Abstract
Skin related diseases comprise a major health challenge to the practicing physician, and constitute a significant psychological, social and financial burden to the society. Further, skin cancer, especially non-melanoma skin cancer is currently the leading type of malignancy in the Western world. Given the huge burden of skin diseases, there is growing emphasis on understanding their pathophysiology, and towards their early detection. Mucins are high molecular weight O- and N-linked glycoproteins that have emerged in recent years as important molecules in maintaining health and in promoting or protecting against inflammation and cancer. They have also begun to emerge as highly specific diagnostic and prognostic markers and novel therapeutic targets in several malignant disorders. However, their role in cutaneous pathologies has remained largely obscured. The present review provides the expression patterns and proposed role of mucins in the healthy skin and various benign and malignant skin diseases. The review has immense clinical significance as the availability of highly specific reagents including monoclonal antibodies against mucins makes them extremely attractive targets for specific diagnosis and/or immunotherapy of benign and malignant cutaneous diseases.
Keywords: Mucin, cutaneous diseases, diagnosis
INTRODUCTION
Skin-related diseases comprise the most common health problem reported worldwide and constitute a significant psychological, social and financial burden to the society in general and patients in particular.[1] In the United States, they account for more than 25 million visits to the physician's office each year, according to the National Health Statistics Report published in 2008.[2] According to the same report, a skin rash was rated by patients as one of the top 20 causes for their visits to the physician, accounting for nearly 10 million visits (or 1.1 percent of all patient visits) in 2006.
Mucins comprise a large family of high molecular weight glycoproteins characterized structurally by a high content of proline (P), serine (S) and threonine (T) residues concentrated in one or more regions of the molecule (PST regions) and by O-linked glycosylation of the serine and threonine residues in these PST regions. [3, 4] There are two categories of mucins: epithelial and endothelial. The epithelial mucins comprise 17 members (MUC1–8, MUC12, MUC13, MUC15–17, MUC19 and MUC20) and are divided into two subtypes, membrane-bound and secreted.[3, 5–7] Endothelial or leukocyte mucins include CD34, CD45RA, GlyCAM-1 and PSGL-1(CD162).[4] Epithelial mucins are distinguished by the presence of a variable number of tandem repeats ranging from 8–169 amino acids in length and occurring within one or more of their mucin domains. [4] The endothelial mucins or mucin-like glycoproteins lack these repeat domains but possess O-linked glycosylation clustered on the serine and threonine residues.
The structure of mucins and their role in several diseases has been reviewed in earlier articles. [3, 8–11] However, their significance in diseases of the skin and its appendages has never been discussed. The present review discusses the expression and proposed role of the epithelial mucins in cutaneous pathologies.
The Skin: A multifunctional organ
The skin is the largest organ in the human body and serves as the first line of defense against external agents. It also resists mechanical shocks, regulates heat loss and mediates various tactile sensations including those of touch, pain, pressure and temperature. Histologically, it is comprised of three layers: an outer epidermis, an inner dermis and the innermost layer of subcutaneous adipose tissue. The epidermis is almost entirely composed of keratinocytes, with other cells, including melanocytes, the neuroendocrine Merkel cells and unmyelinated axons, making up only about 10 percent of the remaining cellular components. The epidermis is comprised of three distinct zones: the stratum corneum, the stratum spinosum and the stratum granulosum. The stratum granulosum or basal layer is the innermost layer and consists of columnar cells that are oriented perpendicular to the surface. Mitosis is restricted to this layer of the epidermis and all other layers arise from this layer of cells. The basal layer is also significant for the presence of melanocytes, which are large cells with a clear cytoplasm and small regular nuclei. The stratum spinosum lies above the basal layer and is important in the formation of keratin. The stratum corneum forms the outermost layer of the skin and is comprised of flattened keratinized cells which are usually anucleate. This layer plays a critical role in regulating the response of the skin to radiation exposure as well as the reaction to extremes of temperature and humidity.
The dermis is separated from the epidermis by an indistinct basement membrane and is comprised predominantly of a supporting matrix of collagen and glycosaminoglycans. It is divided into a more superficial papillary dermis that lies subjacent to the epidermal rete ridges, and the deeper reticular dermis. The dermis contains glands (apocrine and eccrine sweat glands and sebaceous glands), muscles, blood vessels, nerves and lymphatics. Functionally, the epidermis and dermis form a single functional unit, as reflected in the involvement of both layers by most cutaneous diseases. The dermis is also home to histiocytes, scavenger cells that phagocytose melanin, lipids and other debris and mast cells that play an important role in inflammation. [12]
An important function of the skin is to absorb harmful radiation. The untanned epidermis absorbs most of the ultraviolet radiation from sunlight while allowing the visible and near infrared radiations to pass through. Tanning however markedly reduces the amount of radiation transmitted through the skin and is shown to protect against the development of radiation-induced skin cancers. [13] Regulating a constant body temperature despite marked changes in external temperature is another critical function served by the skin, specifically the eccrine sweat glands and cutaneous blood vessels. The eccrine glands produce a hypotonic secretion that cools the skin as it evaporates off the surface, while the blood vessels constrict or dilate to conserve or dissipate heat, respectively, to the surroundings. Developmentally, these glands arise from the epidermis and then descend through the dermis, finally coming to rest near the junction of the dermis and subcutaneous fat. Histologically, a sweat gland is a hollow tube which is comprised of a coiled secretory portion lying in the dermis, followed by a duct which is initially coiled and then straightens out, finally leading to the surface after spiraling through the epidermis. Unlike apocrine glands, which store the secretions temporarily within their ducts, eccrine sweat glands release their secretion almost instantaneously to the surface upon stimulation by a heat stress. In humans as in other higher primates, the true sweat glands are eccrine in nature.
Sebaceous glands serve another important function, to waterproof the skin. These holocrine glands arise from the outer root sheath of hair follicles and thus are generally associated with a hair follicle. Each gland consists of a cluster of flask shaped glandular elements similar to the lung alveoli that are tightly packed with cells containing oil droplets. These cells disintegrate, releasing sebum either into the hair follicle or directly onto the skin surface.
Apocrine glands are coiled tubular glands of poorly understood function that are localized to specific areas: the axilla, areola of the mammary glands, labia minora, the periumblical and periscrotal areas and in the external ear canal and eyelids (glands of Moll). In animals, they serve as sexual scent glands. In humans, they remain rudimentary until the individual reaches puberty, when they enlarge significantly and begin to produce secretions rich in androgens and cholesterol.
The MUC family of mucins - Structural and functionally diverse molecules
Epithelial mucins are divided into two families: secreted or gel-forming (comprises MUC2, MUC5AC, MUC5B, MUC6 and MUC19) and membrane-tethered mucins (MUC1, MUC3A, MUC3B, MUC4, MUC11–12, MUC16 and MUC17). [14] The chromosomal location of the mucin encoding genes is now well defined. Of the membrane-bound mucins, the gene encoding MUC1 is located at 1q21, while MUC4 was mapped to the chromosome locus 3q29. MUC3A, MUC3B, MUC11, MUC12 and MUC17 genes were found clustered on 7q22.1 and the MUC16 gene was at locus 19p13.2. [14, 15] The secreted mucins MUC2, MUC5AC, MUC5B and MUC6 are encoded by genes located in a cluster on chromosome 11p15. [16] All the secreted or gel-forming mucins (with the exception of MUC7) form oligomeric structures owing to intermolecular disulphide bonds between highly conserved cysteine residues present in domains similar to that seen in the von Willebrand factor (vWF). This enables them to produce a thick viscous gel that lines and lubricates most luminal surfaces. The membrane-bound mucins, on the other hand, are monomeric glycoproteins located primarily on the cell surface. A review of the literature revealed that MUC1 was the most examined mucin family member in cutaneous pathologies, mostly under the name of epithelial membrane antigen (EMA), followed by MUC5AC. Here, we will review some salient features of these two mucins that represent the sub-classes of membrane-bound and secreted mucins, respectively.
MUC1 was the first mucin gene to be cloned. [17–20] It is a type 1 transmembrane glycoprotein with an N-terminal signal peptide, a central large tandem repeat domain (makes up 50% to 80% of the total length of the protein according to the inherited polymorphism in the number of repeats), a SEA module, potential N-glycosylation sites, a transmembrane domain and a cytosolic tail. Several splice variants of MUC1 have been described of which the MUC1/Y isoform lacks the tandem repeat domain. MUC1 is normally expressed on the apical surface of glandular and ductal elements in several organs including the mammary gland, lungs and the pancreas.
MUC5AC is the major mucin present in respiratory secretions. It is expressed mostly by the goblet cells of the surface epithelium lining the air passages. Thus, an upregulation of MUC5AC expression is commonly observed in respiratory diseases like cystic fibrosis, which are characterized by an increase in the goblet cell population.[21] Further, transgenic mice that overexpress IL-4 selectively in the lungs, were found to have significantly elevated levels of MUC5AC mRNA in their lungs by Northern blot, suggesting that it may have a role in IL-4 induced bronchial asthma [22]. MUC5AC has also been shown to be involved in neoplastic progression. For instance, while it is expressed by the normal gastric mucosa, its expression (together with that of MUC1 and MUC6) is lost in type I intestinal metaplasia, a pre-malignant lesion that precedes gastric adenocarcinoma [23]. Like other polymeric mucins present in the airway secretions, the protein backbone of MUC5AC possesses a large number of disulfide bridges and adopts a random coil conformation in solution. The gene coding for this polymeric mucin is comprised of a single exon encoding the large mucin domain, characterized by PST-rich tandemly repeated units, while the 5'and 3' regions of the gene code for cysteine-rich domains that resemble the cysteine-rich domains present in the von Willebrand factor (vWF). These cysteine-containing domains are believed to be important in the polymerization of the mucin following its secretion, thus creating a mesh in which the particulate matter entering the airways is trapped. [24]
Functions of mucins in health and disease
Mucins serve several functions in healthy tissues which include providing lubrication, maintaining tissue hydration, protecting underlying cells from infection, and promoting or inhibiting cell attachment to the substratum (Figure 1). The peripheral region of the oligosaccharides attached to the mucin core proteins play a key role in determining the antigenicity of these large molecules. As the characteristic tandem repeat regions of mucins are heavily O-glycosylated, variations in the length (VNTR polymorphism) and the degree of glycosylation of these domains has been proposed to play an important role in their immune modulatory function. [7]
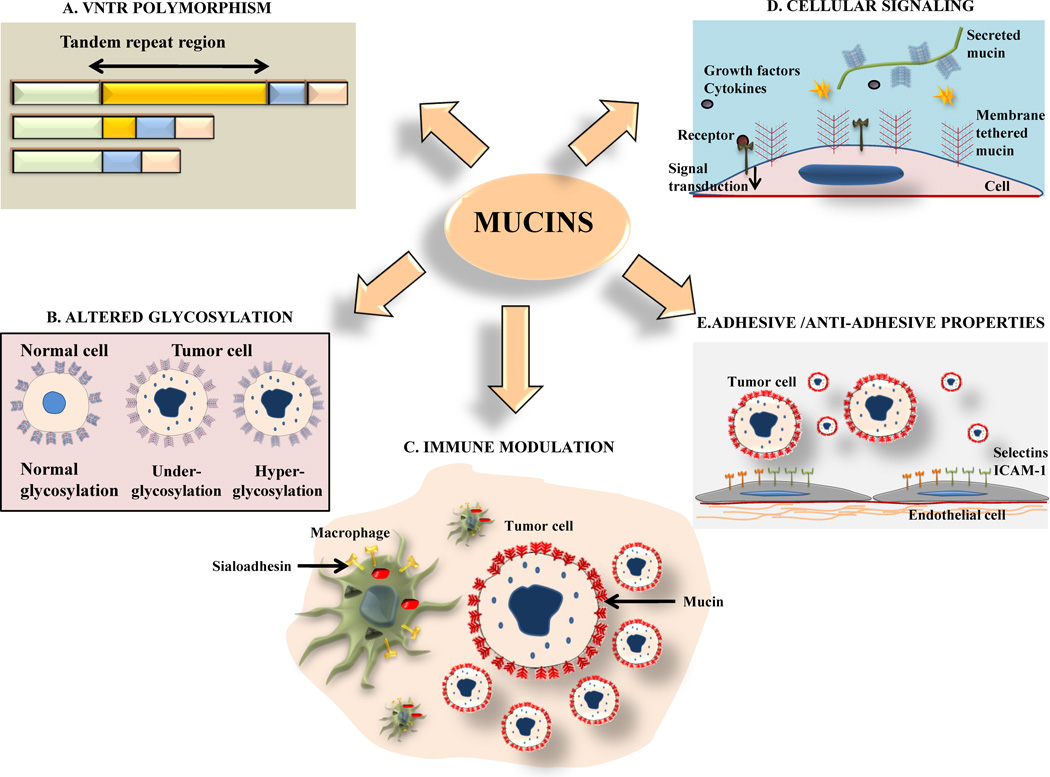
Figure 1. Mechanisms underlying mucin-mediated modulation of cutaneous biology in health and disease.
