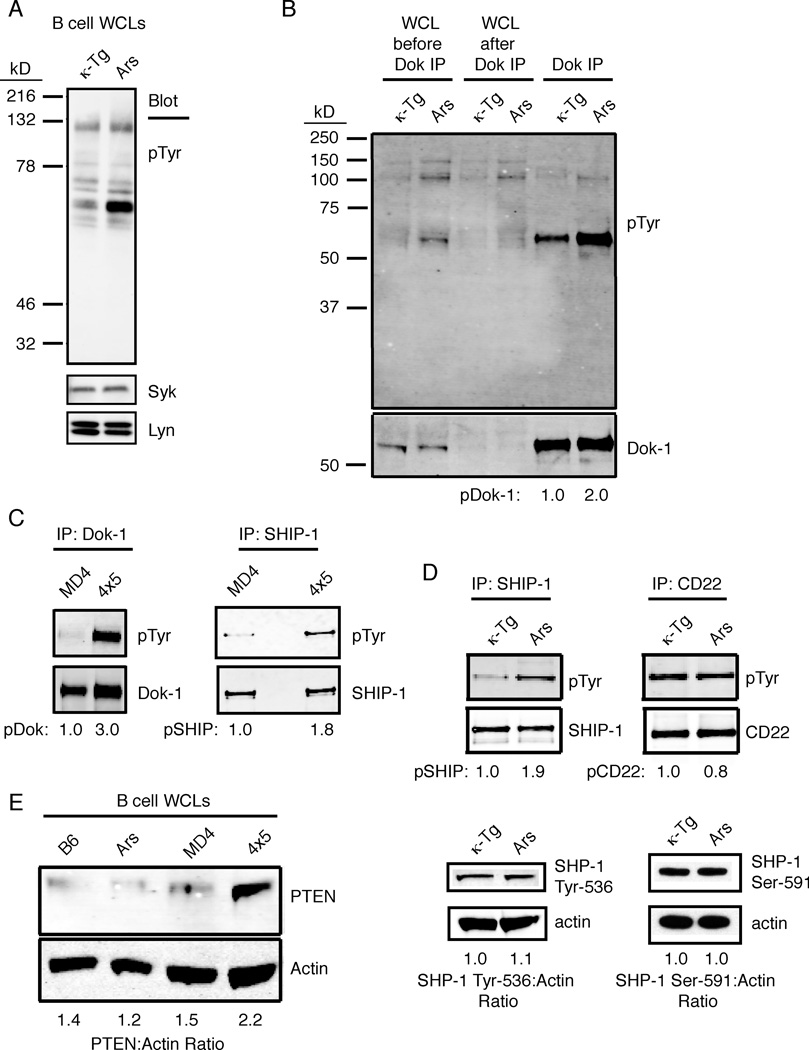
In naive B cells antigen receptors transduce signals via the proximal activation of tyrosine kinases, and therefore it seems plausible that anergy-enforcing signals transduced in anergic B cells might also be mediated by tyrosine phosphorylation, perhaps targeting unique substrates. To explore this possibility we compared basal protein tyrosine phosphorylation in immediately ex vivo anergic B cells from Ars/A1 mice to that of naïve B cells from animals transgenic for the κ light chain used to construct the Ars/A1 B cell antigen receptor (Benschop et al., 2001). When whole cell lysates were compared, the most significant difference seen was increased phosphorylation of a substrate of a ~62kDa protein (Fig. 1A). Based on its relative mass, we hypothesized that this phosphoprotein might be the adaptor Dok-1 and/or its closely related homolog Dok-3, both of which are expressed in B cells (Lemay et al., 2000). Further analysis indicated that this protein was not Dok-3, which migrates at a distinctly lower relative mass than phosphoprotein observed in anergic cells (supplemental figure 1). Indeed, when lysates were immunodepleted using an antibody reactive with Dok-1 prior to analysis, this band was depleted (Fig. 1B, first 4 lanes), and the immunoprecipitate contained the 62kDa phosphoprotein (Fig. 1B, lanes 5 and 6). Further analysis revealed that Dok-1 phosphorylation was also elevated in anergic B cells from MD4.ML5 mice (Fig. 1C, left panel). These findings are consistent with the reported increase in Dok-1 phosphorylation in naturally occurring AN1 anergic B cells (Merrell et al., 2006), and suggests that elevated tyrosine phosphorylation of Dok-1 is a general feature of anergic B cells.
Figure 1.
Chronic activation of the SHIP-1-Dok-1 inhibitory pathway in anergic B cells. (a) B cell lysates (0.5 × 106 cell equivalents) from the CD43-negative fraction of spleens from κTg or Ars/A1 (Ars) mice were lysed and immunoblotted to examine their ex-vivo basal expression of phosphoproteins (pTyr) as well as Syk and Lyn as loading controls. (b) Dok-1-sufficient or Dok-1-depleted B cell lysate samples were probed with anti-pTyr antibodies to confirm the identity of the elevated pTyr band in Fig. 1a, and the immunoprecipitated Dok-1 from either κTg or Ars B cell lysates is shown on the right of the blot. (c) Purified B cells (10 × 106) from MD4 and anergic MD4.ML5 mice were immunoprecipitated with anti-Dok and anti-SHIP-1 and probed with anti-pTyr antibody to examine basal pDok-1 or pSHIP-1 expression. (d) Purified B cells (10 × 106) from κTg or Ars/A1 spleens were immunoprecipitated with anti-SHIP-1 or anti-CD22 and immunoblots were probed with anti-pTyr (top panel), whole cell lysates (106 cell equivalents) were blotted for SHP-1 Y536 and SHP-1 S591; (e) B cells enriched from spleens of C57BL/6, Ars/A1, MD4 or MD4.ML5 mice were lysed and immunoblotted with anti-PTEN or anti-actin as a loading control. Blots are representative of one of at least 3 experiments.
Dok-1 functions as an adaptor for multiple effectors including the SH2-containing inositol 5-phosphatase SHIP-1 and ras GTPase p120 rasGAP (Helgason et al., 1998; Mashima et al., 2009). In view of a previous report stating that anergic B cells fail to accumulate PtdIns3,4,5P3 following stimulation (Browne et al., 2009), we were interested in the possible role of SHIP-1, the dominant SHIP isoform in B cells, in anergy. We found that in both MD4.ML5 and Ars/A1 anergic B cells SHIP-1 phosphorylation was elevated relative to controls (Fig. 1C, right, and 1D, left).
The SH2-containing phosphotyrosine phosphatase SHP-1 has also been implicated in regulation of BCR signaling and in maintenance of tolerance. In this regard, B cell targeted ablation of SHP-1 leads to lupus-like disease (Pao et al., 2007). To begin to explore possible roles in anergy of SHP-1 and immunoreceptor tyrosine-based inhibitory motif (ITIM)-containing receptors that engage this phosphatase, we analyzed tyrosine phosphorylation of CD22 (Ferry et al., 2005), and SHP-1 (Liu et al., 2007). Phosphorylation of CD22 is required for its recruitment of inhibitory effector molecules (Blasioli and Goodnow, 2002), and phosphorylation of SHP-1 Y536 and S591 has been shown to occur rapidly following antigen receptor stimulation (Zhang et al., 2003; Liu et al., 2007). We did not detect changes in phosphorylation of CD22, or SHP-1 in anergic cells, arguing against their function in maintaining anergy (Fig. 1D).
Recently, the inositol 3-phosphatase PTEN was proposed as a mediator of the unresponsive state of anergic B cells based on the fact that its expression is increased in anergic B cells from MD4.ML5 mice (Browne et al., 2009). To address this possibility further we sought to confirm the findings of Browne and Rickert (Browne et al., 2009) and extend them to the Ars/A1 model. As shown in figure 1E increased PTEN was detected in anergic MD4.ML5 B cells. However, we detected no increase in PTEN in anergic Ars/A1 B cells. These findings suggest that PTEN upregulation may be unique to B cells that are chronically stimulated by very high avidity autoantigens. While the affinity of MD4 anti-HEL for hen egg lysozyme is high (Ka > 2 × 109 M−1), the affinity of Ars/A1 BCR for ssDNA, its only known autoantigen, is much lower (<106 M−1).
