Abstract
Background
Recent studies in mice and patients suggest that posttransplantation induction of autoimmune responses to tissue-specific antigens contributes to the rejection of major histocompatibility complex mismatched allotransplants. The relevance of this phenomenon to the rejection of major and minor histocompatibility-mismatched allografts performed in large-animal models remains to be established.
Methods
Miniature swine were immunized with cardiac myosin (CM) in Freund’s adjuvant and received heterotopic, minor antigen-mismatched heart transplants. T-cell (proliferation and delayed type hypersensitivity [DTH]) and B-cell (antibody) responses specific to CM were measured. The rejection of heart transplants was assessed histologically.
Results
Three of four swine that were immunized with CM before receiving a minor antigen-mismatched heart transplant exhibited potent DTH, T-cell proliferation and antibody responses to CM and rejected their grafts acutely. The fourth swine, which failed to mount a significant DTH response to CM and displayed low and transient anti-CM antibody titers, demonstrated long-term allograft survival.
Conclusions
This large-animal study supports the relevance of autoimmunity to CM in the rejection of minor antigen disparate cardiac allotransplants.
Keywords: Heart transplantation, Swine, Autoimmunity, Cardiac myosin
The transplantation of allogeneic organs triggers a massive inflammatory immune response mediated by recipient T lymphocytes recognizing donor major histocompatibility complex (MHC) molecules. Allorecognition takes place primarily in the recipient’s secondary lymphoid organs where T cells interact with intact allo-MHC molecules present on donor dendritic cells (direct allorecognition) and with donor MHC peptides processed and presented by recipient antigen presenting cells (indirect allorecognition) (1). In addition, minor histocompatibility antigens, defined as peptides derived from non-MHC donor polymorphic proteins, are presented directly or indirectly, and can trigger an alloimmune response (2). The activation of alloreactive CD4+ and CD8+ T cells triggers a cascade of events including cytotoxic T-cell differentiation, delayed type hypersensitivity (DTH) reactions, and anti-donor antibody production that lead to the rejection of allografts. On the other hand, the involvement of tissue-specific antigens in anti-graft immunity has long been postulated (3). Although some of these antigens have been identified, little is known regarding their contribution to the rejection of allografts.
Recent studies in our laboratory and others’ have demonstrated that rodents receiving allogeneic heart or lung transplants mount an autoimmune response to graft tissue-specific antigens (4–6). In a mouse heart transplant model, we have reported that the T-cell response to donor MHC class I molecules is followed by an autoimmune response to cardiac myosin (CM), a contractile protein expressed by cardiomyocytes (4). CM also represents the target autoantigen in experimental autoimmune myocarditis (EAM), a mouse model of autoimmune heart disease (7). These findings suggest that anti-CM immunity may cause damage to the transplanted heart, thereby contributing to its rejection by the host. There is a report supporting this view that induction of an autoimmune response to CM in mouse recipients before transplantation accelerates the rejection of allogeneic heart transplants (4). In this case, the anti-CM autoimmune response was mediated by activated MHC class II-restricted CM-specific CD4+ TH1 cells (4). In addition, high titers of IgG1 anti-CM autoantibodies were detected in the serum of transplanted mice (4). Most importantly, tolerance induction to CM and collagen type V has been shown to significantly prolong the survival of heart and lung transplants, respectively (8, 9). Finally, several studies have provided evidence suggesting the relevance of CM autoimmunity in clinical cardiac transplantation (10–13). Collectively, these studies suggest that secondary immunity to tissue-specific antigens may represent an essential component of allograft rejection. However, the actual relevance of this phenomenon to the rejection of minor antigen-mismatched allografts in a large-animal model remains to be established.
In this study, we investigated the influence of CM autoimmunity on the rejection of minor antigen-mismatched cardiac allografts performed in a swine model. We observed that the induction of an anti-CM response led to the rejection of minor antigen-mismatched cardiac allografts. The implications of these findings for the design of novel diagnostic tools and therapies in transplantation are discussed.
RESULTS
In this study, we investigated the effect of CM sensitization on the rejection of minor antigen-mismatched cardiac allografts in miniature swine. To address this question, we compared DTH, T-cell proliferation and antibody responses with CM and transplant rejection in swine immunized with CM emulsified in Freund’s adjuvant and control nontreated or adjuvant-immunized recipients.