(A) Mucins are characterized by the presence of tandem repeats (TR), which are short stretches of amino acids repeated several times in tandem. These TR regions are rich in Proline, Serine and Threonine. Serine and Threonine are sites for O-glycosylation. Polymorphisms in the TR region (termed variable number of tandem repeat or VNTR polymorphism) give rise to different splice variants of mucins. The VNTR polymorphism of MUC1 has been shown to affect the likelihood of patients developing a severe form of acne. (B) Mucins are heavily glycosylated proteins and there is an alteration (both under and hyperglycosylation) in their degree of glycosylation in malignant cutaneous diseases. Increased glycosylation masks peptide epitopes on the tumor cell surface and has been suggested as a mechanism employed by tumors to evade the immune system. (C) Mucins have a key role in immune response and can modulate the function of both innate and aquired immune response. MUC1, for instance, interacts with sialoadhesion, a molecule restricted to macrophages and thus promotes recruitment of these scavenger cells to the sites of inflammation. (D) Mucins have diverse roles in modulating intracellular signaling events by interacting with both extracellular receptors (like epidermal growth factor receptor-1 (EGFR) and ErbB2) and intracellular adaptors (like Grb2, p53 and c-Abl). The resultant signals modulate vital cellular processes, including survival, apoptosis, invasion, motility and metastases. (E) The binding of their oligosaccharide side chains to adhesion molecules (e.g. selectins and inter-cellular adhesion molecule-1 (ICAM-1)) allows mucins to act as adhesive agents facilitating the attachment of tumor cells to the vascular endothelium. At the same time, these oligosaccharides inhibit the interaction of tumor cells with extracellular matrix components (e.g. kalinin and laminin) and thus act as anti-adhesive agents.
In the case of MUC1, this VNTR polymorphism gives rise to several alleles. Of these, the 4000 base pair allele is the most common allele observed in both healthy subjects and those with diseases associated with an overexpression of MUC1. [25] In a study comprised of Japanese patients with either mild or severe acne or atopic dermatitis, it was found that while the 4000 bp allele was still the predominant allele in all cases, there was a significant increase in the frequency of the allele with >5000 base pairs in patients with severe acne (compared to healthy subjects) but not in those with mild acne or atopic dermatitis. Further, while the frequency of the >5000 base pair allele was 31% in blood samples from healthy Japanese subjects, among healthy Europeans the frequency was observed to be nearly 50%. [25] This value closely resembled the frequency of the >5000 base pair allele seen in Japanese subjects with severe acne (59%). These studies suggest that a difference in allele length in healthy individuals demonstrates ethno specificity. Further, within a given ethnic or racial group, the variation in the length of the tandem repeat region could alter the function of the mucin, which, in turn, could affect their susceptibility to disease. Although the exact mechanism by which the VNTR polymorphism affects the function of MUC1 in the skin is not known, one hypothesis is that the larger size of the allele may interfere with its anti-adhesive properties, thereby allowing bacteria to colonize the skin. In support of this hypothesis, mucins have been shown to prevent binding of the bacterium Pseudomonas aeruginosa to the mucosa in the tracheobronchial tree. [26]
In addition to size, the degree of the glycosylation of mucins also plays an important role in the pathogenesis of various diseases, both benign and malignant. For instance, the prototype mucin, MUC1 expression is highly upregulated in glandular cells upon their malignant transformation. During this process, there is an alteration in its glycosylation, most commonly underglycosylation. However, both hyper and hypoglycosylated MUC1 has been shown to stimulate cytotoxic T-lymphocytes (CTL’s) derived from ovarian cancer patients. These anti-MUC1 specific CTL's have the ability to kill tumor cells and therefore provide long-term immunity against cancer in animal models. In cutaneous squamous cell carcinoma (both sporadic and that associated with epidermolysis bullosa) and Bowen’s disease, the tumor cells express a hyperglycosylated form of MUC1 (identified with glycosylation status specific antibodies). [27] This altered glycosylation of mucins, chiefly hyperglycosylation, has been suggested as a possibly mechanism used by tumor cells to avoid detection by the immune system possibly by masking of the peptide epitopes, thus contributing to their continued survival. The importance of MUC1 in the immune response pathway is further confirmed by its ability to modulate the adhesion of intramural polymorphonuclear leucocytes to the epithelium in vitro. [28] MUC1 may also play a role in recruiting macrophages to the sites of inflammation by its specific interaction with sialoadhesion, an adhesion molecule restricted to macrophages.
MUC1 has also been shown to have adhesive and anti-adhesive properties. The former is mediated by the binding of its oligosaccharide side chains with specific adhesion molecules (like selectins) and of the tandem repeat domain to ICAM-1. Both these mechanisms have been proposed to contribute to the metastases of cancer cells by promoting their binding to the vascular endothelium. In contrast, it also inhibits the interaction of cells with the extracellular matrix components like laminin and Kalinin, thus acting as an anti-adhesive agent.
The cytoplasmic domain of MUC1 plays a key role in intracellular signaling through its interactions with several regulatory molecules, including β-catenin, c-src, Grb2/SOS and the epidermal growth factor (EGF) receptors 1–4. MUC1 also upregulates cyclin-D gene expression, suggesting its possible role in regulating cell cycle progression. [5]
Expression of mucins in the normal skin
The normal skin exhibits a rather restricted expression of mucins (Figure 2). Several studies have demonstrated that the epithelial cells of the normal epidermis do not express sialomucins. [29, 30] MUC1, also known as epithelial membrane antigen (EMA), episialin, DF3 antigen, polymorphic epithelial mucin (PEM) and human milk fat globulin (HMFG) antigen is the most studied mucin in benign and malignant skin disorders. [31] Neither MUC1 (EMA) nor MUC5AC core proteins are detected in the squamous epithelium of the normal adult epidermis. However, MUC1 is expressed on the luminal surface and ductal structures of the eccrine, apocrine and Bartholin's glands, and in the Toker cells (of the nipple). [32] The degenerate cells comprising the holocrine secretions of sebaceous glands in the skin are also positive for MUC1/EMA [33]. MUC1 expression has also been reported in the glands of Moll, which are specialized apocrine glands located in the margin of the eyelids. MUC1 expression in these glands was strongest in the apical portion of active glandular elements, with a weak to medium intensity noted in the cytosol. In the inactive glandular cells, a strong expression of MUC1 was noted specifically in the apical portion of the cells. Additionally, MUC1 also stained the irregular floccular structures present in the lumen of these glands [34]. Lymphocytes present in the normal skin reacted specifically with an antibody (mAb SM-3) that recognizes the hypoglycosylated form of MUC1. [27] Merkel cells present in the normal epidermis and hair follicles were immunolabeled by antibodies that recognized the underglycosylated (mAb BM-7) or unglycosylated (mAb BM-2) forms of MUC1 as well as those that were not specific for its glycosylation status (VU-2G7). [35] Using these antibodies, the acinar elements of both the sweat and sebaceous glands were brightly positive, while the ductal cells were uniformly negative. Another antibody (mAb MA552),, which recognized MUC1 independent of its glycosylation status did not stain the Merkel cells and only weakly stained the luminal portion of sweat gland acini. MUC5AC is expressed only by Bartholin's glands but not the other ductal structures, while MUC2 and MUC6 are not expressed by any of the glandular elements of the normal skin [32, 36]. A study examining MUC6 expression in various normal human tissues found no expression in the normal adult skin by immunohistochemical analysis. [37]
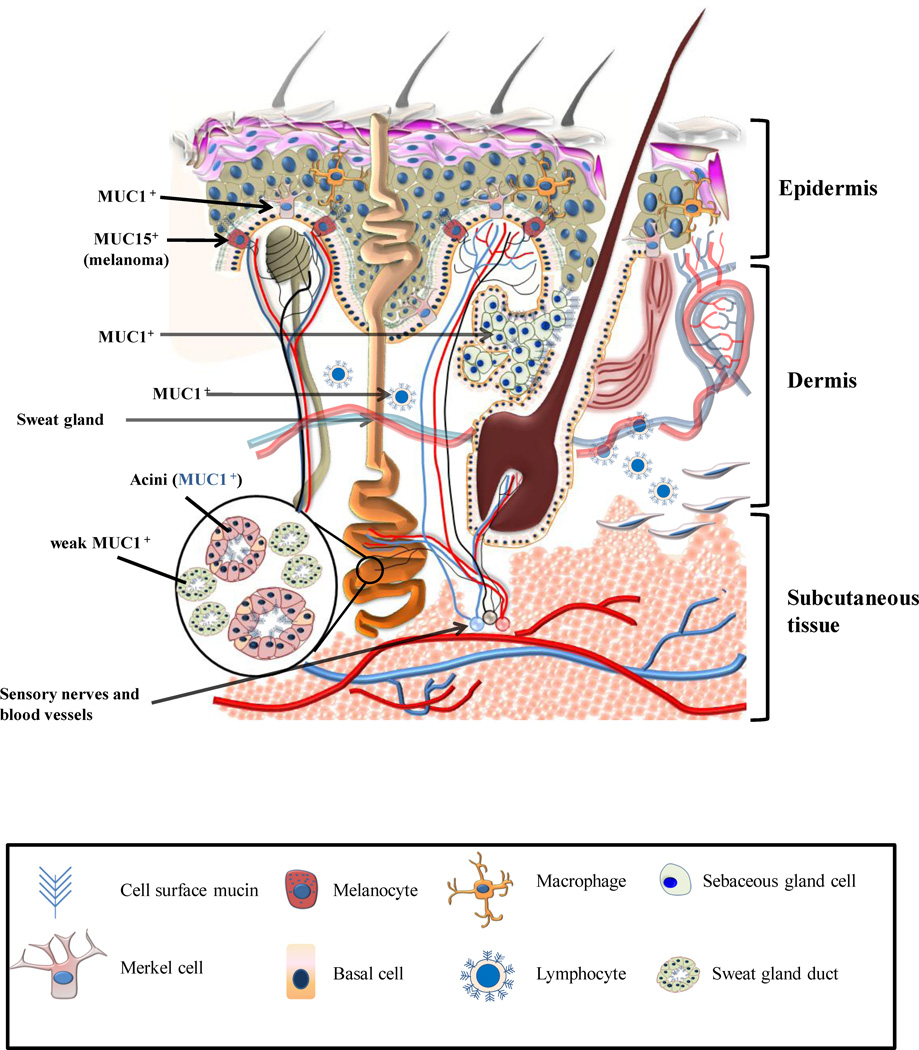
Figure 2. Schematic diagram of the normal skin showing an expression of various mucins.
The normal skin is comprised of three layers, the epidermis (most superficial), the dermis and the subcutaneous tissue (innnermost layer). MUC1, MUC2, MUC5AC and MUC6 are not expressed by the normal epidermis. MUC4 is weakly expressed by the epidermis in a proportion of tissue sections. The luminal surfaces of eccrine and apocrine glands and the degenerate cells comprising the secretions of the sebaceous glands are positive for MUC1. In apocrine glands (e.g. Moll’s glands of the eyelids), MUC1 is strongly expressed on the cell membrane and weakly in the cytoplasm of active glandular elements. MUC1 staining is also observed in the secretions present in the lumen of these glands. The acini of both sebaceous and sweat glands express MUC1 but not MUC2, MUC6 or MUC5AC (Except Bartholin’s glands which express MUC5AC). Ducts of these glands, however, are either negative (using antibodies specific for underglycosylated MUC1) or weakly positive (using antibodies that recognize MUC1 independent of its glycosylation status) for MUC1. Merkels cells express an under-glycosylated form of MUC1. Lymphocytes present in the normal skin express an under-glycosylated form of MUC1. Melanocytes normally do not express mucins but MUC15 mRNA expression was shown to be aberrantly upregulated in thin melanomas.