Consistent with chronic activation of SHIP-1 (and PTEN upregulation in MD4.ML5 mice), basal Akt phosphorylation (as an indirect measurement of PtdIns3,4,5P3 accumulation) was reduced in anergic cells relative to controls, while basal CD19 phosphorylation was increased (supplemental figure 2). BCR stimulation of phosphatidylinnositol-3 (PI3) kinase is partially dependent on tyrosine phosphorylation of CD19 (Buhl and Cambier, 1999; Inabe and Kurosaki, 2002) and anergic B cells receive constant signals through the BCR due to association with self-antigen (Gauld et al., 2005). Increased basal phosphorylation of CD19 in the face of reduced basal activation of Akt is suggestive of chronically increased PtdIns3,4,5P3 hydrolysis in anergic cells rather than decreased PtdIns3,4,5P3 production. Additional aggregation of their BCR led to a modestly reduced CD19 phosphorylation in anergic B cells compared to naïve cells and reduced Akt phosphorylation. Taken together these data support the idea that increased hydrolysis of PtdIns3,4,5P3 by SHIP-1 plays a role in maintaining anergy.
B cell-targeted deletion of SHIP-1 results in severe lupus-like autoimmunity
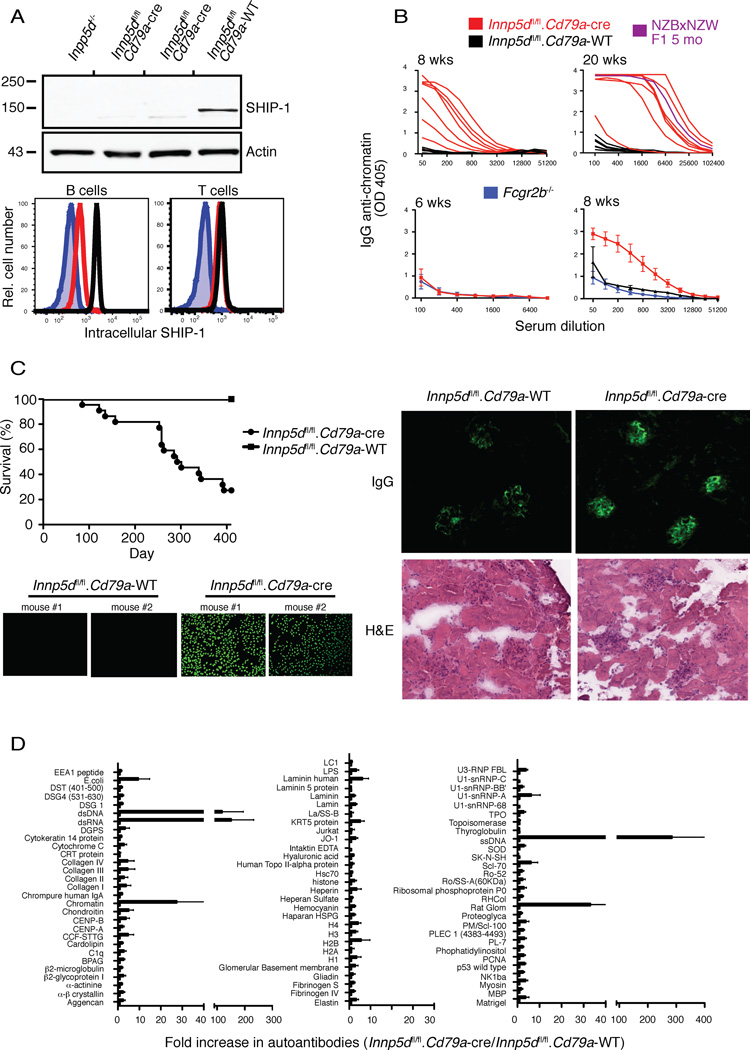
Given the evidence that the SHIP-1 pathway is active in anergic B cells, we next explored the affect of B cell-targeted Inpp5d (inositol polyphosphate-5-phosphatase D, ship-1 gene) ablation on immune tolerance. Mice in which the Inpp5d gene is flanked by LoxP sites (Karlsson et al., 2003) were crossed to mice expressing CRE recombinase under control of the Cd79a promoter (Hobeika et al., 2006). As shown in figure 2A, analysis of splenic B cells by both anti-SHIP-1 immunoblotting and immunofluorescence revealed that SHIP-1 expression is reduced by >90% in splenic B cells but unchanged in T cells. Thus conditional Inpp5d gene ablation in these mice is B cell-specific and, furthermore, most SHIP-1 protein decays between the pro-B cell when the Cd79a promoter is activated, and mature stages.
Figure 2.
Specific loss of SHIP-1 in the B cell compartment of Inpp5dfl/fl.Cd79a-cre mice induces autoimmunity in vivo. (a) B cell lysates from Inpp5d−/−, Inpp5dfl/fl.Cd79a-cre (2 mice shown) or Inpp5dfl/fl.Cd79a-WT mice were examined for SHIP-1 protein expression by Western blotting with an anti-SHIP-1 or actin as a loading control. B cells were separately examined by flow cytometry for intracellular SHIP-1 (lower panel); B cells (B220+) or T cells (CD3+) were gated. Inpp5d−/− (blue filled), Inpp5dfl/fl.Cd79a-cre (red), Inpp5dfl/fl.Cd79a-WT (black). (b) Anti-chromatin (IgG) ELISAs were used to assess serum autoantibody in young (6–8 wk old) or older (20 wk old) Inpp5dfl/fl.Cd79a-cre (red), Inpp5dfl/fl.Cd79a-WT (black), or Fcgr2b−/− (blue) mice. NZWxNZB F1 5 month old (blue) mice. NZWxNZB F1 5 month old mice (purple) were used as a positive control. Data from individual mice are plotted in the top panels. (n=7–10 mice/group). (c) Survival curve plotted for Inpp5dfl/fl.Cd79a-cre or Inpp5dfl/fl.Cd79a-WT mice (top panel to the left). Anti-nuclear antibody (ANA) production in 5-month-old Inpp5dfl/fl.Cd79a-WT or Inpp5dfl/fl.Cd79a-cre mice (lower panel to the left). Kidney sections were probed with a fluorescent anti-IgG to detect IgG deposition in the glomeruli of either 4 mo old Inpp5dfl/fl.Cd79a-WT or Inpp5dfl/fl.Cd79a-cre mice; H&E stains of consecutive sections (right panel). (d) An antibody array was utilized to detect serum autoantibodies in 5-month old Inpp5dfl/fl.Cd79a-cre or Inpp5dfl/fl.Cd79a-WT mice, n=3 of each mice. Relative autoantibody is expressed as a fold-increase in Inpp5dfl/fl.Cd79a-cre serum compared to Inpp5dfl/fl.Cd79a-WT control mice. Data are representative of three (a–c) or two (d) independent experiments.
We next examined the appearance of autoantibodies in mice in which B cells were ablated for Inpp5d. Chromatin autoantibodies were readily detectable at 8 weeks of age (Fig. 2B). At 20 weeks of age, B cell-targeted Inpp5d−/− mice expressed chromatin antibodies at amounts comparable to those seen in NZB/NZW mice of the same age. Sera contained anti-nuclear antibodies (ANA), and there was clear evidence of IgG deposition in kidney glomeruli (Fig. 2C). Furthermore, mice with B cell-targeted Inpp5d deletion died at a similar rate as reported for NZB/NZW (Auborn et al., 2003) with a <40% survival rate at 400 days, compared to 100% survival of mice that maintained normal SHIP-1 expression.
