Abstract
Social isolation and poor sleep quality are independent predictors of poor health outcomes and increased biological risk for disease. We previously found in a small sample of older women that the presence of social ties compensated for poor sleep in associations with the inflammatory protein interleukin 6 (IL-6). The current study extended those findings to a national sample of middle-aged and older men and women. Using both subjective and objective sleep assessments, we found that in men, but not in women, social engagement moderated the association of subjective sleep complaints with both IL-6 and the soluble adhesion molecule E-selectin. Social engagement also moderated the link between sleep efficiency—assessed by actigraphy—and IL-6 levels in men, but not in women. These results extend our previous work and bolster the suggestion that positive psychological functioning may compensate for other risk factors in predicting advantageous profiles of biological risk in aging adults.
Keywords: sleep quality, actigraphy, interleukin 6, E-selectin, social well-being
Introduction
Circulating levels of inflammatory proteins, which have been cross-sectionally and prospectively linked to a range of disease conditions,1–3 are influenced by myriad social, psychological, and behavioral factors. Studies documenting these relationships typically focus on the independent association of one or more of these determinants with inflammatory proteins. None of these determinants exists in isolation of the others, however, and it is therefore important to consider the ways in which they may interact in predicting inflammation. This study weaves together three separate lines of inquiry involving sleep and social factors and examines the points at which they intersect in their associations with inflammation.
First, studies using multiple assessment techniques and approaches have established robust links between sleep and inflammation. Major depression, for example, is often associated with increased circulating levels of inflammatory proteins4,5 and significantly impaired sleep,6 and recent evidence suggests that impaired sleep may be a better predictor of inflammation than the presence or absence of major depression.7 Similarly, adults with excessive daytime sleepiness have higher circulating levels of IL-6 than those with better sleep.8 Experimental studies involving sleep restriction report higher levels of IL-69,10 along with increased expression of genes linked to increased inflammation.11 In community dwelling adults, poor sleep is associated with higher circulating levels of IL-612 and with increased release of inflammatory proteins from stimulated lymphocytes.13 Collectively, these results show that impaired or curtailed sleep, achieved either naturally or experimentally, predict higher circulating levels of inflammatory proteins.
The second line of inquiry focuses on the association of social well-being and inflammation. Isolation from social contact has been linked with increased risk of mortality in epidemiological studies14–16 and with impaired host defense in experimental studies.17 Recent evidence suggests that loneliness is associated with increased activity in genetic pathways regulating the production of inflammatory proteins, such as NF-κB.18 A complementary literature has shown that strong social relationships in women with ovarian cancer19 and social engagement in older community dwelling women20 predict lower levels of IL-6. These studies suggest that social ties are important predictors of inflammation; the latter studies in particular suggest that high levels of social well-being may have compensatory properties, predicting lower levels of inflammation in those at risk of greater inflammation due to illness or advancing age.
Finally, recent studies from Cacioppo et al. suggest associations between social relationships and sleep. Specifically, individuals who report high levels of loneliness also had reduced sleep efficiency as assessed by self report21 or using the Nightcap sleep system.22 Another study using daily diary assessments showed that loneliness predicted impaired daytime functioning independently of the amount of sleep the prior night.23 One hypothesis stemming from this work is that loneliness is evolutionarily linked to feelings of vulnerability, one result being increased nighttime vigilance, which in turn reduces the restorative quality of sleep.24
These three lines of inquiry converge on the hypothesis that sleep and social relationships, which are independently linked to inflammation and to one another, may interact in predicting inflammation. We tested this hypothesis in a sample of older women living in Wisconsin, and we found that while greater sleep efficiency and higher levels of social engagement independently predicted lower levels of IL-6, the presence of one compensated for the lack of the other; the only women in the sample at risk of higher levels of IL-6 were those with low sleep efficiency and low social engagement.25 We now extend this research to a national sample of middle-aged and older men and women—the Survey of Mid-Life in the United States (MIDUS)26—that combines measures of subjective and objective sleep quality. We also extend these analyses with measurement of E-selectin, a soluble adhesion molecule that, like IL-6, is implicated in age-related morbidity, especially cardiovascular disease,27–30 and is elevated by reduced sleep related to sleep apnea31 and experimental sleep deprivation.32
We hypothesized that poor sleep quality would predict higher levels of IL-6 and E-selectin, while greater social engagement would predict lower levels. More centrally, we hypothesized that sleep quality and social engagement would interact so that higher levels of social engagement would compensate for poor sleep quality—and vice versa—in predicting inflammation. Finally, as noted below, we observed significant differences between men and women in sleep, inflammation, and their interaction. Therefore, consistent with other studies examining links between social factors and inflammation,33 we conducted separate analyses for men and women.
