Patients with diabetes have a several-fold higher risk of cardiovascular events than non-diabetic patients. The susceptibility to heart failure, lower limb amputations, and other manifestations of tissue ischemia in diabetes is due to an increased risk of atherothrombotic disease as well as impaired neovascularization and collateral formation through arteriogenesis or angiogenesis (1, 2). Abnormal angiogenesis in diabetes, however, is complex since increased neovascularization is the hallmark of proliferative diabetic retinopathy, another major complication of the disease.
The mechanisms responsible for this range of abnormal angiogenesis in different tissues in patients with diabetes are poorly understood, but relate at least in part to differential expression of hypoxia-induced angiogenic factors like vascular endothelial growth factor (VEGF). Cardiac expression of VEGF and its receptors are decreased in diabetic rats (3, 4) and in patients with diabetes (3), while VEGF and its receptors, paradoxically, are increased in the diabetic retina, even in animal models where proliferative diabetic retinopathy does not occur (3, 5). Both insulin resistance and hyperglycemia, present in most patients with diabetes, may affect angiogenic potential. Insulin action is necessary for appropriate expression of VEGF (6) (see figure), and rats with obesity-associated non-diabetic insulin resistance have decreased expression of VEGF and VEGF receptors (3), and decreased capillary density in the heart (6). Hyperglycemia itself may inhibit angiogenesis, for example through formation of advanced glycation end-products (AGE). Modification of (basic) fibroblast growth factor-2 (FGF2) by AGE (7), as shown by several independent groups, inhibits its angiogenic action. Even though many studies have shown that AGE increase proangiogenic functions of cultured endothelial cells, the compound effect of AGE in vivo may be an impairment of ischemia-induced angiogenesis because inhibition of AGE formation improves angiogenesis in diabetic mice (1).
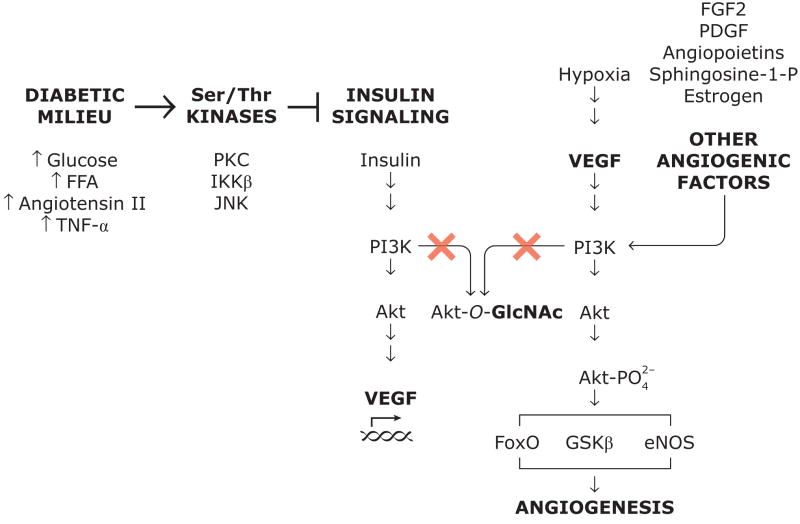
Figure 1.
Proposed mechanisms for impairment of angiogenesis through inhibition of insulin signaling and through O-GlcNAc modification (glycosylation) of Akt. Glycosylated Akt is not activated by PI3K and consequently cannot increase expression of VEGF or mediate the effects of VEGF or other proangiogenic factors. Abbreviations: AGE, advanced glycation end-products; FFA, free fatty acids; FGF2, (basic) fibroblast growth factor; GlcNAc, N-acetylglycosamine; IKKβ; inhibitor κB kinase; JNK, c-Jun NH2-terminal kinase; PDGF, platelet-derived growth factor; PI3K, 1-phosphatidylinositol 3-kinase; PKC, protein kinase C; Sphingosine-1-P, sphingosine-1-phosphate; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
In this issue of Arterioscler Thromb Vasc Biol, Luo et al. (8) examined another mechanism of impaired angiogenesis in diabetes, protein modification by O-linked β-N-acetylglucosamine (O-GlcNAc). Such O-linked glycosylation may affect nuclear and cytosolic proteins as a post-translational modification on serine or threonine residues. This modification is dynamic, enzymatically regulated, and often modifies protein function (9). It can be reciprocal to phosphorylation of the same residue because glycosylation prevents phosphorylation (9). O-linked glycosylation of certain proteins is increased in diabetes because increased cellular uptake of glucose activates the hexosamine pathway, which metabolises glucose as an alternative to other metabolic pathways like glycolysis. A product of the hexosamine pathway is uridine-diphospho-N-acetyl-glucosamine (UDP-GlcNAc), a substrate in the reaction that forms O-linked glycosylation (10). Activation of the hexosamine pathway is not only a result of increased substrate availability during hyperglycemia. In patients with diabetes, the expression of glutamine:fructose-6-phosphate amidotransferase (GFAT), the rate-limiting enzyme for the hexosamine pathway, is increased in several tissues prone to complications (11). Modulation of proteins by O-GlcNAc has been shown to modify DNA binding, enzyme activity, protein-protein interactions, the half-life of proteins, and subcellular localization.