We considered the possibility that autoantibodies arise in these animals because the inhibitory signaling function of FcγRIIB, which is SHIP-1 dependent (Ono et al., 1996), would be mitigated, and Fcgr2b ablation causes a lupus-like autoimmunity in C57BL/6 mice (Bolland and Ravetch, 2000). It is noteworthy that studies shown in Figure 2 utilized B cell-targeted Inpp5D ablation on C57BL/6.129 mixed background, a situation in which Fcgr2b genetically targeted mice do not develop disease (Bolland and Ravetch, 2000). Suggestive of an FcR independent role for SHIP-1 in anergy, autoantibodies were not detectable in 8 week-old Fcgr2b −/− mice on pure C57BL/6 background, a time when autoantibodies were readily detected in mixed background Inpp5Dfl/fl.Cd79a-cre mice (Figure 2B). Further, a recent study showed that conventional Inpp5D−/− BALB/C mice produce anti-nuclear autoantibodies by 30 weeks of age, while Fcgr2b−/− BALB/C mice do not produce autoantibodies at any age (Bolland and Ravetch, 2000; Maxwell et al., 2011). This finding lends further support to the contention that FcγRIIB-independent SHIP-1 functions maintain B cell tolerance.
B cell SHIP-1 deficiency might be expected to compromise tolerance to all self-antigens. To explore the possibility that autoantibodies of additional specificities are made in the conditionally ablated mice we analyzed sera from 20-week old B cell-targeted Inpp5d−/− mice using an autoantigen array (Li et al., 2007). Autoantibodies produced were restricted to specificities seen in lupus, with the strongest reactivities detected against ssDNA, dsDNA, dsRNA and chromatin (Fig. 2D). Thus autoantibodies are produced selectively against autoantigens that carry linked ligands for toll-like receptors (TLRs) 7 and 9. These findings suggest that in normally silenced autoreactive B cells, SHIP-1 may function to inhibit BCR signals required to complement TLR signaling in B cell activation.
SHIP-1 is required to maintain B cell anergy
Inpp5d deletion could subvert tolerance by allowing autoreactive cells to evade receptor editing, deletion or anergy. To begin to explore these possibilities we analyzed the effect B cell-targeted Inpp5d ablation on anergy in Ars/A1 transgenic mice (Ars.Inppd5fl/fl.Cd79a-cre) and Inpp5d sufficient controls (Ars.Inpp5dWT.Cd79a-cre). Since immune tolerance to ssDNA required that B cell express SHIP-1 (fig. 2), we thought that anergy of Ar/A1 B cells might be disrupted in the absence of SHIP-1. In Ars/A1 mice in which B cells lacked SHIP-1, peripheral B cell numbers were reduced by ~70%, suggesting enhanced negative selection (data not shown). Since these mice are conventional Ig transgenics and are therefore unable to undergo receptor editing, this presumably reflects increased clonal deletion. Increased sensitivity to deletion is consistent with removal of a negative regulator of BCR signaling. Based on this result one might have expected that the tolerance of this clonotype might be better enforced in the conditionally ablated mice. However, as discussed below this is not the case.
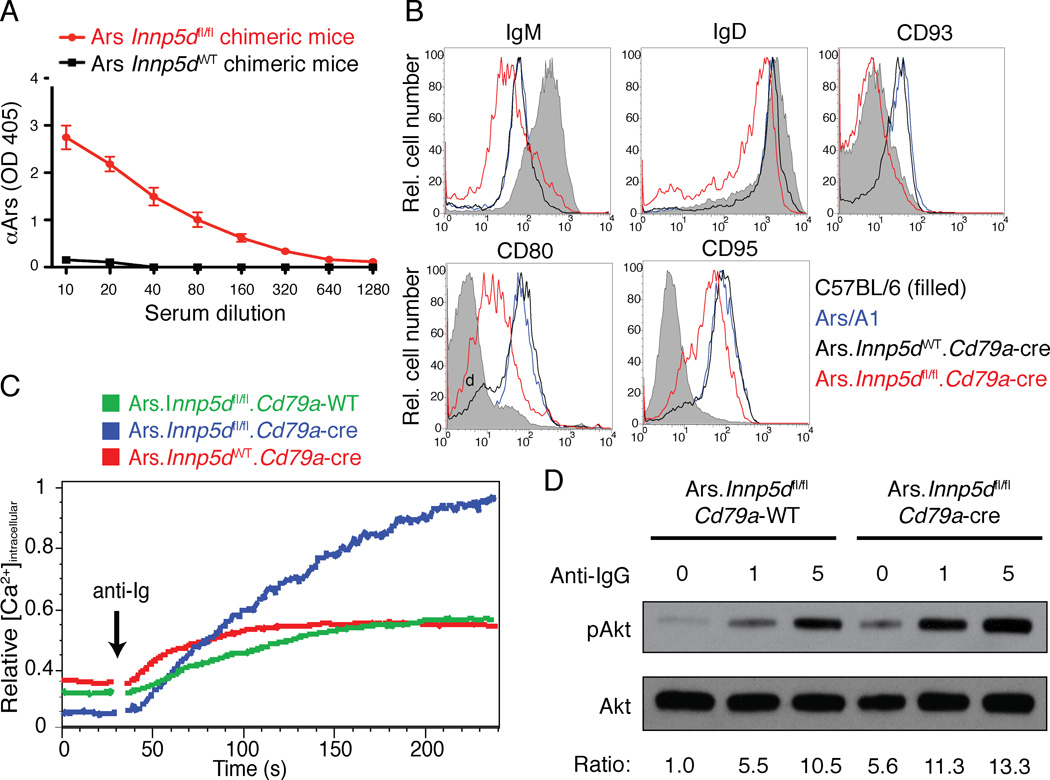
Analysis of autoantibody production in B cell-targeted Inpp5d−/− mice revealed loss of tolerance (Figs. 2B and 2D). By 20 weeks of age Ars/A1.Inpp5d−/fl/fl.Cd79a-cre mice spontaneously expressed ~4 fold increased autoantibody relative to Inpp5d sufficient littermates (data not shown). The seemingly modest increase in autoantibody in the conditionally targeted mice was somewhat surprising and we considered the possibility that autoantibody production or detection might be limited by antigen neutralization. To test this possibility we created bone marrow chimeric mice in which ~3–5% of splenic B cells were derived from Ars/A1 Inpp5d sufficient or conditional Inpp5d−/− Ars/A1 mice, while the remainder of the repertoire was diverse. As shown in figure 3A, by 20 weeks following bone marrow reconstitution, Inpp5d−/− Ars/A1 B cells gave rise to >100 fold more autoantibody than Ars/A1 Inpp5d-sufficient control mice.
Figure 3.
Exclusive deletion of Inpp5d in B cells reverses their anergic phenotype. (a) Bone marrow chimeras were generated and resulted in the following reconstitution ratio: Ars.Inpp5dfl/fl.Cd79a-cre (2.7%), C57BL/6 (97.3%) (red), and Ars.Inpp5dWT.Cd79a-cre (4.9%), C57BL/6 (95.1%) (black). These mice were evaluated at 5 months post-bone marrow transfer for the production of serum autoantibody (IgMa anti-arsonate) by ELISA. (b) B cells derived from C57BL/6 (black filled), Ars/A1 (blue), Ars.Inpp5dWT.Cd79a-cre (black) or Ars.Inpp5dfl/fl.Cd79a-cre (red) mice were evaluated for surface phenotype by flow cytometry. (c) Intracellular calcium mobilization was determined in B220+ B cells from Ars.Inpp5dWT.Cd79a-WT (green), Ars.Inpp5dfl/fl.Cd79a-cre (blue) or Ars.Inpp5dWT.Cd79a-cre (red) mice stimulated with 10 µg/ml anti-IgG (H+L) F(ab’)2. (d) Purified B cells from Ars.Inpp5dfl/fl.Cd79a-WT or Ars.Inpp5dfl/fl.Cd79a-cre mice were stimulated (0, 1 or 5 minutes) with 10 µg/ml anti-IgG (H+L) F(ab’)2, and lysates were immunoblotted with anti-pAkt (S473), or anti-pan Akt antibody. Data are representative of at least 2 experiments.