Methods
Participants
The Survey of MIDUS comprises a national probability sample of noninstitutionalized English-speaking adults (N = 3,487) living in the coterminus United States and recruited by random digit dialing (RDD). A sample of monozygotic and dizygotic twin pairs (N = 1,914) was also recruited from a national twin registry. A follow-up study was completed 9–10 years later (MIDUS 2). Mortality-adjusted retention from the original study was 75%. All respondents completed telephone interviews and self-administered questionnaires. An oversample of African American adults living in the Milwaukee County (N = 592) was added at MIDUS 2. They completed in-person interviews and self-administered questionnaires.
A subsample of MIDUS 2 respondents (N = 1,229) participated in a detailed clinic-based assessment of health, disease-related biomarkers, and physiologic function (“biomarker sample”). Participation in the biomarker sample was open to all MIDUS 2 respondents who had completed the interviews and self-administered questionnaires and were willing to travel to a General Clinical Research Center (GCRC) for an overnight stay. Compared to the full MIDUS 2 sample, the biomarker sample was more likely to have graduated from a four-year college and less likely to smoke, but they were similar to MIDUS 2 respondents on all other demographic and health characteristics.34 Although inclusion of respondents from the twins sample has not influenced the results of previous analyses involving MIDUS data,35 we accounted for possible effects of genetic and familial relatedness among the twins here by including a variable indicating twin status in all analyses.
Three regional GCRCs participated in the MIDUS biomarker study—one on the West coast, one in the Midwest, and one on the East coast—and participants were invited to stay overnight at whichever GCRC imposed the least travel burden. At the GCRC, each respondent provided a detailed medical history interview with a clinician and completed a set of self-administered questionnaires. Participants were also asked to bring all current medications with them, and these were inventoried by project staff. Fasting blood samples were obtained the next morning between 8:00 AM and 10:00 AM. Serum was isolated from all samples, aliquoted, frozen at –80° C, shipped on dry ice to the appropriate laboratory, and stored at –80° C for assay. Participants at the Midwest regional GCRC were further invited to participate in a sleep study, which involved wearing an actigraphy watch for seven consecutive nights once they returned to their own homes.
Collection of data for MIDUS 2 and analysis of those data for the current study were both approved by the Health Sciences Institutional Review Board at the University of Wisconsin–Madison.
Chronic conditions
Participants indicated whether they had received a physician diagnosis for any of 12 chronic conditions, including autoimmune disorders, cardiovascular and cerebrovascular disease, hypertension, arthritis, asthma, diabetes, gastrointestinal diseases, liver disease, and cancer. From these responses, an index of chronic disease burden was constructed with responses ranging from 0 to 12. A continuous variable indicating total burden of chronic conditions was used in all analyses.
Inflammatory proteins
Serum IL-6 and E-selectin from fasting blood samples were measured using high-sensitivity enzyme-linked immunosorbent assay according to manufacturer guidelines (R&D Systems, Minneapolis, MN). The laboratory intra- and inter-assay coefficients of variance for both proteins were in acceptable ranges (<10%). The distribution for IL-6 was positively skewed, and data were ln-transformed for statistical analyses. Due to missing data, analyses for IL-6 involved 988 participants, while those for E-selectin involved 987 participants (one E-selectin value that exceeded two standard deviations from the mean was dropped from analyses).
Social well-being
Assessments of social well-being were based on responses to the positive relations with others seven-item subscale from Ryff's Psychological Well-Being (PWB) inventory.36 Examples of items from this scale include “I know that I can trust my friends, and they know they can trust me” and “Maintaining close relationships has been difficult and frustrating for me” (reverse scored). Internal validity was 0.78.
Hedonic well-being
Positive affect was measured using the High Positive Affect sub-scale from the Mood and Anxiety Symptom Questionnaire (MASQ).37,38 Here, respondents are asked to indicate how much in the past week they felt or experienced 14 items (e.g., felt cheerful, felt optimistic) using a five-point scale (1 = not at all; 5 = extremely). Internal validity (determined from the biomarker sample) was 0.93 for the positive affect scale.
A negative affect was assessed using the Depressive Symptoms sub-scale from the MASQ. Here, respondents are asked to indicate how much in the past week they felt or experienced 12 items (e.g., felt hopeless, felt sluggish or tired) using the same five-point scale as for positive affect. Internal validity for the depressive symptoms subscale was 0.90 for the biomarker sample.
The life satisfaction variable was a composite of items asking respondents to rate their health, work situation, relationship with spouse/partner, relationship with children, and life overall (1 = worst possible; 10 = best possible). Internal validity for this measure was 0.65 for the MIDUS 2 sample.
While measures of hedonic well-being and ill-being were of interest in their own right, in order to determine independent associations between different domains of psychological functioning and inflammation, we also included them in analyses focused on eudaimonic well-being (and vice versa).
Subjective sleep quality
Questionnaires completed during the overnight clinic stay included the Pittsburgh Sleep Quality Index (PSQI).39,40 The PSQI, a widely used instrument for the evaluation of sleep quality and sleep pathology, consists of seven component scores—subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, and use of sleep medication—that are aggregated into a global score with a range of 0–21. Global scores in excess of five are considered indicative of sleep pathology, although we treated global PSQI scores as a continuous variable in this study.