MUC4 expression was detected only in the embryonic skin in the early stages of development, but disappeared later, and was absent from the normal adult skin. [38] Recent studies in our laboratory using the monoclonal antibody against the tandem repeat domain of human MUC4 (clone 8G7) have revealed that a weak expression of MUC4 is observed in the epidermis of nearly 37% of normal skin tissues. [39] Significantly, a moderately strong expression was observed in a small percentage (2/21) of normal skin tissues adjacent to a cutaneous malignancy. This data suggests a possible modulation of epidermal MUC4 expression by adjacent malignant cells.
Mucin expression in benign skin disorders
Benign diseases with an overexpression of mucins include mucocutaneous neuromas, [40] generalized sclerodermatous graft-vs.-host disease, [41] follicular mucinosis (both idiopathic and lymphoma associated types) [42] and morphea profunda. [43]
Ectopic hamartomatous thymoma (EHT) is a benign tumor believed to arise from the remnants of the primitive ectoderm of the branchial arch. [44] It is comprised of epithelial elements, spindle cells and adipocytes and can often be confused with other benign or malignant spindle cell tumors that contain fatty tissue. In one case of EHT, [45] the epithelial elements exhibited features resembling the glandular structures found in the normal skin. Immunohistochemical examination of tissue sections revealed that the sebaceous cells and the luminal borders of the eccrine and apocrine glands were strongly stained by the antibody to MUC1/EMA, while all other components of the tumor were negative for MUC1. This suggests that MUC1/EMA is a marker of cells with sebaceous differentiation and for eccrine and apocrine glands in benign and malignant neoplasms arising from the skin and its adnexa. MUC1 expression was also reported in the stratified ciliated columnar epithelium lining the cystic cavity in a case of the rare but benign cutaneous ciliated cyst. [46]
First described in 1972 by Reed et al., palisaded encapsulated neuroma (PEN) of the skin is a benign, solitary papular lesion that preferentially occurs near the mucocutaneous junctions in the face, although it is not limited to this region alone. [47] One of the factors suggested to play a role in their genesis is chronic trauma. In an attempt to resolve this query, Argenyi et al. examined eight cases of traumatic neuroma (TN) and 12 cases of PEN. [48] While in TNs the EMA expressing perineural cells surrounded the nerve fascicles, the perineural cells were present predominantly in the capsular areas and rarely in the fascicles in case of the PEN lesions.
MUC1 (EMA) is also expressed in other benign cutaneous lesions including the rare clear-cell papulosis observed only in Taiwanese children [49] and inflammatory dermatitis.[33] In the latter condition, a strong circumferential staining for MUC1 was observed in the cell membrane of the keratinocytes, with a weaker cytoplasmic positivity. A deficiency of MUC1 production is seen in the corneal epithelium of patients with Sjogren’s syndrome and has been implicated in the ocular manifestations of this autoimmune disease, possibly by increasing the viscosity and decreasing the stability of the tear film. [50]
We observed moderately strong MUC4 expression in a subset of chronically inflamed cutaneous specimens [39]. Further, we observed a strong MUC4 expression in a proportion of cutaneous hyperplasia of the vulval skin and in condyloma acuminate of the vulva. The latter is associated with infection by human papillomavirus types 6, 11 (predominantly) and to a lesser extent other HPV types (including 31, 33, 35, 39, 40.42–45, 51–56 and 58). MUC4 expression, both in inflammation and hyperplastic conditions, was observed to be both in the cytoplasm and membrane.
Mucin expression in malignant neoplasms arising in the skin
Mucin overexpression is also observed in numerous types of cutaneous malignancies, including glomus tumors, [51] the rare primary mucinous carcinoma of the skin (MCS), [52] and cutaneous metastases from mucin-expressing tumors. [53–55]
Primary mucinous carcinoma of the skin (MCS) is a rare tumor of skin appendages that is believed to arise from the apocrine glands. In a case report of MCS, the mucinous material was identified as being a sialomucin, although its exact identity was not investigated. [56] Ishida et al. reported a case of MCS with endocrine differentiation arising in the face, unassociated with any other internal malignancy, which was positive for MUC2 and MUC6 in addition to immunoreactivity for estrogen and progesterone receptors. [57]
Lymphoma is the most common malignancy among adolescents, accounting for >25% of newly diagnosed cancers in the 15–19 year age group and includes the Hodgkin and Non-Hodgkin subtypes. [58] Non-Hodgkin lymphoma (NHL) is the fourth most common malignancy among adolescents in the United States, accounting for nearly 8% of all cancers in those between 15 and 19 years of age. Anaplastic large cell lymphoma (ALCL), a subtype of NHL comprises nearly 10% of NHLs in children up to 14 years old, reaching a peak incidence of 17% in adolescents. However, it is less common in adults (aged 24 and older), with adult cases accounting for only 5% of all NHLs. [59] Based on clinical features and molecular markers, ALCL is divided into three types: primary systemic ALCL (anaplastic lymphoma kinase (ALK)-positive), ALK-negative primary systemic ALCL and primary cutaneous ALCL (usually ALK-negative). [60] Primary cutaneous anaplastic large cell lymphoma (C-ALCL) belongs to the group of CD30-positive primary cutaneous lymphomas and is characterized by the development of multifocal skin lesions that have a tendency to regress spontaneously and relapse, however, with a favorable prognosis (10-year survival rate of nearly 90%). [61] Ten percent of C-ALCL cases disseminate extracutaneously (termed as systemic ALCL, S-ALCL). S-ALCL is characterized by a strong expression of MUC1 (EMA), while a weak or non-expression of MUC1 is the feature of C-ALCL. [62] An ALK-positive phenotype is also characteristic of systemic dissemination (S-ALCL) and is usually associated with EMA positivity. [63] However, in a case report of a 47-year-old Japanese woman with ALK and EMA positive neoplastic cells and prominent neutrophil infiltration, the ALCL lesion was localized to the skin (cutaneous ALCL). [64] Although meticulous assessment showed no detectable involvement of lymph nodes or visceral organs in this patient, subsequent assessment six months later revealed regional lymph nodes positive for neoplastic cells. This case illustrates the importance of an aggressive search for another visceral malignancy in cases of C-ALCL with ALK+/EMA+ tumor cells even though the primary lesion appears to be limited to the skin. In cases with no apparent metastases, a close watch should be maintained to detect early metastatic lesions. Kadin et al. [62] reported another case of MUC1-positive C-ALCL wherein the tumor cells were positive for the activated form of ALK in the cytoplasm. It was suggested by these authors that activated ALK could be responsible for the induction of MUC1 expression in systemic and some rare cases of C-ALCL. This is supported by the strong association of ALK positivity with EMA positivity in systemic ALCL and by the observation that stable transduction of the oncogenic form of ALK (nucleophosmin-ALK, NPM-ALK) into cell lines are derived from Hodgkin’s lymphoma and C-ALCL induced the de novo expression of MUC1. [65]
MUC1 is not only expressed in established cutaneous malignancies but also in pre-malignant lesions like Epidermolysis bullosa and Bowen’s disease (Figure 3a). Epidermolysis bullosa (EB) is a group of inherited disorders of the skin characterized by cutaneous blistering following minor trauma. [66] Patients with dystrophic and junctional epidermolysis bullosa (DEB and JEB), lesions characterized by the occurrence of blisters in the papillary dermis and within the lamina lucida of the skin respectively, are at a significantly higher risk for subsequent development of cutaneous squamous cell carcinoma (SCC). [67] Using monoclonal antibodies that recognize the differentially glycosylated forms of MUC1, it was observed that all of the DEB/JEB associated SCC's (n=30) and 95 percent of sporadic SCCs (n=55) exhibited MUC1 positivity in the tumor cells. Ninety-seven percent of tissues from cases of sporadic Bowen’s disease (n=30) were also positive for MUC1. MUC1 expressed by the neoplastic cells both in SCC (sporadic as well as DEB/JEB associated) and Bowen's disease was determined to be hyperglycosylated, as indicated by a greater reactivity of the tumor cells with the antibody specific for the hyperglycosylated form of the mucin (mAb HMFG1). MUC1 was detected predominantly in the cell membrane of the tumor cells, with occasional weak cytoplasmic staining. Further, it was present throughout the entire cell membrane of the malignant epithelial cells, unlike the apical polarity observed in glandular epithelial cells. Interestingly, some of the non-neoplastic areas of the skin adjacent to the malignant regions in sporadic SCC and Bowen’s disease were also positive for MUC1. In order to examine whether this could have arisen following recent exposure to ultraviolet (UV) radiation, MUC1 expression was compared between non-irradiated and areas of healthy skin irradiated with 200mJ cm−1 of UVB 24 hours earlier (n=12). However, no significant difference was observed following the treatment in any subject and the percentage of MUC1-positive cells was very low in the healthy skin (< 5 percent), suggesting that short term radiation with UVB did not induce MUC1 expression in the skin. [68] All 15 invasive SCCs, 11 sebaceous carcinomas and 12 of the 15 porocarcinomas were positive for MUC1 (EMA) in another study. [69]
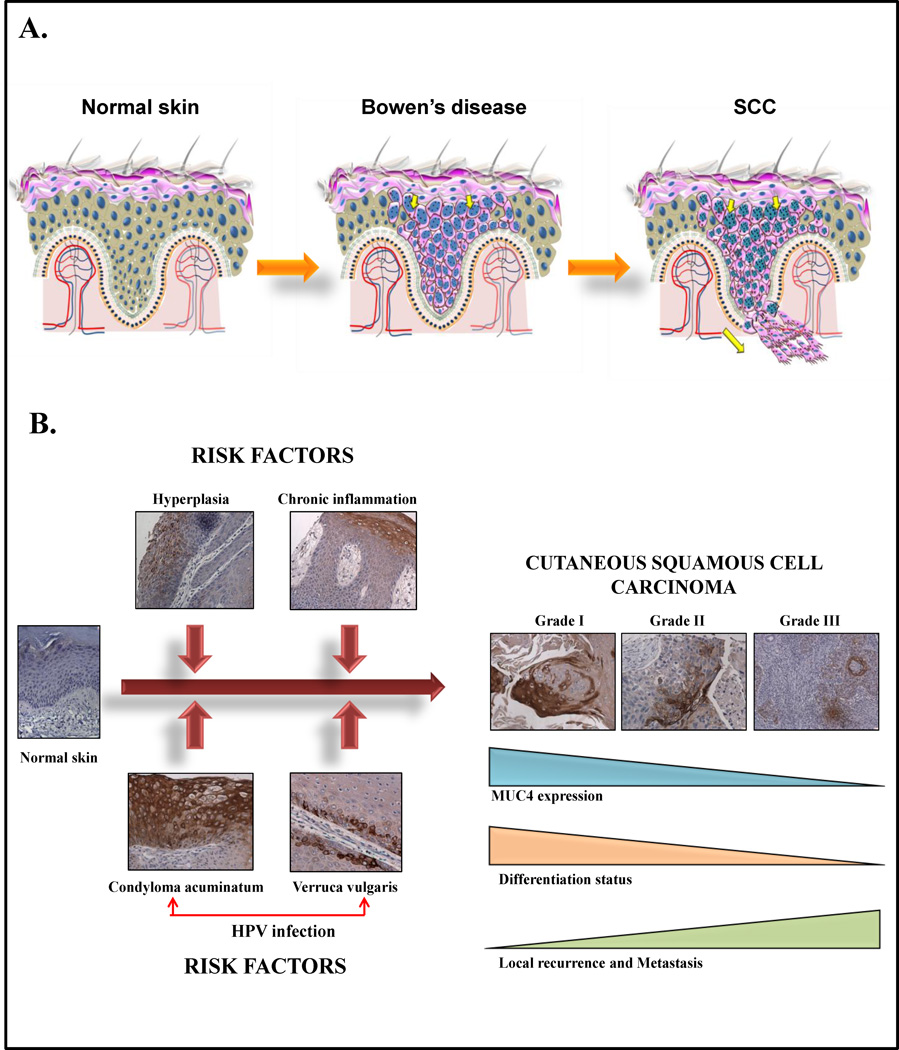
Figure 3. Role of mucins in the development of cutaneous squamous cell carcinoma.
(A) MUC1 is not expressed by the normal epidermis, but is expressed by the dysplastic epithelial cells in pre-malignant conditions like Bowen’s disease (or squamous carcinoma in situ, middle) and Epidermolysis bullosa (EB). MUC1 is also expressed in nearly all cases of both sporadic as well as EB-associated squamous cell carcinoma (SCC). Characterstically, the distribution of MUC1 in cutaneous SCC is predominantly membranous with a weaker cytoplasmic staining. Unlike adenocarcinomas where MUC1 is localized to the apical portion of the malignant cell, in SCCs it is distributed all around the periphery of the tumor cell.