To more directly address the anergic status of the B cells in B cell-targeted Inpp5d−/− Ars/A1 mice, we analyzed their surface phenotype. Cell surface mIgM and mIgD were reduced 50% in conditionally Inpp5d deleted ex vivo B cells relative to anergic B cells from Ars/A1.Inpp5dWT.Cd79a-cre mice (Fig. 3B). This effect was consistent with continued in vivo exposure to antigen and resultant BCR modulation from the cell surface. Consistent with loss of anergy, Inpp5d -deficient B cells expressed reduced CD93, CD95 and CD80, the expression of which is normally increased in anergic B cells (Borrero and Clarke, 2002; Merrell et al., 2006). Thus surface phenotype of ex vivo B cells from conditional Inpp5d−/− Ars/A1 mice was indicative of loss of anergy. Further analysis revealed an increase in frequency of Ars/A1 B cells with activated phenotype (CD86+) (3.6 % vs 0.93% of B cells) in Inpp5d deleted vs sufficient B cells. Similarly we detected an increased frequency of Ars/A1 plasma cells (identified as CD138+, intracellular Ars/A1 idiotypehi) in the Inpp5d deficient Ars/A1 population (4.96% of B cells) compared to Inpp5d sufficient Ars/A1 B cells (0.04%).
To address BCR signaling function in Inpp5d deficient B cells from the Ars/A1 mice, we analyzed their ability to mobilize calcium in response to co-aggregation of mIgM and mIgD using F(ab’)2 anti-mouse H and L chain antibodies. B cells lacking SHIP-1 mounted robust calcium mobilization responses relative to anergic control populations (Fig. 3C). We further examined Akt activation in response to BCR ligation with antibody. Conditionally Inpp5d deleted Ars/A1 B cells underwent more rapid and robust Akt phosphorylation than anergic controls. Furthermore, these cells exhibited a >5 fold increase in basal Akt phosphorylation consistent with functional autoantigen-induced BCR signaling in vivo. Taken together these findings indicate that ssDNA reactive Ars/A1 B cells lacking SHIP-1 lose features of anergy, including antigen unresponsiveness measured by BCR aggregation-induced Akt activation and calcium mobilization, and autoantibody production. Importantly however, since virtually all peripheral B cells lose phenotypic features of anergy but only a small proportion proceed to the plasma cell stage, additional compromising events must be required to fully break anergy.
BCR signaling in anergic B cells drives ITAM monotyrosylphosphorylation and inhibitory signaling
We then explored upstream BCR signaling events that might bias downstream responses toward activation of the SHIP-1-Dok-1 inhibitory signaling circuit. The CD79a and b (Ig-αβ) subunits of the BCR each contain an immunoreceptor tyrosine-based activation motif (ITAM) with two conserved embedded tyrosine residues. We have previously shown that dual phosphorylation of these ITAMs is required for engagement of the Syk tyrosine kinase and stimulation of B cell activation (Kurosaki et al., 1995). It is known that Syk activation is lost in anergic cells (Cooke et al, 1994; Vilen et al., 1997). Therefore we considered the possibility that chronic BCR signals are mediated by ITAM monophosphorylation, and that these promote activation of the SHIP-1-Dok-1 circuit. Consistent with this possibility, monophosphorylation of ITAM tyrosines appears sufficient to promote activation of Lyn and possibly other Src-family kinases (Pao et al., 1998) and Lyn is required to maintain B cell tolerance to dsDNA (Chan et al., 1997). Thus ITAM monophosphorylation could, in part by further stimulating Src kinase activity, propagate signals that maintain anergy.
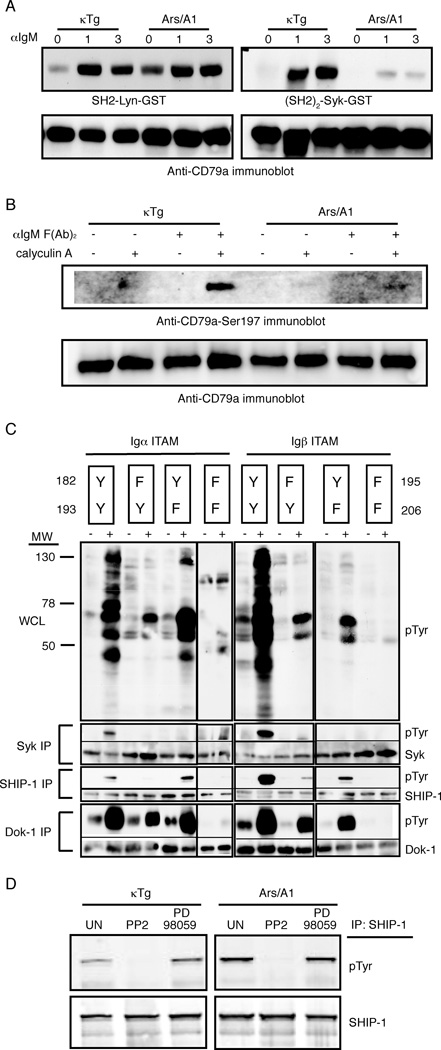
To explore this possibility we first assessed basal ITAM phosphorylation in anergic cells, analyzing in particular the relative amount of dual phosphorylation versus monophosphorylation of ITAM tyrosines. Since phosphorylation-dependent anti-CD79a and b ITAM tyrosine antibodies useful in immunoblotting are not available, we approached the problem by far-western blotting (Wienands et al., 1995). Specifically, we used the Lyn SH2 domain and the Syk (SH2)2 domain to probe electrophoretic transfers of SDS-PAGE fractionated CD79 co-immunoprecipitated with IgM from naive and anergic (Ars/A1) B cells. The Lyn SH2 binds all phosphorylated ITAM tyrosines while the Syk (SH2)2 binds only dually phosphorylated ITAMs (Adachi et al., 2007). This allowed estimation of total versus dual ITAM phosphorylation. As shown in Figure 4A, CD79a in anergic B cells exhibited increased basal ITAM phosphorylation relative to naïve cells as detected by binding of the Lyn SH2 probe. Dual CD79a tyrosine phosphorylation (as detected by binding of the (SH2)2-Syk-GST recombinant protein to the blot) was detected in lysates from unstimulated naïve (κ only tg) B cells but not in anergic Ars/A1 B cells, indicating that the increased basal CD79a ITAM phosphorylation in anergic cells is monophosphorylation. Phosphorylation of CD79b, which is normally low relative to CD79a, was not detected in these experiments (data not shown). Thus anergic B cells display elevated antigen receptor ITAM monophosphorylation.
Figure 4.
Increased ITAM monophosphorylation may transduce an inhibitory signal in anergic B cells. (a) The CD79a and b subunits of the BCR each contain an ITAM with two tyrosine residues. We used the Lyn SH2 domain and the Syk (SH2)2 domain as probes in a far western assay of CD79a and b co-immunoprecipitated with surface IgM from naïve κTg and anergic Ars/A1 B cells. The Lyn SH2 protein domain binds all phosphorylated ITAM tyrosines while the Syk (SH2)2 binds only dually phosphorylated ITAMs. (b) 107/ml κTg and Ars/A1 B cells were incubated for 17 min with 50nM calyclin A, 20µg/ml F(Ab’)2 anti-IgM, both or no stimulation. WCLs were probed with antibodies against CD79aS197 and total CD79. (c) The cytoplasmic tail of CD79a or CD79b was fused to the extracellular domain of the MHC class II (I-Ak) alpha or beta chains then expressed in B cell lymphoma lines. Either one or both ITAM tyrosines from each chimeric receptor were mutated to phenylalanine to assess the affect of monophosphorylation following receptor aggregation via anti-MHC class II (I-Ak) antibodies. B cells expressing I-Ak-CD79a or I-Ak-CD79b chimeric receptors with or without single or double mutations in the ITAM tyrosines within the cytoplasmic tails were treated with biotin-anti-I-Ak and crosslinked at 37°C with streptavidin. Lysates were used for immunoprecipitation with anti-Syk, anti-SHIP-1 or anti-Dok-1 beads and immunoblotting was done with anti-pTyr (4G10), anti-Syk, anti-SHIP-1 and anti-Dok-1. (d) Purified B cells were incubated with or without inhibitors (PP2, 10 µM; PD98059, 20 µM) for 30 minutes at 37°C and lysed. Lysates were used for immunoprecipitation with anti-SHIP-1 beads and immunoblotting was done with anti-pTyr (4G10) and anti-SHIP-1.