To study the effect of diabetes, Luo et al. (8) used aorta from animal models of human type 1 and type 2 diabetes, respectively, namely mice with streptozotocin-induced diabetes and mice fed a high-fat diet for 3 months. To study the effects of actvation of the hexosamine pathway more directly, they incubated mouse aorta with medium containing glucosamine, which is a substrate for the hexosamine pathway. The aortas all exhibited increases of approximately 50% in immunoreactive O-GlcNAc in aorta lysate and in sprouting of vascular cells from rings of aorta incubated in Matrigel.
The authors then described the effects of O-GlcNAc protein modification in cell culture studies of pro-angiogenic cell function. Glucosamine incubation increased O-GlcNAc immunoreactivity in cell lysate and decreased cell migration and tube formation in a transformed endothelial-like cell line and in primary human umbilical vein endothelial cells. The effects of glucosamine were likely due to O-GlcNAc protein modification since a pharmacological inhibitor of β-O-linked N-acetylglucosaminidase (O-GlcNAcase) inhibited cell migration and tube formation, whereas adenoviral-mediated expression of O-GlcNAcase increased these cell functions.
Finally, Luo et al. examined the O-GlcNAc of Akt, which they considered an important candidate for pro-angiogenic cell function. In particular, the Akt1 isoform has been shown to be critical for angiogenesis induced by ischemia or VEGF (12). They found increased O-GlcNAc modification and decreased Ser473 phosphorylation of Akt in endothelial cells grown in glucosamine. Inhibition of O-GlcNAcase increased O-GlcNAc glycosylation of Akt and decreased Akt activity, whereas overexpression of O-GlcNAcase decreased O-GlcNAc glycosylation of Akt and increased Akt phosphorylation and activity. Since activation of Akt mediates angiogenic actions of several factors, among them VEGF, FGF2, and estradiol (see figure), this mechanism of Akt inactivation could affect angiogenic actions of many hormones, growth factors, and cytokines. It is readily appararent from the O-GlcNAc immunoblots of aorta lysate from diabetic animals (fig. 1 in Luo et al.’s paper (8)) that several proteins are glycosylated, as discussed by the authors (8), and the decreased proangiogenic function observed by Luo et al. may be explained in part by glycosylation that impairs other signaling than the PI3K/Akt pathway.
Taken together, Luo et al.’s work is significant because it demonstrates for the first time that O-GlcNAc modification of vascular proteins may be a determinant of impaired angiogenesis in diabetes. Most of the evidence linking O-GlcNAc glycosylation and vascular function has been obtained in cell culture studies, and Luo et al.’s experiments in vascular tissue from animal models of both type 1 and 2 diabetes are valuable because they offer an alternative to approximating the diabetic milieu by increasing glucose concentrations in cell culture studies. Other mechanisms involving O-GlcNAc modification may operate than those described by Luo et al. For example, hyperglycemia, production of reactive oxygen species, and O-GlcNAc modification can increase expression of of angiopoietin-2 (13) which has pro-angiogenic effects. Therefore, confirmation of Luo et al.’s findings in vivo is critical, both because of the complexity of the true diabetic condition and because angiogenesis is determined by a range of factors beyond vascular cell proliferation and morphology, including production of growth factors and cytokines by interstitial cells and immune cells, and modification of the extracellular matrix. It will also be important to dermine quantitatively how much the mechanism of O-GlcNAc modification in diabetes contribute to inhibition in endothelial cells of the PI3K/Akt pathway, which can also be affected by other mechanisms, like activation of PKC (14) (see figure). Furthermore, the author’s results cannot explain the paradoxically increased angiogenesis in proliferative diabetic retinopathy despite the fact that O-GlcNAc protein modification is probably observed in all vascular tissues, including the retina.