Actigraphy
Actigraphy data were collected from MIDUS 2 respondents who stayed at the GCRC at the University of Wisconsin–Madison (N = 440) using wrist actigraphs (Mini Mitter, Bend, OR, USA). Respondents began recording at 7:00 AM on the first Tuesday morning after the GCRC visit and continued for seven consecutive nights. Counts of movements were collected in 30 sec epochs. Respondents used an event marker on the actiwatch to indicate when they started trying to fall asleep (bedtime) and when they arose in the morning (rise time). They also recorded bedtime and rise time using a sleep diary. Upon return of the actiwatches to MIDUS project staff, manufacturer algorithms for detecting sleep activity were applied, and several dimensions of sleep were derived from the activity data, including total sleep time (total amount of time asleep during period from bedtime to rise time), latency to sleep onset (time from bedtime to first sleep bout), and sleep efficiency (percent of time asleep between bedtime and rise time). The values for sleep duration and sleep efficiency were normally distributed, but because of a strong positive skew, values for sleep latency were ln-transformed for analyses.
Covariates
In addition to age, marital status, and race, analyses controlled for the following.
Education
As part of the telephone interview, participants were asked their highest level of educational attainment. Responses were grouped into 12 categories ranging from “no school/some grade school” to “PhD, MD, JD, or other professional degree.” This 12-category variable was included in all analyses.
Obesity
Height and weight were measured by GCRC staff and used to calculate body mass index (BMI; weight in kilograms divided by the square of height in meters). A continuous measure of BMI was included in all analyses. Waist and hip circumference were measured directly on skin or over a single layer of clothing by GCRC staff. The waist-to-hip ratio was then calculated by dividing waist circumference by hip circumference. A continuous measure of the waist-to-hip ratio was used in all analyses
Medications
Antihypertensive,41,42 cholesterol lowering,43 steroid, and antidepressant44 medications have all been shown to have anti-inflammatory properties. Dichotomous variables indicating the use of any of these medications were included in all analyses.
Statistical analyses
Preliminary analyses indicated significant age-adjusted gender differences in subjective sleep quality and actigraphic sleep assessments—women reported poorer subjective sleep but higher levels of sleep efficiency—and circulating levels of E-selectin were higher in men. Moreover, regression analyses adjusting for age and race showed significant interactions between gender and sleep in predicting inflammatory markers. For these reasons, we report results of analyses stratified by gender as well as for the full sample. Although there were racial differences in some parameters, we did not observe interactions with race, so we did not stratify the sample by race. Multivariate linear regression models were used to estimate the independent and interactive associations of sleep and positive relations with others with inflammatory proteins—separate models were estimated for subjective and objective sleep assessments and for IL-6 and E-selectin. In each set of analyses, the initial model examined direct and interactive associations of the sleep parameter and positive relations adjusting for age, gender, race, and educational attainment. The subsequent full model added adjustments for health status, health behavior, and positive and negative affect. The threshold for identifying statistically significant associations was set at α = 0.05.
Results
Descriptive statistics for all variables are shown in Table 1; results for men and women are shown separately. Men and women differed significantly on most measures, with the exception of IL-6, depressive symptoms, exercise, BMI, and use of blood pressure medication.
Table 1.
Descriptive statistics for sample (N = 1,229)
| Mean (SEM) |
% |
||||
|---|---|---|---|---|---|
| Variable | Men | Women | Men | Women | t or χ2 value |
| Age | 57.9 (0.5) | 56.9 (0.4) | 1.49 | ||
| Race (% nonwhite) | 17.5 | 24.4 | 8.72** | ||
| Education (% HS grad or GED) | 24.0 | 31.0 | 7.48** | ||
| PSQI global score | 5.7 (0.2) | 6.6 (0.1) | 4.03*** | ||
| Actigraphy (weekly average) | |||||
| Sleep duration (hours) | 5.9 (0.1) | 6.4 (0.1) | 4.42*** | ||
| Sleep latency (min) | 37.2 (2.7) | 27.9 (1.8) | 3.02** | ||
| Sleep efficiency (%) | 76.6 (0.9) | 80.9 (0.6) | 4.24*** | ||
| Positive relations with others | 39.5 (0.3) | 41.3 (0.3) | 4.44*** | ||
| Serum IL-6 (pg/mL) | 2.0 (1.0) | 2.2 (1.0) | 1.69# | ||
| Serum E-selectin (ng/mL) | 45.0 (1.0) | 41.4 (0.8) | 2.80** | ||
| CES-D | 6.5 (1.0) | 7.0 (1.0) | 1.21 | ||
| Marital status (% married) | 73.8 | 54.6 | 69.60*** | ||
| Smoking (% current smokers) | 16.5 | 13.7 | 10.13** | ||
| Alcohol (% nondrinkers) | 29.1 | 39.7 | 38.51*** | ||
| Exercise (%) | 77.9 | 75.5 | 0.99 | ||
| BMI (% >30) | 40.0 | 42.1 | 0.53 | ||
| Waist-hip ratio | 0.97 (0.0) | 0.84 (0.0) | 27.36*** | ||
| Medication (% yes) | |||||
| Blood pressure | 34.3 | 38.0 | 1.81 | ||
| Cholesterol | 35.2 | 22.2 | 26.24*** | ||
| Steroid | 4.1 | 18.2 | 58.12*** | ||
| Depression | 10.7 | 16.5 | 8.74** | ||
P < 0.10
P < 0.01
P < 0.001.