In this experiment we also assessed the ability of further BCR stimulation to induce CD79 phosphorylation in anergic cells. Interestingly BCR stimulation led to additional CD79a monophosphorylation, but little dual phosphorylation. Phosphorylation of CD79b was not detected (data not shown). Thus, anergic B cells have increased basal ITAM monophosphorylation, presumably driven by chronic autoantigen stimulation, and upon further BCR stimulation ITAMs undergo additional mono-, but not dual phosphorylation,
It is unclear what molecular mechanism could limit ITAM phosphorylation in anergic cells. However, one possibility is suggested by the recent demonstration that antigen receptor stimulation can lead to serine phosphorylation of CD79a serine 197, and that this phosphorylation limits output of calcium signals by the receptor (Heizmann et al., 2010). Given the proximity of S197 to Y193 in the CD79a ITAM we thought that S197 phosphorylation might occur chronically in anergy cells, and might limit CD79a ITAM tyrosine phosphorylation. Seemingly inconsistent with this possibility is evidence that S197 phosphorylation is mediated by Syk, which is not activated in anergic cells. Despite this we analyzed S197 phosphorylation in naive and anergic B cells by immunoblotting. Similar, S197 phosphorylation was detected in unstimulated naive and anergic cells provided the phosphatase inhibitor calyculin was used to stabilize phosphor-Serine (pS197) (figure 4B). Chronically autoantigen stimulated anergic B cells did not contain elevated pS197, and thus S197 phosphorylation can explain neither their unresponsiveness nor their chronic ITAM monophosphorylation. Interestingly, BCR stimulation led to S197 phosphorylation in naive cells but not in anergic B cells. This finding, as well as failure of BCR stimuli to induce ITAM biphosphorylation in anergic cells, is consistent with previous work showing impaired Syk activation in anergic B cells (Noorchashm et al., 1999).
To address the possibility that monophosphorylation of ITAM tyrosines is a driver of activation of the SHIP-1-Dok-1 circuit we employed previously characterized chimeric receptors composed of I-Ak α and β extracellular and transmembrane domains fused to the cytoplasmic tails of CD79a or b respectively (Pao et al, 1998). Each chimeric chain was expressed with a tailless complementary chain to construct surrogate receptors that contained one CD79a or one CD79b tail. These chimeras were expressed in the M12 B cell lymphoma (I-Ad), and resultant I-Ak positive lines were stimulated using biotinylated anti-I-Ak and avidin. By systematically mutating the ITAM tyrosines to phenylalanine we were able to titrate the affects of ITAM mono- and bi-phosphorylation on downstream signaling. Aggregation of the wild-type CD79 ITAMs led to tyrosine phosphorylation of many downstream proteins including Syk, SHIP-1 and Dok-1 (Fig. 4C). In contrast, aggregation of ITAMs containing only a single motif tyrosine led to very limited downstream phosphorylation. Importantly, monophosphorylation was associated with phosphorylation of SHIP-1 and Dok-1 but, as expected, not Syk. Stimulation of SHIP-1 and Dok-1 phosphorylation required that only a single conserved ITAM tyrosine be present, suggesting that their activation may be mediated by increased Src-family kinase activation afforded by ITAM monophosphorylation (Pao et al., 1998). The response was most obvious with the CD79a ITAM containing tyrosine 182, but was also noted with tyrosine 193. Tyrosine 182 is the preferred Lyn substrate in CD79a and thus is most likely to be phosphorylated in anergic B cells (Pao et al., 1998). Similar tyrosine dependency was seen using I-Ak CD79b chimeric receptors. However, greater SHIP-1 and Dok-1 activation occurred when only tyrosine 195 was present than when only tyrosine 206 was present, consistent with earlier findings that tyrosine 195 is the preferred site of CD79b phosphorylation (Pao et al., 1998). Taken together these findings demonstrate that chronic antigen stimulation of anergic B lymphocytes leads to biased BCR ITAM monophosphorylation, and this monophosphorylation drives activation of the SHIP-1-Dok-1 inhibitory circuit, likely through activation of Src-family kinases.
Chronic BCR-mediated SHIP-1 phosphorylation in anergic B cells is Src-family kinase dependent
Previous findings have demonstrated that in normal B cells activation of SHIP-1 and Dok-1 tyrosine phosphorylation by co-aggregation of BCR and FcγRIIB is Lyn dependent (Malbec et al., 1998). It therefore seems likely that SHIP-1 and Dok-1 phosphorylation in anergic B cells is also Src-family kinase dependent. To address this hypothesis we treated ex vivo Ars/A1 splenic B cells with the Src family kinase inhibitor PP2 and assessed the effect on SHIP-1 phosphorylation (Fig. 4D). As shown, the Src-family tyrosine kinase inhibitor but not the MEK1 and 2 inhibitor blocked basal SHIP-1 tyrosine phosphorylation. These data are consistent with a mechanism wherein chronic BCR stimulation leads to biased Lyn-mediated receptor ITAM monophosphorylation, leading in turn to activation of SHIP-1 and Dok-1. However, they do not formally exclude the action of other Src-family kinases in this pathway.
Discussion
In this study we explored the nature of signals that emanate from chronically occupied BCR on anergic B cells, and the role such signals play in maintaining unresponsiveness to antigen. We showed that in anergic B cells antigen receptor ITAMs are tyrosine mono-phosphorylated, and that further stimulation of these cells leads to additional mono- but not dual-tyrosine phosphorylation of their ITAMs. We further showed that in anergic cells the adaptor Dok-1 and its binding partner SHIP-1 are tyrosine phosphorylated, a condition associated with activation of their inhibitory signaling function (Ono et al., 1996; Tamir et al., 2000), and that this can be a consequence of receptor ITAM monophosphorylation and activation of Src-family tyrosine kinases, e.g. Lyn. Finally we showed that disabling this inhibitory circuit by B cell-targeted deletion of Inpp5d leads to loss of the anergic phenotype and to development of severe lupus-like autoimmunity. Taken together, these findings demonstrate that a consequence of chronic occupancy of antigen receptors on anergic B cells is ITAM monophosphorylation, and this monophosphorylation drives the activation of inhibitory signaling circuitry involving the inositol lipid phosphatase SHIP-1 and its adaptor Dok-1. Activation of this signaling circuit is critical for prevention of autoimmunity. It is noteworthy that this bias towards inhibitory signaling is seen in both extant immunoglobulin transgenic models of anergy, Ars/A1 and MD4.ML5, as well as in naturally occurring anergic cells (Merrell et al., 2006), and it is therefore likely that activation of this mechanism is a common feature of anergic B cells.
The observations raise multiple important questions regarding maintenance of anergy, including how in molecular terms receptors are re-wired to activate ITAM monophosphorylation but not biphosphorylation; how monophosphorylated ITAMs recruit the Dok-1 and SHIP-1 circuit; and finally whether and how this biased proximal signaling signature contributes to the previously described anergy-associated pattern of downstream events.