(B) Recent findings from our laboratory suggest that MUC4 may play a role in the development and progression of cutaneous squamous cell carcinoma. While the normal epidermis is generally negative or weakly positive for MUC4, a significant upregulation of its expression is observed in certain inflammatory (chronic inflammation, cutaneous hyperplasia) and infectious conditions (verruca vulgaris and condyloma acuminatum) that are known to be risk factors for the development of cutaneous squamous cell carcinoma. In established squamous cell carcinoma lesions, MUC4 expression decreases progressively with the increasing grade of the tumor, with the highest expression in grade 1 and 2 SCCs. In positive cases, MUC4 is expressed both by the cytoplasm and the cell membrane. The strong MUC4 expression observed in conditions known to be caused by human papilloma virus (HPV) suggests a possible, novel association between HPV infection and MUC4-induced transformation in HPV-associated cutaneous and extra-cutaneous malignancies.
MUC1 overexpression is also observed in other cutaneous malignancies, including clear cell eccrine carcinomas, [70, 71] primary mucinous carcinoma [72] and metastatic mucinous adenocarcinomas of the skin [73] and breast. [74] Focal MUC1/EMA expression was reported in one of the cases of the myxoid variant of dermatofibrosarcoma protuberans, a locally aggressive mesenchymal neoplasm of the skin and subcutaneous tissue, [75] while the fibrosarcomatous variant exhibited EMA positivity in cells with a myoid differentiation in another study. [76] Adenoid (acantholytic) SCC, commonly seen on the sun-exposed areas of older individuals, is often confused with cutaneous angiosarcoma on histologic examination. In a report comparing the clinical and microscopic features of six cases each of adenoid SCC and cutaneous angiosarcoma, it was observed that while all of the former were positive for cytokeratin and EMA (MUC1), the latter were uniformly negative. [77] EMA positivity was also reported in the neoplastic cells in tumor sections [78, 79] and percutaneous fine needle aspirates of primary and metastatic lesions of cutaneous Merkel cell carcinoma (MCC). [80]
MCC is an uncommon, yet aggressive cutaneous malignancy affecting elderly Caucasians with a propensity for local recurrence and regional lymph node metastases. [81] Using antibodies that detect the differentially glycosylated forms of MUC1, Kurzen et al. [35] observed that although the glycosylation of MUC1 in MCC was altered, the staining pattern with antibodies specific for the various glycan forms was not consistent (e.g. while mAbs IE4 and BM-7 both recognize glycosylated epitopes in the tandem repeat region of MUC1, MCC tumors were immunostained only by the latter antibody). Hypoglycosylation of the tandem repeat region of MUC1, however, appears to be a consistent phenomenon, with both mAb 2D7 and BM-2 (recognizing APDTR and PDTRP epitopes on the hypoglycosylated MUC1 respectively) staining the malignant cells. The staining in the tumor cells was predominantly cytoplasmic with allantibodies against which a reaction was observed, with only a weak membrane staining, unlike the strong membrane labeling observed in normal glandular elements. In some of the tumors, a paranuclear punctuate staining was also observed. It has been suggested that this represents the Golgi apparatus where most of the mucin glycosylation occurs within the cell. Further, using a semiquantitative scoring system, the composite score for MUC1 positivity was significantly higher in metastatic MCC compared to non-metastatic MCC only in sections stained with the BM-2 (hypoglycosylated form specific) and BM-7 (recognizes glycosylated epitope) monoclonal antibodies (no significant difference being observed with the others). Thus, while nearly 85% of all MCCs expressed MUC1 (when defined as reactivity toward at least one antibody), it appears that the role of MUC1 in metastasis of this aggressive malignancy (median time to metastasis being four months and that to death from the time of detection of metastases 13.5 months in this study) is related to the extent of glycosylation of this mucin.
Clear cell carcinoma arising from the penile foreskin is a highly aggressive malignancy with a tendency for widespread metastasis and a tendency for multiple recurrences. In a series of six clear cell carcinomas of the penile foreskin, all the tumors showed intense positivity with an anti-MUC1 antibody. [82] In one case, tumor cell emboli with a clear-cell appearance observed in the afferent lymphatics were also positive for MUC1.
Basal cell carcinomas of the skin are generally entirely negative for MUC1 except in cases with a sebaceous or keratotic differentiation pattern. [69] Tumors negative for MUC1 expression include the uncommon primary cutaneous solitary fibrous tumor. [83]
Paget's disease is an intraepidermal adenocarcinoma that develops over the nipple or areola (mammary Paget’s disease) or in the skin around the perianal and anogenital regions and axilla (extramammary Paget’s disease). While MPD is almost always associated with an underlying carcinoma of the breast, the occurrence of EMPD with an underlying malignancy is less frequent. [84]
Immunohistochemical analysis of paraffin-embedded sections from cases of MPD (n=60) and EMPD (n=23) revealed that MUC1 was the most frequently expressed mucin in MPD with 100 percent of the cases positive. MUC2 (5 percent), MUC3 (75 percent), MUC4 (10 percent), MUC7 (7 percent) and MUC8 (4 percent) were less often detected in the MPD tissue sections. MUC1 was also the most commonly expressed mucin in EMPD with nearly 96 percent of the cases examined being immunoreactive. MUC3, MUC5AC and MUC8 were the other mucins whose expression was detected in the EMPD tissues (15 percent, 42 percent and 5 percent cases positive, respectively). Expression of MUC1 together with low-molecular weight cytokeratins (CK-7 and 8/18) and the absence of high-molecular weight cytokeratins (CK-20) emerged as the combination most useful to discriminate MPD from EMPD. Interestingly, the mammary Paget cells shared the same staining profile as that of the underlying high grade ductal carcinoma in situ (DCIS grade 3) for MUC1, 3, 5AC, 5B and 6, suggesting the possibility of a common cell of origin of both the lesions. [85]
In another study comparing the mucin expression profile in MPD with that in EMPD, MUC1 was expressed by most cases of PD (irrespective of location), while MUC5AC was expressed by most cases of EMPD, but it was absent in MPD. Further, in all cases with an MPD-associated breast cancer (n=13), there was a common mucin expression profile with both the Paget cells and carcinoma cells being positive for MUC1 and negative or MUC2 and MUC5AC. A similar expression of the three mucins is also observed in Toker cells present in the epidermis of normal nipples, suggesting that these cells could be responsible for giving rise to the neoplastic cells of MPD. Toker cells, identified in about 10 percent of normal nipples are clusters of non-neoplastic cells that are considered to be ectopic mammary elements located in the epidermis of the nipple. [86] MUC2 was expressed by all three cases of perianal PD associated with rectal adenocarcinoma examined, while MUC1 and MUC5AC expression was more variable. Further, cases of PD without an underlying malignancy were positive for MUC1 and MUC5AC but negative for MUC2. [87] The identical pattern of mucin expression in PD with that of the underlying adenocarcinoma suggests that the neoplastic cells in at least a subset of PD arise from an underlying ductal carcinoma.
Examination of the skin appendages normal vulva revealed that only the Bartholin's glands had a mucin phenotype identical to that of intraepidermal EMPD. Thus, it is possible that intraepidermal EMPD in the anogenital region may originate from cells of the Batholin's glands and possibly from other glandular elements in the skin. Similarly, the consistent expression of MUC2 by PD of the perianal region (compared to PD in other areas which is MUC2 negative) suggests a possible origin of this entity from the colorectal mucosa (also MUC2 positive). [87] Thus, a specific mucin profile associated with PD and EMPD holds promise as a tool to identify the histogenesis of these lesions and possibly as a target for their immunotherapy.
The Paget cells, which are cancer cells with enlarged nuclei and pale cytoplasm, are scattered singly in the affected epidermis and typically contain sialylated mucin which is positive for Alcian blue or zirconyl hematoxylin stain. In a comparison of MUC1 and MUC5AC staining between a typical (sialic acid positive) and an atypical case of EMPD (sialic acid negative), all the Paget’s cells in both the cases were strongly positive for MUC1, while most cells were reactive for MUC5AC. [88] However, immunoreactivity with the MUC5AC antibody was less reliable, as another study using double the concentration of the same antibody failed to detect MUC5AC staining in more than half the Paget's cells. [85] Thus, MUC1 (EMA) is considered to be a more reliable marker for EMPD than MUC5AC. A significant observation was the positivity of certain cells resembling keratinocytes for MUC1 and MUC5AC core proteins. This was seen in both the typical and atypical EMPD cases but in none of the control skin tissues, suggesting that Paget’ cells in EMPD possibly originate from these cells rather than Toker cells (MUC1-, MUC5AC-) as suggested by other studies [89]. In the case of triple-extramammary Paget’s disease (lesions on the areola and in the axilla and genitalia) in a 91-year old man, the Paget’s cells expressed MUC1 and MUC5AC but not MUC2 [90]. In the same study, expression of MUC1 but not MUC5AC was observed, albeit focally in two cases of MPD stained simultaneously. It is proposed that immunohistochemical detection of MUC1 (EMA) positive incipient cells in the epidermis before they attain Paget cell morphology could make the determination of surgical margins more accurate and thereby reduce the rate of recurrence.
Paget cells usually spread either horizontally in the epidermis or vertically into the contiguous epithelium of the hair follicles, sebaceous glands and sweat ducts. A distinction between intraepithelial and invasive Paget’s disease is of great prognostic significance as patients with the former condition can be cured completely by resection of the lesion, while the outcome is less favorable for the latter group. [91] In a study on 36 cases of EMPD, MUC1 was expressed in intraepithelial Paget cells (limited to the epidermis and/or adnexal epithelium) at high levels in 30 of 36 lesions, at moderate levels in four, low levels in one and one lesion was negative. [92] In 13 out of the 36 cases, microinvasive lesions were present. MUC1 was strongly expressed in 11 of the microinvasive Paget’s cells and at moderate levels in one case. The one case with low MUC1 expression had also exhibited low MUC1 positivity in the intraepithelial Paget's cells. However, there was no difference in the intensity or pattern of MUC1 expression between microinvasive and intraepithelial Paget cells. Six of the 36 cases also had invasive lesions in the dermis. Five of the six cases had high MUC1 expression in all three types of Paget’s cells: intraepithelial, microinvasive and invasive. One case had high MUC1 expression in the invasive but low expression in the intraepithelial and microinvasive Paget’s cells.
MUC5AC expression in the intraepithelial Paget’s cells was intense in 32 of the 36 lesions, moderate in three and low in one. In the lesions with features of microinvasion, MUC5AC expression in the Paget’s cells was either as intense (8 of 13 lesions) or lower (5 of 13 lesions) than that in the intraepithelial Paget’s cells. In the lesions with invasive Paget’s cells, MUC5AC expression was interestingly decreased or completely lost compared to the intraepithelial and microinvasive Paget’s cells in the same lesions. MUC5AC was expressed only in the Paget’s cells but in no other structure of the skin, suggesting that it is a highly specific marker for intraepithelial Paget’s cells. No difference in the pattern of MUC1 or MUC5AC expression was observed between EMPD lesions from the penis, scrotum, vulva or the perianal area. Neither MUC2 nor MUC6 were expressed in any of the EMPD lesions. This study demonstrated that MUC5AC expression is downregulated in invasive Paget’s cells compared to those in intraepithelial Paget's cells, suggesting that it could be useful as a potential prognostic marker in this disease.
Basosquamous carcinoma is considered an aggressive type of basal cell carcinoma. It is estimated that this type of cutaneous cancer comprises about 12 percent of all nonmelanomatous skin cancers. However, it was reported to have a recurrence rate of 12–51 percent and a five percent risk of metastasis within 30 years. [93] In some cases, the distinction between basosquamous and squamous cell carcinoma can be difficult. [94] EMA expression was noted in 22 of 23 SCC cases examined in one study, while only one of the 13 cases of basosquamous carcinoma had focal EMA positivity confined to the areas of squamous differentiation (epithelial pearls).[95] Thus, the antigen expression pattern of basosquamous carcinoma resembles that of basal cell carcinoma, which is uniformly negative for EMA. In the SCCs, EMA staining was noted predominantly in the membrane and variably in the cytoplasm. It was suggested that a combination of EMA negativity and Ber EP4 (an epithelial antigen) positivity can distinguish basosquamous carcinoma from squamous cell carcinoma and thus could be extremely useful to make an accurate diagnosis in difficult cases. EMA expression in melanoma is more restricted, with a mere 2–10% of melanomas staining positive for this epithelial marker. [96]
Hidardenocarcinoma is an uncommon malignant intredermal tumor of sweat glands commonly involving the face and extremities. [97] Nash et al. reported the case of a 44-year-old man with a solitary clear cell hidradenocarcinoma on the right chest which was positive for MUC5AC but negative for MUC2. [98] Staining for EMA was present in the areas of ductal differentiation.