Bivariate associations among key variables in men and women are shown in Table 2. Higher PSQI global scores, indicating poorer subjective sleep quality, were associated with shorter actigraphic sleep duration in women, greater sleep latency, and reduced sleep efficiency. Higher global scores also predicted higher levels of both IL-6 and E-selectin. Greater sleep duration and sleep efficiency and shorter sleep latencies predicted lower levels of IL-6 in women, but not in men; greater sleep efficiency also predicted lower E-selectin levels in women. Finally, higher scores on positive relations with others predicted lower levels of IL-6 and E-selectin in women, but not in men.
Table 2.
Zero order correlations among key variables. Values for men (upper number) and women (lower number) are shown separately.
| Variable | PSQI | Sleep duration | Sleep latency | Sleep efficiency | Positive relations | IL-6 |
|---|---|---|---|---|---|---|
| PSQI global score | ||||||
| Actigraphy | ||||||
| Sleep duration | –0.05 | |||||
| –0.17* | ||||||
| Sleep latency | 0.18* | –0.35*** | ||||
| 0.23** | –0.23*** | |||||
| Sleep efficiency | –0.26** | 0.70*** | –0.72*** | |||
| –0.37*** | 0.53*** | –0.60*** | ||||
| Positive relations with others | –0.25*** | –0.05 | –0.19* | 0.16* | ||
| –0.18*** | 0.10 | –0.12# | 0.19** | |||
| Serum IL-6 | 0.11* | 0.07 | 0.02 | –0.07 | 0.03 | |
| 0.14*** | –0.18** | 0.25** | –0.29*** | –0.09* | ||
| Serum E-selectin | 0.15** | –0.11 | –0.05 | –0.07 | –0.06 | 0.18*** |
| 0.11** | –0.04 | 0.06 | –0.13** | 0.20*** |
P < 0.10
P < 0.05
P < 0.01
P < 0.001.
Subjective sleep quality
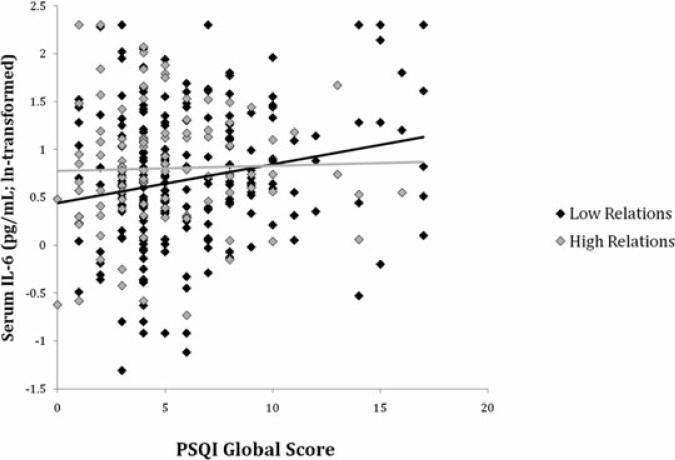
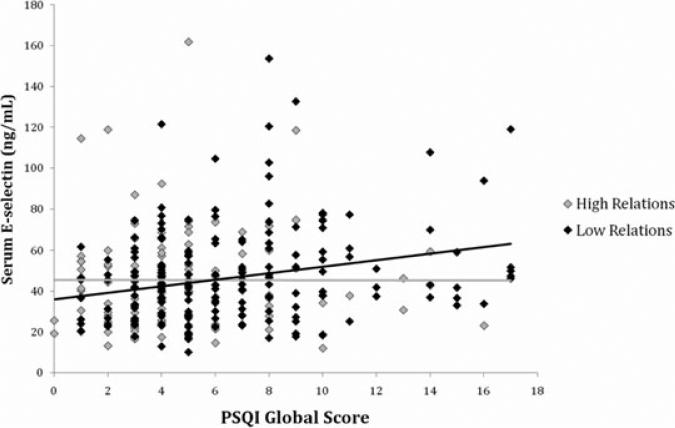
Tables 3 and 4 display the results of multivariate regression analyses involving PSQI global scores. In men, but not in women, PSQI global scores and its interaction with positive relations with others were significantly associated with IL-6 (Table 3) and E-selectin (Table 4) in the base model adjusting for demographic characteristics (model 1) and the full model adjusting for health status, health behavior, and positive and negative affect (model 2). In particular, the interaction showed that as subjective sleep quality declined (higher PSQI scores), IL-6 and E-selectin levels were less likely to rise in men reporting higher positive relations scores (Figs. 1 and 2). Positive relations with others was positively associated with IL-6 and E-selectin in men but only after the interaction term was included in the model, suggesting a complex relationship among these predictor variables.