It is fair to say that we understand very little regarding how in anergic B cells antigen receptor signaling is biased toward receptor ITAM monophosphorylation. Earlier reports showed that antagonist peptides can induce partial T cell receptor (TCR) zeta chain ITAM tyrosine phosphorylation (Kersh et al., 1999). Thus altered patterns of ITAM phosphorylation can be determined by both affinity of antigen-receptor interactions (T cells) as well as chronicity of stimulation (B cells), as shown here. Parenthetically, incomplete phosphorylation of both BCR and TCR ITAMs has been shown previously to lead to generation of inhibitory signals, but the nature of these signals had been unknown (Kersh et al., 1999; Pao et al., 1998). A basis for altered antigen receptor coupling to ITAM phosphorylation is suggested by findings that BCR on anergic B cells are structurally destabilized, resulting in inefficient co-immunoprecipitation following detergent lysis (Vilen et al., 1999). Receptors in which this destabilized state is approximated by introduction of mutations in the transmembrane domains of membrane immunoglobulin heavy chains are functionally defective (Grupp et al., 1993). Consistent with the degree of receptor occupancy needed to maintain anergy, B cells in which as few as 15–20% of BCR are disabled by such mutations display reduced receptor tyrosine phosphorylation and calcium mobilization in response to antigen stimulation (Vilen et al., 2002). Thus bias toward receptor monophophorylation may be the result of poor quality of antigen receptor aggregates consequent to short receptor dwell time or destabilization. Another possibility is suggested by observations that antigen receptors on anergic B cells are endocytosed at a greatly accelerated rate upon antigen binding (Blery et al., 2006). This could result in premature termination of signaling leaving ITAMs incompletely phosphorylated. Other findings suggest a role for inhibitory BCR co-receptors such as CD72 and CD22, which function by recruiting SHP-1, in maintaining anergy, since genetically targeted mice lacking these molecules exhibit an autoimmune phenotype (Li et al., 2008; O'Keefe et al., 1999; Pao et al., 2007; Shultz et al., 1984). Active SHP-1 could, by differential dephosphorylating ITAMs (Veillette et al., 2002), tip the balance toward monophosphorylation. We note, however, that, as shown here, we have been unable to detect increased SHP-1 activation in anergic B cells. Clearly, this is important area for future study.
A molecular basis for monophosphoryl-ITAM activation of the SHIP-1-Dok-1 circuit is suggested by previous findings that SHIP-1 binds via its SH2 domain to monophosphorylated FcsR1 beta chain (Kimura et al., 1997) and DAP12 (Peng et al., 2010). This could lead to SHIP-1 phosphorylation by src-family kinases as occurs during FcyRIIB activation, and to subsequent recruitment of Dok-1 (Tamir et al., 2000), which is in turn phosphorylated. Dok-1 contains a pleckstrin homology domain (PH) and a phosphotyrosine binding (PTB) domain, and has several tyrosines in its COOH-terminal region that are phosphorylated (Mashima et al., 2009; Tamir et al., 2000). These phosphorylations allow Dok-1 to act as an adaptor for proteins including p120 rasGAP (via the YXXP motif), SHIP-1, Nck, and Csk (Mashima et al., 2009). While multiple Dok-1 coupled pathways may thus be activated in anergic cells, the effect of SHIP-1 knockout suggests that this particular Dok-1 effector may play a uniquely important role in maintaining anergy. It is noteworthy that Dok-1 knockout mice do not exhibit autoimmunity (J Cambier, unpublished), presumably due to expression of redundant proteins including Dok-1~Dok-3>>Dok-2 (Lemay et al., 2000) and J. Cambier unpublished). Mice in which both Dok-1 and Dok-2 are ablated (in all cells) suffer lupuslike disease (Yasuda et al., 2007). Further studies are needed to test the role of other Dok effectors in anergy.
The mechanism by which SHIP-1 and Dok-1 cooperate to maintain anergy is suggested by findings that following cell stimulation Dok-1 is translocated to the plasma membrane via a PI3K-dependent mechanism (Zhao et al., 2001). Further, this translocation is mediated by its PH domain. SHIP-1 and Dok-1 associate via SHIP-1 SH2 interaction with phosphotyrosines in pDok-1 and Dok-1 PTB interactions with phosphotyrosines in pSHIP-1, presumably forming a bi-dentate complex (Tamir et al., 2000). Thus it seems logical that the function of pDok-1 in this context is to target associated pSHIP-1 to areas of the plasma membrane that are enriched in PtdIns3,4,5 P3, a SHIP-1 substrate. Dok targeting of SHIP-1 would promote hydrolysis of PtdIns3,4,5 P3 thereby limiting the translocation of Bruton’s tyrosine kinase (BTK) and phospholipase C (PLCγ2) to the plasma membrane, which would consequently block calcium mobilization. It is possible that PtdIns3,4,5 P3 depletion also causes the previously reported BCR destabilization (Vilen et al., 1999), as well as biased CD79a monophosphorylation seen in anergic B cells. Ongoing studies in our laboratory address this hypothesis.
Loss of anergy in B cell-targeted SHIP-1 knockout mice provides evidence of the central importance of the suppression of the PtdIns3,4,5P3 pathway in anergy. Consistent with this concept are previously reported findings that mice haploinsufficient in the PTEN gene spontaneously develop lupus-like autoimmunity (Di Cristofano et al., 1999). Homozygous knockout of PTEN might be expected to oppose the anergy-maintaining effects of SHIP-1 by further increasing PtdIns3,4,5P3 amounts. However, this does not occur apparently due to requirements for PTEN for expression and/or function of activation-induced cytidine deaminase (Suzuki et al., 2003). Further linking reduction of PtdIns3,4,5P3 to maintenance of anergy are observations of Browne et al (Browne et al., 2009) that PTEN expression is increased in anergic B cells from HEL-anti-HEL (MD4.ML5) transgenic mice. Importantly however, as shown here, anergic Ars/A1 B cells do not express elevated PTEN. Interestingly, studies of Moody have shown that haploinsufficiencies of both PTEN and SHIP-1 lead to more pronounced lupus-like autoimmunity than either single haploinsufficiency (Moody et al., 2003). This suggests that PTEN up-regulation and SHIP-1 activation may act cooperatively, where SHIP-1 provides rapidly inducible and reversible regulation while PTEN effects, determined by expression, might be more durable. Differential use of these mechanisms could explain why autoantigen removal from Ars/A1 B cells causes reversal of anergy in minutes while in the MD4.ML5 model it requires many (~48) hours (Gauld et al., 2005; Goodnow et al., 1991). Finally, elevation of PTEN may be involved in maintenance of anergy only in B cells that interact with high avidity autoantigens.
How could decreased accumulation of the PtdIns3,4,5P3 relate to the unique pattern of transcription factor activation previously described in anergic B cells (Healy et al., 1998)? It is well established that in ex vivo B cells antigen receptor-mediated PLCγ activation and consequent calcium mobilization are dependent on PtdIns3,4,5P3 generation. Dolmetsch et al. have shown that downstream signaling pathways emanating from the BCR are differentially activated depending on calcium signal amplitude, e.g. activation of NFAT requires less elevation of [Ca2+]i than activation of NFκB (Dolmetsch et al., 1997). Thus, in anergic cells reduced PtdIns3,4,5P3 could be expected, by limiting PLCγ hydrolysis of PtdIns4,5P2, to cause the differential loss of NFκB activation relative to NFAT. This mechanism may also explain loss of CARD11 activation in anergic cells, since this response is dependent on PKCβ activation, which is in turn dependent on PLCγ-mediated generation of diacyglycerol. Finally, JNK activation, also lost in anergic cells, is negatively regulated by SHIP-1, linking its regulation to availability of PtdIns3,4,5P3 (Robson et al., 2004). Quantitatively distinct requirements of NFAT, NFκB, JNK and CARD11 pathways for PtdIns3,4,5P3 may explain why some, e.g. NFAT, are spared in anergic cells, while others are not.