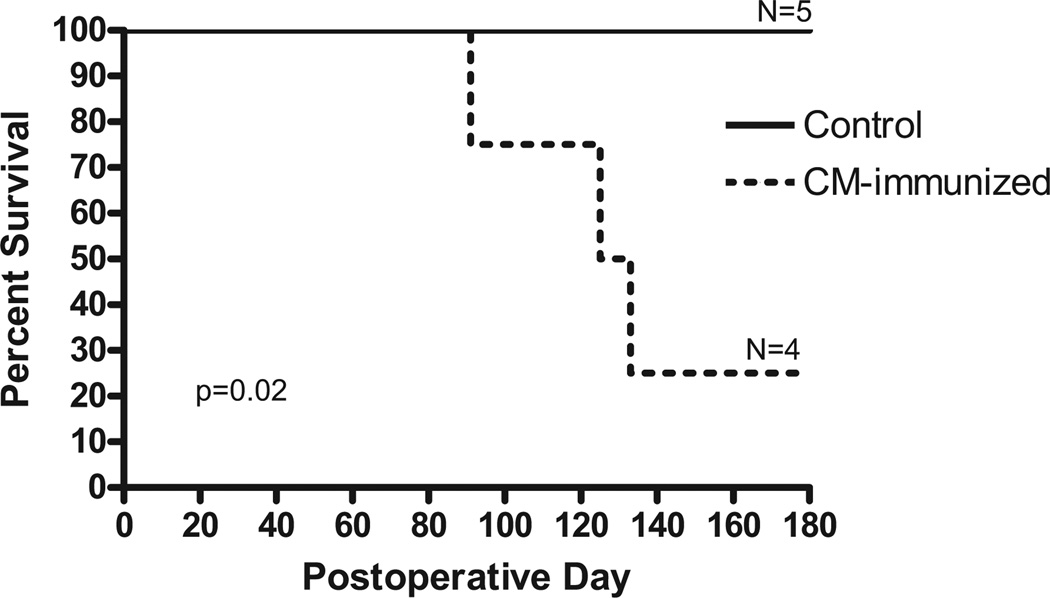
None of the five control (nonimmunized or immunized with adjuvant alone) swine (C1–C5) acutely rejected its graft, an expected result given the poor immunogenicity of minor antigens (Figs. 1 and 2). Although histological examination of these grafts at posttransplant day 60, 120, and 180 revealed some inflammatory cell infiltrates, no signs of cardiac allograft vasculopathy were observed (Fig. 1). No DTH response (induration<1 mm) to CM was detected in the control animals (Table 1). In contrast, experimental swine P1, P2, and P3 mounted potent DTH responses to CM (> 10 mm) (Table 1) and rejected their allografts in an acute fashion (grade 3R, Figs. 1 and 2) (P=0.02). The fourth swine (P4) mounted a poor DTH response after immunization with CM (8 mm) and was diagnosed with a low grade (1R at day 62) acute rejection characterized by focal perivascular or interstitial infiltrate without myocyte damage. The correlation between CM-specific DTH response and acute rejection was statistically significant (two-tailed t test assuming equal variance P=0.0003).
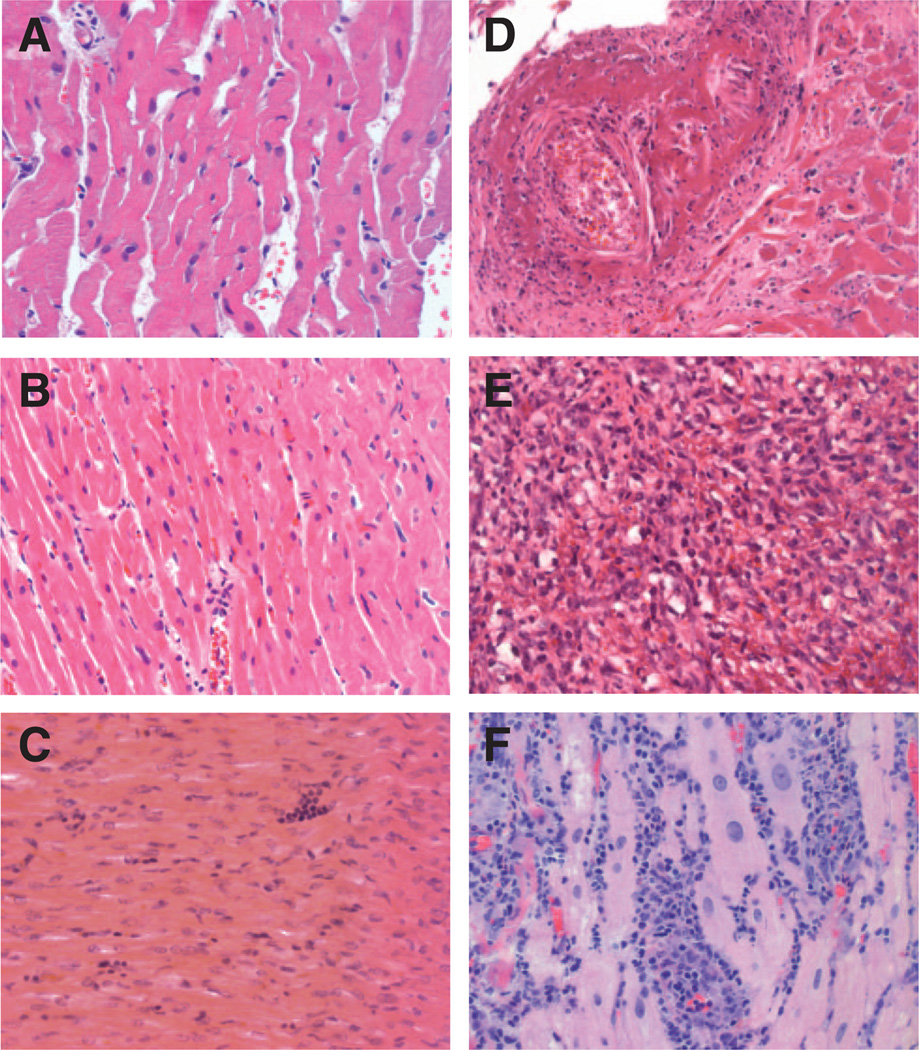
Figure 1.
Histologic examination of heart allograft biopsies for control and experimental animals. Hematoxylineosin staining (magnification ×40) shows no or mild myocardial inflammation and no myocyte necrosis at the time of sacrifice for control animals C1 (International Society for Heart and Lung Transplantation [ISHLT] Grade 0R), C2 (ISHLT Grade 0R), and C5 (ISHLT Grade 1R), respectively (A–C). In contrast, experimental animals P1, P2, and P3 exhibited diffuse myocardial inflammation and necrosis (ISHLT Grade 3R) on postoperative day (POD) 125, POD 133, and POD 91, respectively (D–F).