Spindle cell lesions of the skin present a challenge to the pathologist. Two such lesions which are difficult to distinguish from each other are atypical fibroxanthoma and spindle cell squamous carcinoma. In a study comparing seven cases with sarcoma-like lesions with two cases of unequivocal spindle cell squamous carcinoma of the skin, immunohistochemical analysis revealed that both the spindle cell squamous cell carcinoma cases were positive for EMA and keratin, while five of the seven cases with sarcoma-like features were negative for these two antigens. The remaining two sarcoma-like lesions had intracytoplasmic granules which were strongly positive for both EMA and keratin similar to the spindle cell SCC. These two lesions were also found to be similar histologically to the spindle cell carcinomas. [99] This suggests that while most sarcoma-like lesions are EMA and keratin negative and comprise "true atypical fibroxanthomas" of mesenchymal origin, positivity for EMA and keratin can be used to identify the small number of sarcomatoid tumors which are of epithelial origin. This distinction is important prognostically as atypical fibroxanthomas are biologically benign neoplasms with only rare metastases, while spindle cell SCC is an aggressive tumor with little response to radiotherapy. [100] MUC1 is also positive in other tumors showing a spindle cell morphology of the tumor cells, including perineurioma and meningioma.[101]
Myoepithelial neoplasms of the skin are controversial tumors, although they have been known to arise in the salivary glands, breast and the lungs.[102] Myoepithelial cells are spindle shaped, epithelioid, plasmacytoid or clear-cell in appearance and are normally present around the apocrine and eccrine glands of the skin. They are characterized by their capacity for bidirectional differentiation and exhibiting features of both epithelial and myoid cells. [103] They also exhibit a variable expression of EMA.[104] Cutaneous myoepithelial neoplasms represent an under-recognized entity by themselves although a myoepithelial differentiation pattern is seen in several cutaneous neoplasms including basal cell carcinoma.[105] Benign mixed tumors of the skin (also called chondrioid syringoma), the only accepted cutaneous myoepithelial tumor, is characterized by nests and cords of epithelial cells surrounded by vimentin positive myoepithelial cells. In a study of nine cases of benign mixed tumors of the skin, the neoplastic myoepithelial cells characteristically exhibited a strong expression of EMA while being consistently negative for desmin. [102] This pattern of EMA and desmin expression could be extremely useful clinically to distinguish cutaneous spindle cell myoepithelial neoplasms from smooth muscle and myofibroblastic tumors of the skin.
Although EMA is a marker of epithelial cell lineage, it can also be expressed by cells of primarily mesenchymal origin. In a report, Prierto et al. describe the case of a 50-year-old man diagnosed with malignant mesothelioma who presented with an erythematous eruption one year after treatment. The tumor cells, which were similar to those of the primary tumor, were positive for EMA [106]. In another report of four cases of primary metaplastic carcinoma of the skin (carcinosacroma), EMA expression was detected in both the poorly differentiated epithelium and the chondroid elements in one case. [107]
EMA is normally expressed only by the mature sebocytes in the adult skin. A study comparing the expression of glycoprotein antigens of the carcinoembryonic antigen (CEA) family in normal sebaceous glands and cutaneous neoplasms with a sebaceous differentiation found that both sebaceous and sweat glands expressed EMA, although the expression of the CEA antigens was more restricted.[108] At the ultrastructural level, EMA was demonstrated in the Golgi complex, in small vesicles and on the cell membrane. Further, the expression of EMA was conserved even in reactive, hamartomatous and neoplastic proliferations of adnexal structures with a sebaceous differentiation pattern.
Expression of other mucins in skin diseases
A microarray analysis comparing the gene expression profile in metastatic melanomas with that of various non-metastatic cutaneous tumors, including SCC, basal cell carcinoma and primary cutaneous melanoma (PCM) revealed that primary basal cell carcinomas (15 cases), squamous cell carcinomas (11 cases) and primary non-metastatic or "thin" melanomas (Breslow’s thickness ≤ 1.5 mm, 16 cases) express high levels of MUC15 mRNA.[109] Significantly, the MUC15 mRNA levels were found to be 25-fold lower in metastatic melanoma (40 cases) compared to non-metastatic melanoma, while a 21-fold reduction was observed when compared with localized SCC/basal cell carcinoma. Further, to examine the genes whose expression was deregulated at the "transition point" between non-metastatic and metastatic melanoma, the authors compared the global gene expression profile between PCM lesions (non-metastatic) of different thicknesses and metastatic melanoma until the expression of a gene differentially expressed in PCM reached the levels observed in MM. The most significant difference in MUC15 mRNA levels occurred between intermediate thickness (1–4mm) and thick melanoma (>4mm Breslow's thickness) with a nearly 12-fold decrease in the expression of its mRNA in the latter. There was less than a two-fold decrease in MUC15 mRNA expression when other adjacent regions were compared with each other (i.e., melanoma in situ vs. thin (<1mm), thin vs. intermediate, and thick vs. metastatic melanoma). This report suggested that MUC15 mRNA expression was lost during the transition of malignant melanoma from a localized to metastatic phenotype and possibly has an important role in its pathogenesis.
A possible role for MUC4 in cutaneous and extra-cutaneous malignancies was suggested by the observation that its ectopic expression in A375 melanoma cell lines rendered the cells resistant to the cytotoxic effects of chemotherapeutic drugs like paclitaxel, doxorubicin and vinblastine.[110] We have observed that downregulation of MUC4 in a highly metastatic pancreatic cancer cell line increases gemcitabine mediated apoptosis.[111] The underlying mechanism appears to be inhibition of anti-apoptotic signaling pathways mediated by the receptor tyorsine kinase HER-2 which is downregulated upon silencing of MUC4 expression.[112, 113] These findings suggest that MUC4 may play a role in promoting resistance to gemcitabine induced apoptosis in other epithelial malignancies including those of the skin. Thus, therapeutic strategies that can downregulate MUC4 expression in transformed cells might have a role in restoring or improving the chemosensitivity of malignant cells. In a study to determine the cellular pathways involved in the pathogenesis of cutaneous SCC, Kathpalia et al. found a significant upregulation (nearly three-fold) of MUC4 mRNA in well-differentiated SCCs (by gene microarray analysis) compared to the healthy skin from the same patient [114]. We have observed that moderate to strong cytoplasmic and membrane expression of MUC4 is noted in about 15% of all cutaneous SCCs with a stronger expression in well and moderately differentiated SCCs compared to poorly differentiated ones (Role of MUC4 in progression of SCC is summarized in Figure 3b). However, basal cell papillomas, BCCs and malignant melanomas were either negative or showed weak focal expression [39].
Expression of mucins in skin cancer cell lines
Although MUC1 reactivity has been extensively demonstrated in tissue sections, the literature is rather sparse on the question of its reactivity with cell lines derived from various skin tumors. While examining the expression of MUC1 in tumors developed in severe combined immunodeficient (scid) mice by subcutaneous implantation of human epithelial cancer cell lines, Schumacher and Adam,[115] noted that tumors derived from the A431 epidermoid carcinoma cell line exhibited moderately strong MUC1 staining in up to 20 percent of cells with the HMFG-1 antibody but not with either the HMFG-2 or SM-3 antibodies. These three antibodies recognize overlapping epitopes on MUC1 (PDTR for HMFG-1, DTR for HMFG-2 and PDTRP for SM3).[116] However, their reactivity is modified significantly by the glycosylation pattern of the mucin core protein. Thus, the HMFG-1 antibody reacts well with the mucin expressed by normal cells but the reactivity is decreased in the presence of sialic acid side chains attached to the core protein, a phenomenon observed in neoplastic cells (but not in normal cells). HMFG-2 reacts strongly with the tumor-expressed mucins owing to the shorter length of the glycosyl side chains in these mucins. However, its reactivity is hindered by the longer carbohydrate side chains attached to the mucin core protein expressed by normal cells. The binding site for the SM3 lies between two potential glycosylation sites, but can be influenced by the degree of glycosylation of the flanking regions. Importantly, the SM3 has been shown to specifically recognize the mucin produced by the neoplastic cells but not that expressed by normal cells [117], possibly owing to the masking of its epitope by heavy glycosylation in normal mucins. Interestingly, the authors of this study [115] also observed that pre-treatment of the tissue sections with neuraminidase, which removes the sialic acid moieties, significantly enhanced reactivity to HMFG-1 (but not to HMFG-2), especially enhancing positive staining for MUC1 in the cell membrane. There was considerable heterogeneity in reactivity of the xenograft tumors to the three monoclonal antibodies suggesting that the glycosylation pattern in tumor cells is influenced by several factors, possibly including the tumor microenvironment. This could have important implications in using mucins as targets for antibody-based therapy in cancer and other diseases.
MUC1 mRNA was detected in the human melanoma cell lines SK-MEL28 and SK-MEL-31 by RT-PCR and in SK-MEL28 by Northern blotting. Further, MUC1 expression was also weakly positive in these two cell lines by immunocytochemistry using a monoclonal antibody to the C-terminal region of MUC1 (mAb 139H2).[118] Keratinocytes isolated from punch biopsy of the adult skin were shown to give rise to secretory-type cells expressing Alcian blue positive mucin upon reaching a confluent monolayer in vitro.[119] The proportion of mucin producing cells was low and more in later than early cultures, suggesting that keratinocytes may give rise to the mucin-positive cells during the progression of epidermoid carcinoma.
Regulation of mucin expression
Mucins have a well-defined and site-restricted pattern of expression, which may be disrupted either by environmental insults or intrinsic changes (e.g. epigenetic modifications). While not much is known about the mechanisms underlying the regulation of mucin expression in the skin, considerable literature exists in other organ system pathologies to suggest that their expression is tightly regulated by multiple pathways. The expression of mucin synthesis is mediated through transcriptional, post-transcriptional or other special regulatory pathways (e.g. alternative splicing) by multiple factors including cytokines, bacterial products, growth factors and differentiation agents.[6] Secreted mucins like MUC5AC and MUC5B are secreted by goblet cells in the respiratory tract and their expression is upregulated in chronic inflammatory diseases of the airways (chronic obstructive lung disease (COPD) and asthma) by inflammatory cytokines including tumor necrosis factor α (TNFα), interleukins (ILs)- 1β, 6, 9, 13 and 17, epidermal growth factor (EGF), transforming growth factor α (TGF-α), TGF-β and retinoic acid. Bacteria (e.g. Pseudomonas aeruginosa) and bacterial products (lipopolysaccharide, LPS) have also been shown to upregulate MUC5AC expression.[120] TNF-α also upregulates MUC1 expression in malignant mammary epithelial cells while another cytokine, interferon-gamma (IFN-γ) increases its expression in untransformed mammary epithelial and ovarian cancer cells [121].
We have reported that IFN-γ synergizes with retinoic acid to upregulate MUC4 expression in human pancreatic cancer cells.[122] The mechanism underlying cytokine mediated activation of gene expression involves activation of the Janus kinase (JAK)/ Signal transducer and activator of transcription (STAT) pathway with the STATs (transcription factors) then performing the role of activating mucin gene expression. Bacteria like Pseudomonas on the other hand regulate mucin expression largely through lipopolysaccharides (LPS) which are components of the gram negative bacterial cell wall and promote bacteria-driven inflammation. LPS induced MUC2 upregulation for instance has been demonstrated to involve activation of the Src-dependent Ras/Raf/MEK/ERK/pp90rsk pathway with the transcription factor NF-κB serving as the ultimate effector. The regulation of mucin expression in cancer and inflammatory diseases has been extensively reviewed by us in a recent article.[6]
Role of mucins in diagnosis, prognosis and therapy
Mucins have become increasingly important as specific markers for the diagnosis and predicting the prognosis of epithelial malignancies.[123, 126] Some of the prominent clinically employed mucin-based assays include measurement of serum MUC1 (CA15-3) in breast cancer and serum MUC16 (CA-125) levels for follow-up of patients with ovarian cancer. MUC4 immunohistochemistry on has been shown to be highly specific marker for pancreatic cancer in fine needle aspirate specimens. It also constitues a marker of poor prognosis in pancreatic, and bile duct cancers.[124]
Mucins are also rapidly emerging as targets for therapy, chiefly immunotherapy of malignant epithelial neoplasms. Several MUC-1 based vaccines (L-BLP25 or Stimuvax, TG4010 and PANVAC) are in clinical trials for immunotherapy of non-small cell lung cancer and other malignancies, [125] while antibodies targeting the interaction of MUC16 with mesothelin are being explored as possible therapeutic agents in ovarian cancer [126]. MUC4 has been shown to be a potential target for anti-cancer therapy given the observation that cells expressing rat MUC4 have reduced binding of Trastuzumab, an anti-ErbB2 monoclonal antibody.[127] This, together with its ability to modulate resistance to cytotoxic drugs and increase resistance to immune mediated killing [128] has promoted its emergence as a novel therapeutic target in several cancers including that of the pancreas, breast and ovary. Recently, we have shown that a natural alkaloid Thymoquinone downregulates the expression of MUC4 in vitro, [129] suggesting the possibility of employing this for treatment of MUC4 expressing cancers.