Experimental Procedures
Mice
Except where otherwise indicated six to twelve week old mice were used for all experiments. Ars/A1 (Benschop et al., 2001), κTg (Benschop et al., 2001), Fcgr2b −/−(Takai et al., 1996), Inpp5d−/− (Helgason et al., 1998), MD4, and MD4.ML5 (Goodnow et al., 1988) mice have been described previously. To create mice in which SHIP-1 deficiency is restricted to the B cell lineage mice with “floxed” Inpp5d (Karlsson et al., 2003) were crossed with mice in which cre was targeted into the Cd79a locus (Cd79a-cre) (Hobeika et al., 2006). These mice were kept on a mixed background and are described in more detail in the supplementary data section. All experiments with mice were performed in accordance with the regulations and with the approval of National Jewish Health (Denver, CO) and Institutional Animal Care and Use Committee.
B cell purification
Untouched resting B cells were isolated by depletion of CD43+ cells with anti-CD43-conjugated magnetic beads (MACS anti-mouse CD43; Miltenyi Biotec). B cells were >97% pure by B220+ staining followed by FACS analysis.
Flow cytometry
Cells were resuspended in PBS containing 1% FCS and 0.05% sodium azide and incubated with an optimal amount of biotinylated or directly labeled antibodies described in supplementary data section. Cells were analyzed on a FACSCaliber or LSRII flow cytometer (BD).
Analysis of calcium mobilization
B220-stained splenocytes were loaded with Indo-1 acetoxymethyl (Indo1-AM) (Molecular Probes) and stimulated with 10µg/ml rabbit anti-mouse IgG (H+L) F(ab’)2 (Zymed) as described in the supplementary data section.
Analysis of protein phosphorylation
Purified B cells were lysed in 1% NP-40 lysis buffer. Whole cell lysates were directly mixed with SDS reducing sample buffer or used for immunoprecipitation (IP). Detailed description of IPs, inhibitors used, the far western protocol and the antibodies use for detection after western blot are given in the supplementary data section.
Cell lines
Construction of the MHC class II (I-Ak α and β extracellular domains)-CD79a and b (cytoplasmic tails) fusion proteins and culture conditions for the M12G3R mouse B cell lymphoma lines expressing these proteins has been described previously (Pao et al., 1998).
Enzyme linked immunosorbent assay
Anti-chromatin IgG and anti-Ars IgMa were detected by standard ELISAs, described in the supplementary data section.
ANA Detection
Mouse sera were hybridized to HEp-2 antigen substrate slides (BION) and bound IgG was visualized as described in the supplementary data section.
Immunohistochemistry
Kidneys were flash frozen in Tissue-Tek OCT embedding media (Sakura) and 4 µm sections were stained for IgG as described in the supplementary data section. Consecutive slides were stained with H&E using standard procedures.
Autoantibody array
Autoantibodies were measured on an autoantigen proteomic array that has been described previously (Li et al., 2007) and is described in the supplementary data section.
Supplementary Material
Acknowledgements
We thank Kathy Burke for technical assistance; Dr. Larry Wysocki for reagents; Drs. M. Reth, J. Ravetch and S. Bolland for mice; T. Packard for invaluable technical assistance with Adobe Illustrator and help with editing the figures. This study was supported by the following National Institutes of Health grants: AI022295, AI077597, AG013989 (J.C.C.), an Arthritis Foundation postdoctoral fellowship (S.K.O.), the Swedish Research Council and the Swedish Society for Medical Research (A.G.). J.C.C. is an Ida and Cecil Green Distinguished Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
References
- Adachi T, Wienands J, Tsubata T, Kurosaki T. Interdomain A is crucial for ITAM-dependent and -independent regulation of Syk. Biochem Biophys Res Commun. 2007;364:111–117. doi: 10.1016/j.bbrc.2007.09.100. [DOI] [PubMed] [Google Scholar]
- Auborn KJ, Qi M, Yan XJ, Teichberg S, Chen D, Madaio MP, Chiorazzi N. Lifespan is prolonged in autoimmune-prone (NZB/NZW) F1 mice fed a diet supplemented with indole-3-carbinol. J Nutr. 2003;133:3610–3613. doi: 10.1093/jn/133.11.3610. [DOI] [PubMed] [Google Scholar]
- Benschop RJ, Aviszus K, Zhang X, Manser T, Cambier JC, Wysocki LJ. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity. 2001;14:33–43. doi: 10.1016/s1074-7613(01)00087-5. [DOI] [PubMed] [Google Scholar]
- Blasioli J, Goodnow CC. Lyn/CD22/SHP-1 and their importance in autoimmunity. Curr Dir Autoimmun. 2002;5:151–160. doi: 10.1159/000060551. [DOI] [PubMed] [Google Scholar]
- Blery M, Tze L, Miosge LA, Jun JE, Goodnow CC. Essential role of membrane cholesterol in accelerated BCR internalization and uncoupling from NF-kappa B in B cell clonal anergy. J Exp Med. 2006;203:1773–1783. doi: 10.1084/jem.20060552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- Borrero M, Clarke SH. Low-affinity anti-Smith antigen B cells are regulated by anergy as opposed to developmental arrest or differentiation to B-1. J Immunol. 2002;168:13–21. doi: 10.4049/jimmunol.168.1.13. [DOI] [PubMed] [Google Scholar]
- Browne CD, Del Nagro CJ, Cato MH, Dengler HS, Rickert RC. Suppression of phosphatidylinositol 3,4,5-trisphosphate production is a key determinant of B cell anergy. Immunity. 2009;31:749–760. doi: 10.1016/j.immuni.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl AM, Cambier JC. Phosphorylation of CD19 Y484 and Y515, and linked activation of phosphatidylinositol 3-kinase, are required for B cell antigen receptor-mediated activation of Bruton’s tyrosine kinase. J Immunol. 1999;162:4438–4446. [PubMed] [Google Scholar]
- Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- Cooke MP, Heath AW, Shokat KM, Zeng Y, Finkelman FD, Linsley PS, Howard M, Goodnow CC. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med. 1994;179:425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- Ferry H, Crockford TL, Silver K, Rust N, Goodnow CC, Cornall RJ. Analysis of Lyn/CD22 double-deficient B cells in vivo demonstrates Lyn- and CD22-independent pathways affecting BCR regulation and B cell survival. Eur J Immunol. 2005;35:3655–3663. doi: 10.1002/eji.200535247. [DOI] [PubMed] [Google Scholar]
- Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6:1160–1167. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- Goodnow CC, Brink R, Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 1991;352:532–536. doi: 10.1038/352532a0. [DOI] [PubMed] [Google Scholar]
- Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Grupp SA, Campbell K, Mitchell RN, Cambier JC, Abbas AK. Signaling-defective mutants of the B lymphocyte antigen receptor fail to associate with Ig-alpha and Ig-beta/gamma. J Biol Chem. 1993;268:25776–25779. [PubMed] [Google Scholar]
- Healy JI, Dolmetsch RE, Lewis RS, Goodnow CC. Quantitative and qualitative control of antigen receptor signalling in tolerant B lymphocytes. Novartis Found Symp. 1998;215:137–144. doi: 10.1002/9780470515525.ch10. discussion 144-135, 186–190. [DOI] [PubMed] [Google Scholar]
- Healy JI, Dolmetsch RE, Timmerman LA, Cyster JG, Thomas ML, Crabtree GR, Lewis RS, Goodnow CC. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- Heizmann B, Reth M, Infantino S. Syk is a dual-specificity kinase that self-regulates the signal output from the B-cell antigen receptor. Proc Natl Acad Sci U S A. 2010;107:18563–18568. doi: 10.1073/pnas.1009048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason CD, Damen JE, Rosten P, Grewal R, Sorensen P, Chappel SM, Borowski A, Jirik F, Krystal G, Humphries RK. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci U S A. 2006;103:13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inabe K, Kurosaki T. Tyrosine phosphorylation of B-cell adaptor for phosphoinositide 3-kinase is required for Akt activation in response to CD19 engagement. Blood. 2002;99:584–589. doi: 10.1182/blood.v99.2.584. [DOI] [PubMed] [Google Scholar]