Table 3.
IL-6 regressed on positive relations with others, PSQI global score, and their interaction, and covariates. Standardized regression coefficients are shown
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| Predictor | Men | Women | Men | Women |
| Positive relations with others | 0.17* | –0.04 | 0.21* | –0.05 |
| PSQI global score | 0.57** | 0.13 | 0.47* | –0.01 |
| Positive relations with others × PSQI global score | –0.43* | –0.02 | –0.43* | 0.05 |
P < 0.05
P < 0.01.
Table 4.
E-selectin regressed on positive relations with others, PSQI global score, and their interaction and co-variates. Standardized regression coefficients are shown
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| Predictor | Men | Women | Men | Women |
| Positive relations with others | 0.22* | 0.05 | 0.23** | 0.04 |
| PSQI global score | 0.73** | 0.32 | 0.66** | 0.23 |
| Positive relations with others × PSQI global score | –0.62** | –0.25 | –0.60** | –0.21 |
P < 0.05
P < 0.01.
Figure 1.
Scatter plot of PSQI global scores predicting IL-6 in men. The statistical interaction between PSQI scores and positive relations with others (Table 3) is illustrated here using the top (“high relations”) and bottom (“low relations”) tertiles of scores from the positive relations scale.
Figure 2.
Scatter plot of PSQI global scores predicting E-selectin in men. The statistical interaction between PSQI scores and positive relations with others (Table 4) is illustrated here using the top (“high relations”) and bottom (“low relations”) tertiles of scores from the positive relations scale.
Objective sleep quality
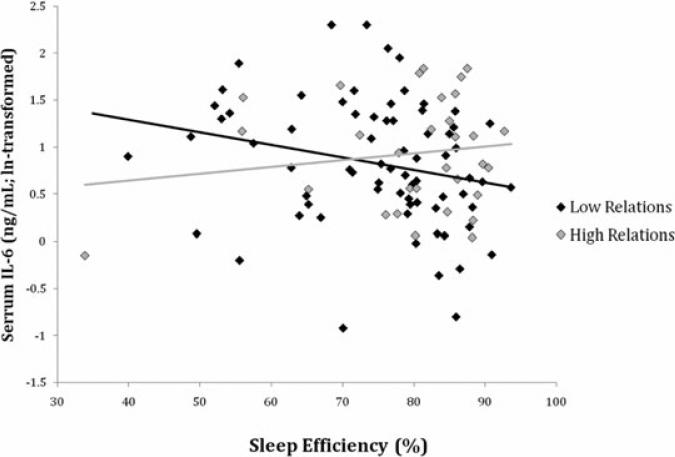
In men, sleep efficiency was unrelated to IL-6 in the model adjusting for demographic characteristics and positive relations with others (β = –0.09, P = 0.27), but it became a significant predictor after the inclusion of the interaction term (β = –0.80, P < 0.05) and remained significantly associated with IL-6 in the full model (β = –0.90, P < 0.05). The sleep efficiency X-positive relations interaction was marginally significant in the model adjusting for demographic characteristics (β = 1.14, P = 0.07), and this association became statistically significant in the full model (β = 1.42, P < 0.05; Table 5). As shown graphically in Figure 3, as sleep efficiency declined, IL-6 tended to increase, but this increase is more pronounced in men with low positive relations scores. In women, sleep efficiency was significantly associated with IL-6 in the base model (β = –0.14, P < 0.05), but not after adjustments for health status, health behavior, and positive and negative affect (β = –0.01, P = 0.95). The sleep efficiency X-positive relations interaction was not a significant predictor of IL-6 in women in either the base model (β = –0.55, P = 0.41) or the full model (β = –0.33, P = 0.56; Table 3).
Table 5.
IL-6 regressed on positive relations with others, sleep efficiency, and their interaction, and covariates. Standardized regression coefficients are shown
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| Predictor | Men | Women | Men | Women |
| Positive relations with others | –0.72# | 0.38 | –0.88* | 0.22 |
| Sleep efficiency (actigraphy) | –0.80* | 0.12 | –0.90* | 0.15 |
| Positive relations with others × sleep efficiency | 1.14# | –0.55 | 1.42* | –0.33 |
P < 0.10
P < 0.05.
Figure 3.
Scatter plot of actigraphic sleep efficiency predicting IL-6 in men. The statistical interaction between PSQI scores and Positive Relations with Others (Table 5) is illustrated here using the top (“high relations”) and bottom (“low relations”) tertiles of scores from the positive relations scale.
Results for E-selectin analyses in men showed that positive relations with others (β = –0.02, P = 0.97), sleep efficiency (β = –0.09, P = 0.82), and the interaction with positive relations (β = –0.01, P = 0.99) were not significantly associated with E-selectin in the base model, and these associations did not change with inclusion of health status, health behavior, and affect variables (data not shown). Sleep efficiency was marginally associated (β = –0.13, P = 0.07) and positive relations with others was significantly associated (β = –0.14, P < 0.05) with E-selectin levels in women after adjusting for demographic characteristics, but not in the full model (sleep efficiency: β = 0.01, P = 1.00; positive relations: β = –0.09, P = 0.20). The interaction term was not significantly associated with E-selectin (β = –0.58, P = 0.46; data not shown).