Conclusions and Perspectives
Mucins comprise a family of glycoproteins with diverse and important functions, including the formation of a mechanical barrier to foreign organisms and transduction of inter and intracellular signals to maintain the integrity of the epithelium. While mucins have been widely studied in other organs, their role in the skin has received considerably less attention. MUC1 is the most extensively studied mucin in cutaneous pathologies and is a prominent target for both diagnostic and therapeutic applications. Recent clinical trials in ovarian and breast cancer patients have shown that vaccination with autologous dendritic cells pulsed with MUC1/EMA derived peptides exhibited a considerable peptide specific T-cell cytotoxic response [31] (role of mucins in anti-cancer therapy has been recently reviewed [123]). Given its expression in a large number of malignant and potentially malignant skin lesions, MUC1 has been suggested as a promising target for the therapy of established cancer as well as a suitable antigen for vaccinating patients at high risk for developing cutaneous malignancies, chiefly SCC [27]. MUC4 has recently emerged as a novel player in cutaneous pathologies and initial studies suggest a possible correlation with HPV-associated skin diseases. MUC15 is overexpressed in melanomas and is a suitable candidate for further investigation in this aggressive malignancy. The role of other mucins is still unclear and needs to be investigated further. With the development of vaccines against MUC1, there is hope that mucins, with their repetitive tandem repeats, will provide novel targets for the development of small molecule inhibitors and antibodies for immunotherapy.
Acknowledgments
We thank Kristi L. Berger for editing the manuscript. The authors on this work are supported, in part, by grants from the U.S. Department of Defense (BC074631 and PC074289) and the National Institutes of Health (RO1 CA78590, UO1 CA111294, RO1 CA131944, RO1 CA133774, RO1 CA138791 and P50 CA127297).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors on this work are supported, in part, by grants from the U.S. Department of Defense (BC074631 and PC074289) and the National Institutes of Health (RO1 CA78590, UO1 CA111294, RO1 CA131944, RO1 CA133774, RO1 CA 138791 and P50 CA127297).
Conflict of interest statement
The authors declare no conflict of interest.
Reference List
- 1.Basra MK, Shahrukh M. Burden of skin diseases. Expert Rev. Pharmacoecon. Outcomes. Res. 2009;9:271–283. doi: 10.1586/erp.09.23. [DOI] [PubMed] [Google Scholar]
- 2.Cherry DK, Hing E, Woodwell DA, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2006 summary. Natl. Health Stat. Report. 2008:1–39. [PubMed] [Google Scholar]
- 3.Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6:D1192–D1206. doi: 10.2741/moniaux. [DOI] [PubMed] [Google Scholar]
- 4.Van Klinken BJ, Dekker J, Buller HA, Einerhand AW. Mucin gene structure and expression: protection vs. adhesion. Am. J Physiol. 1995;269:G613–G627. doi: 10.1152/ajpgi.1995.269.5.G613. [DOI] [PubMed] [Google Scholar]
- 5.Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–476. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim. Biophys. Acta. 2006;1765:189–222. doi: 10.1016/j.bbcan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Rachagani S, Torres MP, Moniaux N, Batra SK. Current status of mucins in the diagnosis and therapy of cancer. Biofactors. 2009;35:509–527. doi: 10.1002/biof.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu. Rev. Physiol. 1995;57:607–634. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 9.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 10.Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 2008;8:477–483. doi: 10.1097/ACI.0b013e32830e6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senapati S, Sharma P, Bafna S, Roy HK, Batra SK. The MUC gene family: their role in the diagnosis and prognosis of gastric cancer. Histol. Histopathol. 2008;23:1541–1552. doi: 10.14670/HH-23.1541. [DOI] [PubMed] [Google Scholar]
- 12.Stal S, Spira M, Hamilton S. Skin morphology and function. Clin. Plast. Surg. 1987;14:201–208. [PubMed] [Google Scholar]
- 13.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 14.Desseyn JL, Tetaert D, Gouyer V. Architecture of the large membrane-bound mucins. Gene. 2008;410:215–222. doi: 10.1016/j.gene.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Chaturvedi P, Singh AP, Batra SK. Structure evolution and biology of the MUC4 mucin. FASEB J. 2008;22:966–981. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagow E, DeSouza MM, Carson DD. Mammalian reproductive tract mucins. Hum. Reprod. Update. 1999;5:280–292. doi: 10.1093/humupd/5.4.280. [DOI] [PubMed] [Google Scholar]
- 17.Batra SK, Metzgar RS, Hollingsworth MA. Human Muc 1 mucin gene expression in the fetal pancreas. Pancreas. 1992;7:391–393. doi: 10.1097/00006676-199205000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Batra SK, Kern HF, Worlock AJ, Metzgar RS, Hollingsworth MA. Transfection of the human Muc 1 mucin gene into a poorly differentiated human pancreatic tumor cell line, Panc1: integration, expression and ultrastructural changes. J. Cell Sci. 1991;100(Pt 4):841–849. doi: 10.1242/jcs.100.4.841. [DOI] [PubMed] [Google Scholar]
- 19.Batra SK, Hollingsworth MA. Expression of the human mucin gene, Muc 1, in normal tissues and metastatic pancreatic tumors. Int. J. Pancreatol. 1991;10:287–292. doi: 10.1007/BF02924167. [DOI] [PubMed] [Google Scholar]
- 20.Lan MS, Batra SK, Qi WN, Metzgar RS, Hollingsworth MA. Cloning and sequencing of a human pancreatic tumor mucin cDNA. J. Biol. Chem. 1990;265:15294–15299. [PubMed] [Google Scholar]
- 21.Groneberg DA, Eynott PR, Oates T, Lim S, Wu R, Carlstedt I, Nicholson AG, Chung KF. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir. Med. 2002;96:81–86. doi: 10.1053/rmed.2001.1221. [DOI] [PubMed] [Google Scholar]
- 22.Temann UA, Prasad B, Gallup MW, Basbaum C, Ho SB, Flavell RA, Rankin JA. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am. J. Respir. Cell Mol. Biol. 1997;16:471–478. doi: 10.1165/ajrcmb.16.4.9115759. [DOI] [PubMed] [Google Scholar]
- 23.Babu SD, Jayanthi V, Devaraj N, Reis CA, Devaraj H. Expression profile of mucins (MUC2, MUC5AC and MUC6) in Helicobacter pylori infected pre-neoplastic and neoplastic human gastric epithelium. Mol. Cancer. 2006;5:10. doi: 10.1186/1476-4598-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 25.Ando I, Kukita A, Soma G, Hino H. A large number of tandem repeats in the polymorphic epithelial mucin gene is associated with severe acne. J Dermatol. 1998;25:150–152. doi: 10.1111/j.1346-8138.1998.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 26.Reddy MS. Human tracheobronchial mucin: purification and binding to Pseudomonas aeruginosa. Infect. Immun. 1992;60:1530–1535. doi: 10.1128/iai.60.4.1530-1535.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper HL, Cook IS, Theaker JM, Mallipeddi R, McGrath J, Friedmann P, Healy E. Expression and glycosylation of MUC1 in epidermolysis bullosa-associated and sporadic cutaneous squamous cell carcinomas. Br. J. Dermatol. 2004;151:540–545. doi: 10.1111/j.1365-2133.2004.06075.x. [DOI] [PubMed] [Google Scholar]
- 28.Rounds S, Likar LL, Harrington EO, Kim KC, Smeglin A, Heins K, Parks N. Nucleotide-induced PMN adhesion to cultured epithelial cells: possible role of MUC1 mucin. Am. J Physiol. 1999;277:L874–L880. doi: 10.1152/ajplung.1999.277.5.L874. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease. J Clin. Pathol. 2000;53:742–749. doi: 10.1136/jcp.53.10.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasai Y, Nakama T, Kasada M. Sialomucin in Paget cells of extramammary Paget’s disease. Histochem. J. 1983;15:987–997. doi: 10.1007/BF01002494. [DOI] [PubMed] [Google Scholar]
- 31.von Mensdorff-Pouilly S, Snijdewint FG, Verstraeten AA, Verheijen RH, Kenemans P. Human MUC1 mucin: a multifaceted glycoprotein. Int. J. Biol. Markers. 2000;15:343–356. doi: 10.1177/172460080001500413. [DOI] [PubMed] [Google Scholar]
- 32.Kuan SF, Montag AG, Hart J, Krausz T, Recant W. Differential expression of mucin genes in mammary and extramammary Paget’s disease. Am. J Surg. Pathol. 2001;25:1469–1477. doi: 10.1097/00000478-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Sloane JP, Ormerod MG. Distribution of epithelial membrane antigen in normal and neoplastic tissues and it value in diagnostic tumor pathology. Cancer. 1981;47:1786–1795. doi: 10.1002/1097-0142(19810401)47:7<1786::aid-cncr2820470711>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Stoeckelhuber M, Stoeckelhuber BM, Welsch U. Human glands of Moll: histochemical and ultrastructural characterization of the glands of Moll in the human eyelid. J. Invest Dermatol. 2003;121:28–36. doi: 10.1046/j.1523-1747.2003.12328.x. [DOI] [PubMed] [Google Scholar]
- 35.Kurzen H, Kaul S, Egner U, Deichmann M, Hartschuh W. Expression of MUC 1 and Ep-CAM in Merkel cell carcinomas: implications for immunotherapy. Arch. Dermatol. Res. 2003;295:146–154. doi: 10.1007/s00403-003-0410-y. [DOI] [PubMed] [Google Scholar]
- 36.Yoshii N, Kitajima S, Yonezawa S, Matsukita S, Setoyama M, Kanzaki T. Expression of mucin core proteins in extramammary Paget’s disease. Pathol. Int. 2002;52:390–399. doi: 10.1046/j.1440-1827.2002.01364.x. [DOI] [PubMed] [Google Scholar]
- 37.Bartman AE, Buisine MP, Aubert JP, Niehans GA, Toribara NW, Kim YS, Kelly EJ, Crabtree JE, Ho SB. The MUC6 secretory mucin gene is expressed in a wide variety of epithelial tissues. J Pathol. 1998;186:398–405. doi: 10.1002/(SICI)1096-9896(199812)186:4<398::AID-PATH192>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Yasin M, Carraway CA, Carraway KL. MUC4 expression and localization in gastrointestinal tract and skin of human embryos. Tissue Cell. 2006;38:271–275. doi: 10.1016/j.tice.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Chakraborty S, Swanson BJ, Bonthu N, Batra SK. Aberrant upregulation of MUC4 mucin expression in cutaneous condyloma acuminatum and squamous cell carcinoma suggests a potential role in the diagnosis and therapy of skin diseases. J Clin. Pathol. 2010;63:579–584. doi: 10.1136/jcp.2010.076125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaffer JV, Kamino H, Witkiewicz A, McNiff JM, Orlow SJ. Mucocutaneous neuromas: an underrecognized manifestation of PTEN hamartoma-tumor syndrome. Arch. Dermatol. 2006;142:625–632. doi: 10.1001/archderm.142.5.625. [DOI] [PubMed] [Google Scholar]
- 41.Penas PF, Jones-Caballero M, Aragues M, Fernandez-Herrera J, Fraga J, Garcia-Diez A. Sclerodermatous graft-vs-host disease: clinical and pathological study of 17 patients. Arch. Dermatol. 2002;138:924–934. doi: 10.1001/archderm.138.7.924. [DOI] [PubMed] [Google Scholar]