Future studies will explore which proteins in vascular tissue are most susceptible to O-GlcNAc modification, and describe in more detail how such glycosylation is regulated. Results from research in this area should provide new targets with the purpose of improving angiogenesis in patients with diabetes, with the hope of preventing or treating heart failure, chronic ulcers, loss of limb, and shortened life span in patients with diabetes. It is likely that O-GlcNAc protein modification is only one piece of a puzzle that make up the complex of vascular abnormalities in diabetes and insulin resistance. More work in this area is clearly needed because of the rapid increase in prevalence of insulin resistance and diabetes in many of the world’s populations.
Acknowledgments
C.R-M. is supported by NIH grants P30 DK036836-21 and K08 EY018677-01. G.L.K. receives NIH grants R01 DK53105 and R01 EY016150.
References
- 1.Tamarat R, Silvestre JS, Huijberts M, Benessiano J, Ebrahimian TG, Duriez M, Wautier MP, Wautier JL, Levy BI. Blockade of advanced glycation end-product formation restores ischemia-induced angiogenesis in diabetic mice. Proc Natl Acad Sci U S A. 2003;100(14):8555–60. doi: 10.1073/pnas.1236929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99(17):2239–42. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 3.Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, Suzuma K, Bowling NL, Vlahos CJ, Aiello LP, King GL. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105(3):373–9. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 4.Jesmin S, Zaedi S, Shimojo N, Iemitsu M, Masuzawa K, Yamaguchi N, Mowa CN, Maeda S, Hattori Y, Miyauchi T. Endothelin antagonism normalizes VEGF signaling and cardiac function in STZ-induced diabetic rat hearts. Am J Physiol Endocrinol Metab. 2007;292(4):E1030–40. doi: 10.1152/ajpendo.00517.2006. [DOI] [PubMed] [Google Scholar]
- 5.Hammes HP, Lin J, Bretzel RG, Brownlee M, Breier G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes. 1998;47(3):401–6. doi: 10.2337/diabetes.47.3.401. [DOI] [PubMed] [Google Scholar]
- 6.He Z, Opland DM, Way KJ, Ueki K, Bodyak N, Kang PM, Izumo S, Kulkarni RN, Wang B, Liao R, Kahn CR, King GL. Regulation of vascular endothelial growth factor expression and vascularization in the myocardium by insulin receptor and PI3K/Akt pathways in insulin resistance and ischemia. Arterioscler Thromb Vasc Biol. 2006;26(4):787–93. doi: 10.1161/01.ATV.0000209500.15801.4e. [DOI] [PubMed] [Google Scholar]
- 7.Giardino I, Edelstein D, Brownlee M. Nonenzymatic glycosylation in vitro and in bovine endothelial cells alters basic fibroblast growth factor activity. A model for intracellular glycosylation in diabetes. J Clin Invest. 1994;94(1):110–7. doi: 10.1172/JCI117296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo B, Soesanto Y, McClain DA. Protein Modification by O-Linked GlcNAc Reduces Angiogenesis by Inhibiting Akt Activity in Endothelial Cells. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.107.159533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446(7139):1017–22. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 10.Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007;73(2):288–97. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nerlich AG, Sauer U, Kolm-Litty V, Wagner E, Koch M, Schleicher ED. Expression of glutamine:fructose-6-phosphate amidotransferase in human tissues: evidence for high variability and distinct regulation in diabetes. Diabetes. 1998;47(2):170–8. doi: 10.2337/diab.47.2.170. [DOI] [PubMed] [Google Scholar]
- 12.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115(8):2119–27. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I, Suske G, Rabbani N, Thornalley PJ, Sarthy VP, Hammes HP, Brownlee M. High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem. 2007;282(42):31038–45. doi: 10.1074/jbc.M704703200. [DOI] [PubMed] [Google Scholar]
- 14.Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JR, Clermont AC, Ueki K, Ohshiro Y, Zhang J, Goldfine AB, King GL. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55(3):691–8. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]