Neither sleep duration nor its interaction with positive relations with others was significantly associated with IL-6 in men (duration: β = 0.02, P = 0.79; duration X-positive relations: β = 0.48, P = 0.47) or in women (duration: β = –0.05, P = 0.46; duration X-positive relations: β = –0.68, P = 0.19). The same was true for E-selectin in men (duration: β = –0.12, P = 0.18; duration X-positive relations: β = 0.92, P = 0.16) and in women (duration: β = –0.06, P = 0.39; duration X-positive relations: β = 0.56, P = 0.32; data not shown).
Sleep latency was not significantly associated with IL-6 in men (β = 0.05, P = 0.55), and the interaction with positive relations with others was approaching significance (β = –0.69, P < 0.10). In women, greater sleep latency predicted higher levels of IL-6 (β = 0.14, P < 0.05), but the interaction with positive relations was not significant (β = –0.20, P = 0.67). E-selectin in men and women was unrelated to sleep latency (men: β = –0.05, P = 0.58; women: β = 0.06, P = 0.40) or to the interaction with positive relations with others (men: β = 0.66, P = 0.13; women: β = –0.15, P = 0.77; data not shown).
Discussion
The central hypothesis of the current study was that sleep quality and social well-being would interact in predicting inflammation in a national sample of middle-aged men and women. The results provide support for this hypothesis. The association between subjective sleep quality and inflammation was significantly weaker in male participants with higher scores on the positive relations with others scale. Positive relations with others also moderated the association of objectively assessed sleep efficiency and IL-6. We previously reported that positive relations with others moderated the relationship between sleep efficiency, assessed using the Nightcap system, and IL-6 in older women.25 We observed the same pattern of results in the current study in additional analyses of women over the age of 60, although the results were not statistically significant (data not shown). In sum, the current results extend our earlier work to a national sample, and they suggest that across a 50-year age range, social engagement may buffer against the proinflammatory effects of poor sleep (and good sleep may compensate for low levels of social engagement), particularly in men.
As expected, poorer subjective sleep quality, as measured by the PSQI, predicted higher levels of both IL-6 and E-selectin in men and women. A number of studies have reported links between poor subjective sleep quality and higher circulating levels of inflammatory proteins,13,45,46 but to the best of our knowledge, this study is the first to document this association in a national sample with broad demographic representation. These associations were also independent of an array of health status and health behavior measures, including factors that are strongly linked to inflammation, such as obesity.47,48 Subjective sleep quality is thus an important independent predictor of biological processes related to age-related disease.
In the subsample that provided actigraphy data, longer sleep durations, shorter latencies to fall asleep, and greater sleep efficiency all predicted lower levels of IL-6 in women, but not in men, in bivariate analyses; greater sleep efficiency was also associated with lower levels of E-selectin in women. In multivariate analyses, however, only sleep efficiency continued to predict IL-6 and E-selectin after adjustments for demographic characteristics, and those associations were eliminated by further adjustment for health status and health behavior. Age is the most likely factor to account for the sleep duration and sleep latency results. IL-6 in particular rises with age,1 and older adults typically sleep less than younger adults.49 Age thus represents a likely confound for these associations. With regard to sleep efficiency, obesity was the strongest predictor of both IL-6 and E-selectin among the health status variables, and as it is strongly linked to disturbed sleep, particularly due to increased risk of sleep apnea,50 obesity is the most likely mediator of the association between sleep efficiency and inflammation. It is worth noting that the relationship between sleep efficiency and inflammation was the most robust of the actigraphic measures. This is consistent with the research on sleep and loneliness, which centers on differences in sleep quality without differences in sleep quantity,24 and with other lines of work examining the links among social factors, sleep, and health more broadly.51
We also found in bivariate analyses that greater subjective sleep quality, greater sleep efficiency, and reduced sleep latency in men were all associated with greater social engagement. These complement earlier studies reporting poorer sleep in young and older adults with higher levels of loneliness,21–23 and suggest that the link between sleep and social contact may involve not merely the presence or absence of loneliness but also the presence or absence of high levels of social engagement. There is rising interest in the positive end of the spectrum of psychological functioning, including flourishing, and the extent to which positive functioning is an independent predictor of mental and physical health above and beyond the lack of negative functioning.52 Nevertheless, this relationship was not a central focus of the current study, and the extent to which these associations are explained by other factors, such as demographic characteristics of health-related variables, remains to be determined.