- 42.Cerroni L, Fink-Puches R, Back B, Kerl H. Follicular mucinosis: a critical reappraisal of clinicopathologic features and association with mycosis fungoides and Sezary syndrome. Arch. Dermatol. 2002;138:182–189. doi: 10.1001/archderm.138.2.182. [DOI] [PubMed] [Google Scholar]
- 43.Fujisawa A, Morita K, Yonezawa MM, Miyachi Y, Utani A. Solitary morphea profunda with a prominent mucinous deposit. Pediatr. Dermatol. 2007;24:201–202. doi: 10.1111/j.1525-1470.2007.00380.x. [DOI] [PubMed] [Google Scholar]
- 44.Rosai J, Limas C, Husband EM. Ectopic hamartomatous thymoma. A distinctive benign lesion of lower neck. Am. J. Surg. Pathol. 1984;8:501–513. [PubMed] [Google Scholar]
- 45.Weinreb I, O’Malley F, Ghazarian D. Ectopic hamartomatous thymoma: a case demonstrating skin adnexal differentiation with positivity for epithelial membrane antigen, androgen receptors, and BRST-2 by immunohistochemistry. Hum. Pathol. 2007;38:1092–1095. doi: 10.1016/j.humpath.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 46.Chong SJ, Kim SY, Kim HS, Kim GM, Kim SY, Jung JH. Cutaneous ciliated cyst in a 16-year-old girl. J. Am. Acad. Dermatol. 2007;56:159–160. doi: 10.1016/j.jaad.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Reed RJ, Fine RM, Meltzer HD. Palisaded, encapsulated neuromas of the skin. Arch. Dermatol. 1972;106:865–870. [PubMed] [Google Scholar]
- 48.Argenyi ZB, Santa CD, Bromley C. Comparative light-microscopic and immunohistochemical study of traumatic and palisaded encapsulated neuromas of the skin. Am. J Dermatopathol. 1992;14:504–510. doi: 10.1097/00000372-199212000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Lee JY, Chao SC. Clear cell papulosis of the skin. Br. J Dermatol. 1998;138:678–683. doi: 10.1046/j.1365-2133.1998.02185.x. [DOI] [PubMed] [Google Scholar]
- 50.Fox RI, Tornwall J, Michelson P. Current issues in the diagnosis and treatment of Sjogren’s syndrome. Curr. Opin. Rheumatol. 1999;11:364–371. doi: 10.1097/00002281-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Godse KV. Glomus tumor with mucinous change. Indian J. Dermatol. Venereol. Leprol. 2005;71:367–368. doi: 10.4103/0378-6323.16798. [DOI] [PubMed] [Google Scholar]
- 52.Karimipour DJ, Johnson TM, Kang S, Wang TS, Lowe L. Mucinous carcinoma of the skin. J. Am. Acad. Dermatol. 1997;36:323–326. doi: 10.1016/s0190-9622(97)80409-4. [DOI] [PubMed] [Google Scholar]
- 53.Chao MW, Gibbs P. Squamous cell carcinoma arising in a giant condyloma acuminatum (Buschke-Lowenstein tumour) Asian J Surg. 2005;28:238–240. doi: 10.1016/S1015-9584(09)60352-3. [DOI] [PubMed] [Google Scholar]
- 54.Kasai T, Moriyama K, Tsuji M, Uema K, Sakurai N, Fujii Y. Adenocarcinoma arising from a mature cystic teratoma of the testis. Int. J. Urol. 2003;10:505–509. doi: 10.1046/j.1442-2042.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz RA, Wiederkehr M, Lambert WC. Secondary mucinous carcinoma of the skin: metastatic breast cancer. Dermatol. Surg. 2004;30:234–235. doi: 10.1046/j.1076-0512.2003.30074.x. [DOI] [PubMed] [Google Scholar]
- 56.Mitsui H, Watanabe T, Jinnin M, Kadono T, Idezuki T, Tamaki K. Mucinous carcinoma of the skin could have either an eccrine or an apocrine origin. Br. J. Dermatol. 2004;151:1285–1286. doi: 10.1111/j.1365-2133.2004.06297.x. [DOI] [PubMed] [Google Scholar]
- 57.Ishida M, Katsura K, Nagata A, Kijima K, Kushima R, Okabe H. A case of primary mucinous carcinoma with endocrine differentiation of the skin. Rinsho Byori. 2008;56:455–458. [PubMed] [Google Scholar]
- 58.Bleyer A, Viny A, Barr R. Cancer in 15- to 29-year-olds by primary site. Oncologist. 2006;11:590–601. doi: 10.1634/theoncologist.11-6-590. [DOI] [PubMed] [Google Scholar]
- 59.Hochberg J, Waxman IM, Kelly KM, Morris E, Cairo MS. Adolescent non-Hodgkin lymphoma and Hodgkin lymphoma: state of the science. Br. J Haematol. 2009;144:24–40. doi: 10.1111/j.1365-2141.2008.07393.x. [DOI] [PubMed] [Google Scholar]
- 60.Stein H, Foss HD, Durkop H, Marafioti T, Delsol G, Pulford K, Pileri S, Falini B. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96:3681–3695. [PubMed] [Google Scholar]
- 61.Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br. J. Dermatol. 2005;153:874–880. doi: 10.1111/j.1365-2133.2005.06905.x. [DOI] [PubMed] [Google Scholar]
- 62.Kadin ME, Pinkus JL, Pinkus GS, Duran IM, Fuller CE, Onciu M, Kawaguchi H, Morris SW. Primary cutaneous ALCL with phosphorylated/activated cytoplasmic ALK novel phenotype: EMA/MUC1+, cutaneous lymphocyte antigen negative. Am. J. Surg. Pathol. 2008;32:1421–1426. doi: 10.1097/PAS.0b013e3181648d6d. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura S, Shiota M, Nakagawa A, Yatabe Y, Kojima M, Motoori T, Suzuki R, Kagami Y, Ogura M, Morishima Y, Mizoguchi Y, Okamoto M, Seto M, Koshikawa T, Mori S, Suchi T. Anaplastic large cell lymphoma: a distinct molecular pathologic entity: a reappraisal with special reference to p80(NPM/ALK) expression. Am. J Surg. Pathol. 1997;21:1420–1432. doi: 10.1097/00000478-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 64.Kato N, Mizuno O, Ito K, Kimura K, Shibata M. Neutrophil-rich anaplastic large cell lymphoma presenting in the skin. Am. J Dermatopathol. 2003;25:142–147. doi: 10.1097/00000372-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Horie R, Watanabe M, Ishida T, Koiwa T, Aizawa S, Itoh K, Higashihara M, Kadin ME, Watanabe T. The NPM-ALK oncoprotein abrogates CD30 signaling and constitutive NF-kappaB activation in anaplastic large cell lymphoma. Cancer Cell. 2004;5:353–364. doi: 10.1016/s1535-6108(04)00084-4. [DOI] [PubMed] [Google Scholar]
- 66.Cooper TW, Bauer EA. Epidermolysis bullosa: a review. Pediatr. Dermatol. 1984;1:181–188. doi: 10.1111/j.1525-1470.1984.tb01113.x. [DOI] [PubMed] [Google Scholar]
- 67.McGrath JA, Schofield OM, Mayou BJ, McKee PH, Eady RA. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J. Cutan. Pathol. 1992;19:116–123. doi: 10.1111/j.1600-0560.1992.tb01352.x. [DOI] [PubMed] [Google Scholar]
- 68.Cooper HL, Cook IS, Theaker JM, Mallipeddi R, McGrath J, Friedmann P, Healy E. Expression and glycosylation of MUC1 in epidermolysis bullosa-associated and sporadic cutaneous squamous cell carcinomas. Br. J. Dermatol. 2004;151:540–545. doi: 10.1111/j.1365-2133.2004.06075.x. [DOI] [PubMed] [Google Scholar]
- 69.Heyderman E, Graham RM, Chapman DV, Richardson TC, McKee PH. Epithelial markers in primary skin cancer: an immunoperoxidase study of the distribution of epithelial membrane antigen (EMA) and carcinoembryonic antigen (CEA) in 65 primary skin carcinomas. Histopathology. 1984;8:423–434. doi: 10.1111/j.1365-2559.1984.tb02354.x. [DOI] [PubMed] [Google Scholar]
- 70.FB FB, Hofman V, Lagier J, Gastaud P, Santini J, Hofman P. Primary signetring cell carcinoma of the eccrine sweat gland in the eyelid. Immunohistochemical and ultrastructural study of a case. J. Fr. Ophtalmol. 2002;25:547–551. [PubMed] [Google Scholar]
- 71.Liegl B, Leibl S, Gogg-Kamerer M, Tessaro B, Horn LC, Moinfar F. Mammary and extramammary Paget’s disease: an immunohistochemical study of 83 cases. Histopathology. 2007;50:439–447. doi: 10.1111/j.1365-2559.2007.02633.x. [DOI] [PubMed] [Google Scholar]
- 72.Yoshida R, Wada N, Nakasute K, Saitoh H, Nagao K, Tanaka M. Dermoscopic features of mucinous carcinoma of the skin. Dermatol. Surg. 2004;30:1138–1139. doi: 10.1111/j.1524-4725.2004.30340.x. [DOI] [PubMed] [Google Scholar]
- 73.Kasai T, Moriyama K, Tsuji M, Uema K, Sakurai N, Fujii Y. Adenocarcinoma arising from a mature cystic teratoma of the testis. Int. J. Urol. 2003;10:505–509. doi: 10.1046/j.1442-2042.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- 74.Schwartz RA, Wiederkehr M, Lambert WC. Secondary mucinous carcinoma of the skin: metastatic breast cancer. Dermatol. Surg. 2004;30:234–235. doi: 10.1046/j.1076-0512.2003.30074.x. [DOI] [PubMed] [Google Scholar]
- 75.Mentzel T, Scharer L, Kazakov DV, Michal M. Myxoid dermatofibrosarcoma protuberans: clinicopathologic, immunohistochemical, and molecular analysis of eight cases. Am. J. Dermatopathol. 2007;29:443–448. doi: 10.1097/DAD.0b013e318145413c. [DOI] [PubMed] [Google Scholar]
- 76.Arican O, Bakaris S, Bulbuloglu E, Ezberci F. Myoid differentiation and EMA expression in fibrosarcomatous dermatofibrosarcoma protuberans. Acta Dermatovenerol. Alp Panonica. Adriat. 2006;15:39–44. [PubMed] [Google Scholar]
- 77.Nappi O, Wick MR, Pettinato G, Ghiselli RW, Swanson PE. Pseudovascular adenoid squamous cell carcinoma of the skin. A neoplasm that may be mistaken for angiosarcoma. Am. J Surg. Pathol. 1992;16:429–438. doi: 10.1097/00000478-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 78.Bayrou O, Avril MF, Charpentier P, Caillou B, Guillaume JC, Prade M. Primary neuroendocrine carcinoma of the skin. Clinicopathologic study of 18 cases. J Am. Acad. Dermatol. 1991;24:198–207. doi: 10.1016/0190-9622(91)70027-y. [DOI] [PubMed] [Google Scholar]
- 79.Drijkoningen M, De Wolf-Peeters C, van LE, Desmet V. Merkel cell tumor of the skin: an immunohistochemical study. Hum. Pathol. 1986;17:301–307. doi: 10.1016/s0046-8177(83)80224-x. [DOI] [PubMed] [Google Scholar]
- 80.Collins BT, Elmberger PG, Tani EM, Bjornhagen V, Ramos RR. Fine-needle aspiration of Merkel cell carcinoma of the skin with cytomorphology and immunocytochemical correlation. Diagn. Cytopathol. 1998;18:251–257. doi: 10.1002/(sici)1097-0339(199804)18:4<251::aid-dc1>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 81.Swann MH, Yoon J. Merkel cell carcinoma. Semin. Oncol. 2007;34:51–56. doi: 10.1053/j.seminoncol.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 82.Liegl B, Regauer S. Penile clear cell carcinoma: a report of 5 cases of a distinct entity. Am. J. Surg. Pathol. 2004;28:1513–1517. doi: 10.1097/01.pas.0000141405.64462.2a. [DOI] [PubMed] [Google Scholar]
- 83.Cowper SE, Kilpatrick T, Proper S, Morgan MB. Solitary fibrous tumor of the skin. Am. J. Dermatopathol. 1999;21:213–219. doi: 10.1097/00000372-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Kanitakis J. Mammary and extramammary Paget’s disease. J. Eur. Acad. Dermatol. Venereol. 2007;21:581–590. doi: 10.1111/j.1468-3083.2007.02154.x. [DOI] [PubMed] [Google Scholar]
- 85.Liegl B, Gully C, Reich O, Nogales FF, Beham A, Regauer S. Expression of platelet-derived growth factor receptor in low-grade endometrial stromal sarcomas in the absence of activating mutations. Histopathology. 2007;50:448–452. doi: 10.1111/j.1365-2559.2007.02634.x. [DOI] [PubMed] [Google Scholar]