- Jun JE, Goodnow CC. Scaffolding of antigen receptors for immunogenic versus tolerogenic signaling. Nat Immunol. 2003;4:1057–1064. doi: 10.1038/ni1001. [DOI] [PubMed] [Google Scholar]
- Karlsson MC, Guinamard R, Bolland S, Sankala M, Steinman RM, Ravetch JV. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J Exp Med. 2003;198:333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersh EN, Kersh GJ, Allen PM. Partially phosphorylated T cell receptor zeta molecules can inhibit T cell activation. J Exp Med. 1999;190:1627–1636. doi: 10.1084/jem.190.11.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Sakamoto H, Appella E, Siraganian RP. The negative signaling molecule SH2 domain-containing inositol-polyphosphate 5-phosphatase (SHIP) binds to the tyrosine-phosphorylated beta subunit of the high affinity IgE receptor. J Biol Chem. 1997;272:13991–13996. doi: 10.1074/jbc.272.21.13991. [DOI] [PubMed] [Google Scholar]
- Kurosaki T, Johnson SA, Pao L, Sada K, Yamamura H, Cambier JC. Role of the Syk autophosphorylation site and SH2 domains in B cell antigen receptor signaling. J Exp Med. 1995;182:1815–1823. doi: 10.1084/jem.182.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemay S, Davidson D, Latour S, Veillette A. Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol Cell Biol. 2000;20:2743–2754. doi: 10.1128/mcb.20.8.2743-2754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DH, Winslow MM, Cao TM, Chen AH, Davis CR, Mellins ED, Utz PJ, Crabtree GR, Parnes JR. Modulation of peripheral B cell tolerance by CD72 in a murine model. Arthritis Rheum. 2008;58:3192–3204. doi: 10.1002/art.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QZ, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR, Mohan C, Wakeland EK, Olsen NJ. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol. 2007;147:60–70. doi: 10.1111/j.1365-2249.2006.03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kruhlak MJ, Hao JJ, Shaw S. Rapid T cell receptor-mediated SHP-1 S591 phosphorylation regulates SHP-1 cellular localization and phosphatase activity. J Leukoc Biol. 2007;82:742–751. doi: 10.1189/jlb.1206736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbec O, Fong DC, Turner M, Tybulewicz VL, Cambier JC, Fridman WH, Daeron M. Fc epsilon receptor I-associated lyn-dependent phosphorylation of Fc gamma receptor IIB during negative regulation of mast cell activation. J Immunol. 1998;160:1647–1658. [PubMed] [Google Scholar]
- Mashima R, Hishida Y, Tezuka T, Yamanashi Y. The roles of Dok family adapters in immunoreceptor signaling. Immunol Rev. 2009;232:273–285. doi: 10.1111/j.1600-065X.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- Maxwell MJ, Duan M, Armes JE, Anderson GP, Tarlinton DM, Hibbs ML. Genetic Segregation of Inflammatory Lung Disease and Autoimmune Disease Severity in SHIP-1−/− Mice. J Immunol. 2011;186:7164–7175. doi: 10.4049/jimmunol.1004185. [DOI] [PubMed] [Google Scholar]
- Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, Cambier JC. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Moody JL, Pereira CG, Magil A, Fritzler MJ, Jirik FR. Loss of a single allele of SHIP exacerbates the immunopathology of Pten heterozygous mice. Genes Immun. 2003;4:60–66. doi: 10.1038/sj.gene.6363903. [DOI] [PubMed] [Google Scholar]
- Noorchashm H, Bui A, Li HL, Eaton A, Mandik-Nayak L, Sokol C, Potts KM, Pure E, Erikson J. Characterization of anergic anti-DNA B cells: B cell anergy is a T cell-independent and potentially reversible process. Int Immunol. 1999;11:765–776. doi: 10.1093/intimm/11.5.765. [DOI] [PubMed] [Google Scholar]
- O’Keefe TL, Williams GT, Batista FD, Neuberger MS. Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. J Exp Med. 1999;189:1307–1313. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- Pao LI, Famiglietti SJ, Cambier JC. Asymmetrical phosphorylation and function of immunoreceptor tyrosine- based activation motif tyrosines in B cell antigen receptor signal transduction. J Immunol. 1998;160:3305–3314. [PubMed] [Google Scholar]
- Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, Humphrey MB. TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci Signal. 2010;3:ra38. doi: 10.1126/scisignal.2000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson JD, Davidson D, Veillette A. Inhibition of the Jun N-terminal protein kinase pathway by SHIP-1, a lipid phosphatase that interacts with the adaptor molecule Dok-3. Mol Cel Biol. 2004;24:2332–2343. doi: 10.1128/MCB.24.6.2332-2343.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Coman DR, Bailey CL, Beamer WG, Sidman CL. “Viable motheaten,” a new allele at the motheaten locus. I. Pathology. Am J Pathol. 1984;116:179–192. [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Kaisho T, Ohishi M, Tsukio-Yamaguchi M, Tsubata T, Koni PA, Sasaki T, Mak TW, Nakano T. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197:657–667. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- Tamir I, Stolpa JC, Helgason CD, Nakamura K, Bruhns P, Daeron M, Cambier JC. The RasGAP-binding protein p62dok is a mediator of inhibitory FcgammaRIIB signals in B cells. Immunity. 2000;12:347–358. doi: 10.1016/s1074-7613(00)80187-9. [DOI] [PubMed] [Google Scholar]
- Veillette A, Latour S, Davidson D. Negative regulation of immunoreceptor signaling. Ann Rev Immunol. 2002;20:669–707. doi: 10.1146/annurev.immunol.20.081501.130710. [DOI] [PubMed] [Google Scholar]
- Vilen BJ, Burke KM, Sleater M, Cambier JC. Transmodulation of BCR signaling by transduction-incompetent antigen receptors: implications for impaired signaling in anergic B cells. J Immunol. 2002;168:4344–4351. doi: 10.4049/jimmunol.168.9.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilen BJ, Famiglietti SJ, Carbone AM, Kay BK, Cambier JC. B cell antigen receptor desensitization: disruption of receptor coupling to tyrosine kinase activation. J Immunol. 1997;159:231–243. [PMC free article] [PubMed] [Google Scholar]
- Vilen BJ, Nakamura T, Cambier JC. Antigen-stimulated dissociation of BCR mIg from Ig-alpha/Ig-beta: implications for receptor desensitization. Immunity. 1999;10:239–248. doi: 10.1016/s1074-7613(00)80024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Wienands J, Freuler F, Baumann G. Tyrosine-phosphorylated forms of Ig beta, CD22, TCR zeta and HOSS are major ligands for tandem SH2 domains of Syk. Int Immunol. 1995;7:1701–1708. doi: 10.1093/intimm/7.11.1701. [DOI] [PubMed] [Google Scholar]
- Yarkoni Y, Cambier JC. Differential STIM1 expression in T and B cell subsets suggests a role in determining antigen receptor signal amplitude. Mol Immunol. 2011 doi: 10.1016/j.molimm.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Bundo K, Hino A, Honda K, Inoue A, Shirakata M, Osawa M, Tamura T, Nariuchi H, Oda H, et al. Dok-1 and Dok-2 are negative regulators of T cell receptor signaling. Int Immunol. 2007;19:487–495. doi: 10.1093/intimm/dxm015. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shen K, Lu W, Cole PA. The role of C-terminal tyrosine phosphorylation in the regulation of SHP-1 explored via expressed protein ligation. J Biol Chem. 2003;278:4668–4674. doi: 10.1074/jbc.M210028200. [DOI] [PubMed] [Google Scholar]
- Zhao M, Schmitz AA, Qin Y, Di Cristofano A, Pandolfi PP, Van Aelst L. Phosphoinositide 3-kinase-dependent membrane recruitment of p62(dok) is essential for its negative effect on mitogen-activated protein (MAP) kinase activation. J Exp Med. 2001;194:265–274. doi: 10.1084/jem.194.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.