There were marked gender differences in sleep quality and inflammation in the MIDUS sample, and this is consistent with a number of earlier studies. Women typically have a greater number of subjective sleep complaints than men, but when sleep quality is assessed objectively using polysomnography or actigraphy, women are often found to sleep better than men.49,53–55 Indeed, while women had significantly higher PSQI scores in the current study, indicating greater sleep pathology, actigraphic assessments showed that compared to men, women took less time to fall asleep, slept longer, and slept for a greater proportion of the night. Men in this sample also had higher levels of E-selectin than women, and this is consistent with other studies.27,56 Inter estingly, while bivariate analyses showed that better subjective and objective sleep quality was more likely to predict lower levels of IL-6 and E-selectin in women, the moderating effect of social engagement on the link between sleep quality and inflammation was apparent only in men. The reasons for this pattern of results are not clear. In some instances, links between psychosocial factors and health are stronger in women.46 On the other hand, there is scant literature on interactive associations among psychological and behavioral factors in predicting inflammation. The current results are similar to those from an earlier study in which exposure to chronic discrimination was linked to higher levels of E-selectin in men from the MIDUS study in spite of the fact that women reported greater exposure to discriminatory treatment.35
Interpretation of these results should be tempered by several limitations of the study. First and foremost, these analyses are cross-sectional, so it is not possible to determine the causal association among the key variables. This is particularly relevant for the link between sleep and inflammation, as inflammatory proteins such as IL-6 are known to impair sleep57 and increase fatigue.58,59 In addition, while this sample was racially diverse, much of that diversity comes from a regional sample of African Americans, and there may be differences between this sample and those from other regions of the country. Finally, inflammatory proteins were only assessed once, which, given variability in their levels within individuals, might produce inaccurate estimates of each person's circulating levels. Nevertheless, such variability would be expected to reduce the likelihood of detecting associations, meaning that the strength of the relationships observed may be underestimated.
In spite of these limitations, the present study adds to the literature on links between sleep and inflammation, social engagement and inflammation, and social engagement and sleep. It also extends our earlier work on interactive associations between sleep and social engagement to a national sample of middle-aged and older adults. These findings underscore the importance of considering the ways in which factors in multiple domains of experience within the same individual interact to affect biological processes related to health. They also highlight the role of positive psychological functioning in the maintenance of advantageous profiles of biological risk as well as the ways in which positive functioning may compensate for the presence of other risk factors.
Acknowledgments
hThis work was supported by grant K01-AG029381 (to EMF) from the National Institute on Aging, and the longitudinal follow-up of the MIDUS investigation was supported in part by grant P01-AG020166 from the National Institute on Aging. The original MIDUS study was supported in part by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. We thank the staff of the Clinical Research Centers at the University of Wisconsin-Madison, UCLA, and Georgetown University for their support in conducting this study. Support comes from the following grants: M01-RR023942 (Georgetown), M01-RR00865 (UCLA) from the General Clinical Research Centers Program, and 1UL1RR025011 (UW) from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 2.Ferrucci L, et al. Serum IL-6 level and the development of disability in older persons. J. Am. Geriatr. Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 3.Gruenewald TL, et al. Combinations of biomarkers predictive of later life mortality. Proc. Natl. Acad. Sci. USA. 2006;103:14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maes M, et al. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–588. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 5.Penninx BW, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol. Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 6.Benca RM, et al. Sleep and mood disorders. Sleep Med. Rev. 1997;1:45–56. doi: 10.1016/s1087-0792(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 7.Motivala SJ, et al. Inflammatory markers and sleep disturbance in major depression. Psychosom. Med. 2005;67:187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, et al. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J. Clin. Endocrinol. Metab. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 9.Redwine L, et al. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J. Clin. Endocrinol. Metab. 2000;85:3597–3603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- 10.Vgontzas AN, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J. Clin. Endocrinol. Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 11.Irwin MR, et al. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch. Int. Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 12.von Kanel R, et al. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer's disease. J. Am. Geriatr. Soc. 2006;54:431–437. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 13.Prather AA, et al. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol. Psychol. 2009;82:12–17. doi: 10.1016/j.biopsycho.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkman LF, et al. Social integration and mortality: a prospective study of French employees of Electricity of France-Gas of France: the GAZEL cohort. Am. J. Epidemiol. 2004;159:167–174. doi: 10.1093/aje/kwh020. [DOI] [PubMed] [Google Scholar]
- 15.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am. J. Epidemiol. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 16.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S. Social relationships and health. Am. Psychol. 2004;59:676–584. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- 18.Cole SW, et al. Social regulation of gene expression in human leukocytes. Genome. Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costanzo ES, et al. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104:305–313. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- 20.Friedman EM, et al. Plasma interleukin-6 and soluble IL-6 receptors are associated with psychological well-being in aging women. Health Psychol. 2007;26:305–313. doi: 10.1037/0278-6133.26.3.305. [DOI] [PubMed] [Google Scholar]