- 86.Toker C. Clear cells of the nipple epidermis. Cancer. 1970;25:601–610. doi: 10.1002/1097-0142(197003)25:3<601::aid-cncr2820250315>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 87.Kuan SF, Montag AG, Hart J, Krausz T, Recant W. Differential expression of mucin genes in mammary and extramammary Paget’s disease. Am. J Surg. Pathol. 2001;25:1469–1477. doi: 10.1097/00000478-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 88.Willman JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am. J Dermatopathol. 2005;27:185–188. doi: 10.1097/01.dad.0000158291.20440.13. [DOI] [PubMed] [Google Scholar]
- 89.Marucci G, Betts CM, Golouh R, Peterse JL, Foschini MP, Eusebi V. Toker cells are probably precursors of Paget cell carcinoma: a morphological and ultrastructural description. Virchows Arch. 2002;441:117–123. doi: 10.1007/s00428-001-0581-x. [DOI] [PubMed] [Google Scholar]
- 90.Ohnishi T, Shibuya S, Nemoto I, Koike S, Takizawa H, Suzuki T, Watanabe S. Evidence from mucin core protein expression that some Paget’s disease on areola can be of extramammary-like histogenesis and part of multisite disease. Br. J Dermatol. 2004;151:688–692. doi: 10.1111/j.1365-2133.2004.06087.x. [DOI] [PubMed] [Google Scholar]
- 91.Lever WF, Elder DE. Lever’s histopathology of the skin. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 92.Yoshii N, Kitajima S, Yonezawa S, Matsukita S, Setoyama M, Kanzaki T. Expression of mucin core proteins in extramammary Paget’s disease. Pathol. Int. 2002;52:390–399. doi: 10.1046/j.1440-1827.2002.01364.x. [DOI] [PubMed] [Google Scholar]
- 93.Garcia C, Poletti E, Crowson AN. Basosquamous carcinoma. J. Am. Acad. Dermatol. 2009;60:137–143. doi: 10.1016/j.jaad.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 94.Schaumburg-Lever G, Lever WF, Lever WF. Color atlas of histopathology of the skin. Philadelphia: Lippincott; 1988. [Google Scholar]
- 95.Beer TW, Shepherd P, Theaker JM. Ber EP4 epithelial membrane antigen aid distinction of basal cell, squamous cell and basosquamous carcinomas of the skin. Histopathology. 2000;37:218–223. doi: 10.1046/j.1365-2559.2000.00999.x. [DOI] [PubMed] [Google Scholar]
- 96.Magro CM, Crowson AN, Mihm MC. Unusual variants of malignant melanoma. Mod. Pathol. 2006;19 Suppl 2:S41–S70. doi: 10.1038/modpathol.3800516. [DOI] [PubMed] [Google Scholar]
- 97.Hasebe T, Mukai K, Yamaguchi N, Ishihara K, Kaneko A, Takasaki Y, Shimosato Y. Prognostic value of immunohistochemical staining for proliferating cell nuclear antigen, p53, and c-erbB-2 in sebaceous gland carcinoma and sweat gland carcinoma: comparison with histopathological parameter. Mod. Pathol. 1994;7:37–43. [PubMed] [Google Scholar]
- 98.Nash JW, Barrett TL, Kies M, Ross MI, Sneige N, Diwan AH, Lazar AJ. Metastatic hidradenocarcinoma with demonstration of Her-2/neu gene amplification by fluorescence in situ hybridization: potential treatment implications. J. Cutan. Pathol. 2007;34:49–54. doi: 10.1111/j.1600-0560.2006.00570.x. [DOI] [PubMed] [Google Scholar]
- 99.Kuwano H, Hashimoto H, Enjoji M. Atypical fibroxanthoma distinguishable from spindle cell carcinoma in sarcoma-like skin lesions. A clinicopathologic and immunohistochemical study of 21 cases. Cancer. 1985;55:172–180. doi: 10.1002/1097-0142(19850101)55:1<172::aid-cncr2820550127>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 100.Gupta R, Singh S, Hedau S, Nigam S, Das BC, Singh I, Mandal AK. Spindle cell carcinoma of head and neck: an immunohistochemical and molecular approach to its pathogenesis. J Clin. Pathol. 2007;60:472–475. doi: 10.1136/jcp.2005.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakamura T, Kawamura T, Nariya S, Fujiwara M. Cutaneous sclerosing perineurioma of the digit. Int. J. Dermatol. 2006;45:1086–1088. doi: 10.1111/j.1365-4632.2004.02514.x. [DOI] [PubMed] [Google Scholar]
- 102.Mentzel T, Requena L, Kaddu S, Soares de Aleida LM, Sangueza OP, Kutzner H. Cutaneous myoepithelial neoplasms: clinicopathologic and immunohistochemical study of 20 cases suggesting a continuous spectrum ranging from benign mixed tumor of the skin to cutaneous myoepithelioma and myoepithelial carcinoma. J Cutan. Pathol. 2003;30:294–302. doi: 10.1034/j.1600-0560.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 103.Hamperl H. The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr. Top. Pathol. 1970;53:161–220. [PubMed] [Google Scholar]
- 104.Franke WW, Schmid E, Freudenstein C, Appelhans B, Osborn M, Weber K, Keenan TW. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980;84:633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suster S, Cajal S. Myoepithelial differentiation in basal cell carcinoma. Am. J Dermatopathol. 1991;13:350–357. doi: 10.1097/00000372-199108000-00005. [DOI] [PubMed] [Google Scholar]
- 106.Prieto VG, Kenet BJ, Varghese M. Malignant mesothelioma metastatic to the skin, presenting as inflammatory carcinoma. Am. J Dermatopathol. 1997;19:261–265. doi: 10.1097/00000372-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 107.Patel NK, McKee PH, Smith NP, Fletcher CD. Primary metaplastic carcinoma (carcinosarcoma) of the skin. A clinicopathologic study of four cases and review of the literature. Am. J Dermatopathol. 1997;19:363–372. doi: 10.1097/00000372-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 108.Metze D, Soyer HP, Zelger B, Neumaier M, Grunert F, Hartig C, Amann U, Bhardwaj R, Wagener C, Luger T. Expression of a glycoprotein of the carcinoembryonic antigen family in normal and neoplastic sebaceous glands. Limited role of carcinoembryonic antigen as a sweat gland marker. J Am. Acad. Dermatol. 1996;34:735–744. doi: 10.1016/s0190-9622(96)90005-5. [DOI] [PubMed] [Google Scholar]
- 109.Riker AI, Enkemann SA, Fodstad O, Liu S, Ren S, Morris C, Xi Y, Howell P, Metge B, Samant RS, Shevde LA, Li W, Eschrich S, Daud A, Ju J, Matta J. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC. Med Genomics. 2008;1:13. doi: 10.1186/1755-8794-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu YP, Haq B, Carraway KL, Savaraj N, Lampidis TJ. Multidrug resistance correlates with overexpression of Muc4 but inversely with P-glycoprotein and multidrug resistance related protein in transfected human melanoma cells. Biochem. Pharmacol. 2003;65:1419–1425. doi: 10.1016/s0006-2952(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 111.Bafna S, Kaur S, Momi N, Batra SK. Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br. J. Cancer. 2009;101:1155–1161. doi: 10.1038/sj.bjc.6605285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chaturvedi P, Singh AP, Moniaux N, Senapati S, Chakraborty S, Meza JL, Batra SK. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol. Cancer Res. 2007;5:309–320. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 113.Chaturvedi P, Singh AP, Chakraborty S, Chauhan SC, Bafna S, Meza JL, Singh PK, Hollingsworth MA, Mehta PP, Batra SK. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68:2065–2070. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kathpalia VP. Genome-wide transcriptional profiling in human squamous cell carcinoma of the skin identifies unique tumor-associated signatures. 2006 doi: 10.1111/j.1346-8138.2006.00075.x. [DOI] [PubMed] [Google Scholar]
- 115.Schumacher U, Adam E. Immunohistochemical detection of the MUC1 gene product in human cancers grown in scid mice. J. Histochem. Cytochem. 1998;46:127–134. doi: 10.1177/002215549804600116. [DOI] [PubMed] [Google Scholar]
- 116.Burchell J, Taylor-Papadimitriou J. Effect of modification of carbohydrate side chains on the reactivity of antibodies with core-protein epitopes of the MUC1 gene product. Epithelial Cell Biol. 1993;2:155–162. [PubMed] [Google Scholar]
- 117.Girling A, Bartkova J, Burchell J, Gendler S, Gillett C, Taylor-Papadimitriou J. A core protein epitope of the polymorphic epithelial mucin detected by the monoclonal antibody SM-3 is selectively exposed in a range of primary carcinomas. Int. J. Cancer. 1989;43:1072–1076. doi: 10.1002/ijc.2910430620. [DOI] [PubMed] [Google Scholar]
- 118.Oosterkamp HM, Scheiner L, Stefanova MC, Lloyd KO, Finstad CL. Comparison of MUC-1 mucin expression in epithelial and non-epithelial cancer cell lines and demonstration of a new short variant form (MUC-1/Z) Int. J. Cancer. 1997;72:87–94. doi: 10.1002/(sici)1097-0215(19970703)72:1<87::aid-ijc13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 119.Papini S, Cecchetti D, Campani D, Fitzgerald W, Grivel JC, Chen S, Margolis L, Revoltella RP. Isolation and clonal analysis of human epidermal keratinocyte stem cells in long-term culture. Stem Cells. 2003;21:481–494. doi: 10.1634/stemcells.21-4-481. [DOI] [PubMed] [Google Scholar]
- 120.Turner J, Jones CE. Regulation of mucin expression in respiratory diseases. Biochem. Soc. Trans. 2009;37:877–881. doi: 10.1042/BST0370877. [DOI] [PubMed] [Google Scholar]
- 121.Grunberg E, Eckert K, Karsten U, Maurer HR. Effects of differentiation inducers on cell phenotypes of cultured nontransformed and immortalized mammary epithelial cells: a comparative immunocytochemical analysis. Tumour. Biol. 2000;21:211–223. doi: 10.1159/000030127. [DOI] [PubMed] [Google Scholar]
- 122.Andrianifahanana M, Agrawal A, Singh AP, Moniaux N, van S, Aubert IJP, Meza J, Batra SK. Synergistic induction of the MUC4 mucin gene by interferongamma and retinoic acid in human pancreatic tumour cells involves a reprogramming of signalling pathways. Oncogene. 2005;24:6143–6154. doi: 10.1038/sj.onc.1208756. [DOI] [PubMed] [Google Scholar]
- 123.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat. Rev. Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chakraborty S, Jain M, Sasson AR, Batra SK. MUC4 as a diagnostic marker in cancer. Expert. Opinion. on Medical. Diagnostics. 2008;2:891–910. doi: 10.1517/17530059.2.8.891. [DOI] [PubMed] [Google Scholar]
- 125.Arlen PM, Gulley JL, Madan RA, Hodge JW, Schlom J. Preclinical and clinical studies of recombinant poxvirus vaccines for carcinoma therapy. Crit Rev. Immunol. 2007;27:451–462. doi: 10.1615/critrevimmunol.v27.i5.40. [DOI] [PubMed] [Google Scholar]
- 126.Kaneko O, Gong L, Zhang J, Hansen JK, Hassan R, Lee B, Ho M. A binding domain on mesothelin for CA125/MUC16. J. Biol. Chem. 2009;284:3739–3749. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Price-Schiavi SA, Jepson S, Li P, Arango M, Rudland PS, Yee L, Carraway KL. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int. J. Cancer. 2002;99:783–791. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- 128.Komatsu M, Yee L, Carraway KL. Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res. 1999;59:2229–2236. [PubMed] [Google Scholar]
- 129.Torres MP, Ponnusamy MP, Chakraborty S, Smith LM, Das S, Arafat HA, Batra SK. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapies. Mol. Cancer Ther. 2010;9:1419–1431. doi: 10.1158/1535-7163.MCT-10-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]