- 21.Cacioppo JT, et al. Loneliness and health: potential mechanisms. Psychosom. Med. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Cacioppo JT, et al. Do lonely days invade the nights? Potential social modulation of sleep efficiency. Psychol. Sci. 2002;13:384–387. doi: 10.1111/1467-9280.00469. [DOI] [PubMed] [Google Scholar]
- 23.Hawkley LC, Preacher KJ, Cacioppo JT. Loneliness impairs daytime functioning but not sleep duration. Health Psychol. 2010;29:124–129. doi: 10.1037/a0018646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann. Behav. Med. 2010;40:218–227. doi: 10.1007/s12160-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman EM, et al. Social relationships, sleep quality, and interleukin-6 in aging women. Proc. Natl. Acad. Sci. USA. 2005;102:18757–18762. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brim OG, Ryff CD, Kessler RC. How Healthy Are We? A National Study of Well-Being at Mid-Life. University of Chicago Press; Chicago: 2004. [Google Scholar]
- 27.Blann AD, Amiral J, McCollum CN. Circulating endothelial cell/leucocyte adhesion molecules in ischaemic heart disease. Br. J. Haematol. 1996;95:263–265. doi: 10.1046/j.1365-2141.1996.d01-1921.x. [DOI] [PubMed] [Google Scholar]
- 28.Hwang S-J, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 29.Ross R. Atherosclerosis—an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 30.Zethelius B, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N. Engl. J. Med. 2008;358:2107–2016. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 31.Zamarron-Sanz C, et al. Plasma levels of vascular endothelial markers in obstructive sleep apnea. Arch. Med. Res. 2006;37:552–555. doi: 10.1016/j.arcmed.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Sauvet F, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J. Appl. Physiol. 2010;108:68–75. doi: 10.1152/japplphysiol.00851.2009. [DOI] [PubMed] [Google Scholar]
- 33.Gruenewald TL, et al. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Soc. Sci. Med. 2009;69:451–459. doi: 10.1016/j.socscimed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Love GD, et al. Bioindiciators in the MIDUS national study: protocol, measures, sample, and comparative context. J. Aging Health. 22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman EM, et al. Chronic discrimination predicts higher circulating levels of E-selectin in a national sample: the MIDUS study. Brain Behav. Immun. 2009;23:684–692. doi: 10.1016/j.bbi.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryff CD, Keyes CL. The structure of psychological well-being revisited. J. Pers. Soc. Psychol. 1995;69:719–727. doi: 10.1037//0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- 37.Watson D, et al. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J. Abnorm. Psychol. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- 38.Watson D, et al. Testing a tripartite model: I. evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J. Abnorm. Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 39.Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry. Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 40.Buysse DJ, et al. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep. 1991;14:331–338. [PubMed] [Google Scholar]
- 41.Fliser D, Buchholz K, Haller H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110:1103–1107. doi: 10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed] [Google Scholar]
- 42.Tatli E, Kurum T. A controlled study of the effects of carvedilol on clinical events, left ventricular function and proinflammatory cytokines levels in patients with dilated cardiomyopathy. Can J. Cardiol. 2005;21:344–348. [PubMed] [Google Scholar]
- 43.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 44.Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int. J. Neuropsychopharmacol. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- 45.Okun ML, Coussons-Read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain Behav. Immun. 2009;23:351–354. doi: 10.1016/j.bbi.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav. Immun. 2008;22:960–968. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bastard JP, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 48.Yudkin JS, et al. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 49.Ohayon MM, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 50.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am. J. Respir. Crit. Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 51.Moore PJ, et al. Socioeconomic status and health: the role of sleep. Psychosom. Med. 2002;64:337–344. doi: 10.1097/00006842-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 52.Keyes CL. The mental health continuum: from languishing to flourishing in life. J. Health Soc. Behav. 2002;43:207–222. [PubMed] [Google Scholar]
- 53.Bixler EO, et al. Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J. Sleep Res. 2009;18:221–228. doi: 10.1111/j.1365-2869.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCrae CS, et al. Sleep complaints, subjective and objective sleep patterns, health, psychological adjustment, and daytime functioning in community-dwelling older adults. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2005;60:P182–P189. doi: 10.1093/geronb/60.4.p182. [DOI] [PubMed] [Google Scholar]
- 55.Van Den Berg JF, et al. Sex differences in subjective and actigraphic sleep measures: a population-based study of elderly persons. Sleep. 2009;32:1367–1375. doi: 10.1093/sleep/32.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blann AD, Daly RJ, Amiral J. The influence of age, gender and ABO blood group on soluble endothelial cell markers and adhesion molecules. Br. J. Haematol. 1996;92:498–500. doi: 10.1046/j.1365-2141.1996.d01-1486.x. [DOI] [PubMed] [Google Scholar]
- 57.Prather AA, et al. Cytokine-induced depression during IFN-alpha treatment: the role of IL-6 and sleep quality. Brain Behav. Immun. 2009;23:1109–1116. doi: 10.1016/j.bbi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bower JE, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin. Cancer Res. 2009;15:5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rief W, et al. Overnight changes of immune parameters and catecholamines are associated with mood and stress. Psychosom. Med. 2010;72:755–762. doi: 10.1097/PSY.0b013e3181f367e2. [DOI] [PMC free article] [PubMed] [Google Scholar]