Figure 2.
Kinetics of cardiac allograft survival in miniature swine. The survival of minor antigen-mismatched cardiac allografts was monitored in five control untreated swine and in four swine immunized with purified cardiac myosin 42 days and 21 days before transplantation. Graft survival was analyzed using the Kaplan-Meier method, and survival curves were compared using the log-rank test.
Table 1.
DTH response to and rejection of minor antigen-mismatched cardiac allografts
| Swine | Immunization | DTH response | Rejection |
|---|---|---|---|
| C1 | – | Negative (<1 mm) | TOL |
| C2 | – | Negative (<1 mm) | TOL |
| C3 | – | Negative (<1 mm) | TOL |
| C4 | – | Negative (<1 mm) | TOL |
| C5 | Adjuvant alone | Negative (<1 mm) | TOL |
| P1 | CM | Positive (13 mm) | ACR (ISHLT grade 3R) |
| P2 | CM | Positive (11 mm) | ACR (ISHLT grade 3R) |
| P3 | CM | Positive (> 10 mm) | ACR (ISHLT grade 3R) |
| P4 | CM | Negative (8 mm) | TOL (grade 1R at day 62) |
Four swine (P1–P4) were immunized twice subcutaneously with CM emulsified in CFA on day −42 and in IFA on day −21 before transplantation with a minor antigen-mismatched heart. Four control nonimmunized swine transplanted with a minor antigen-mismatched heart were also tested (C1– C4). Swine C5 was injected with adjuvant alone (day −42 with CFA, day −21 with IFA) before receiving a minor antigen-mismatched heart transplant. DTH responses >10 mm were considered positive.
CM, cardiac myosin; CFA, complete Freund’s adjuvant; IFA, incomplete Freund’s adjuvant; TOL, tolerant (i.e., allografts displaying normal function >180 d posttransplantation); ACR, acute cellular rejection; DTH, delayed type hypersensitivity; ISHLT, International Society for Heart and Lung Transplantation.
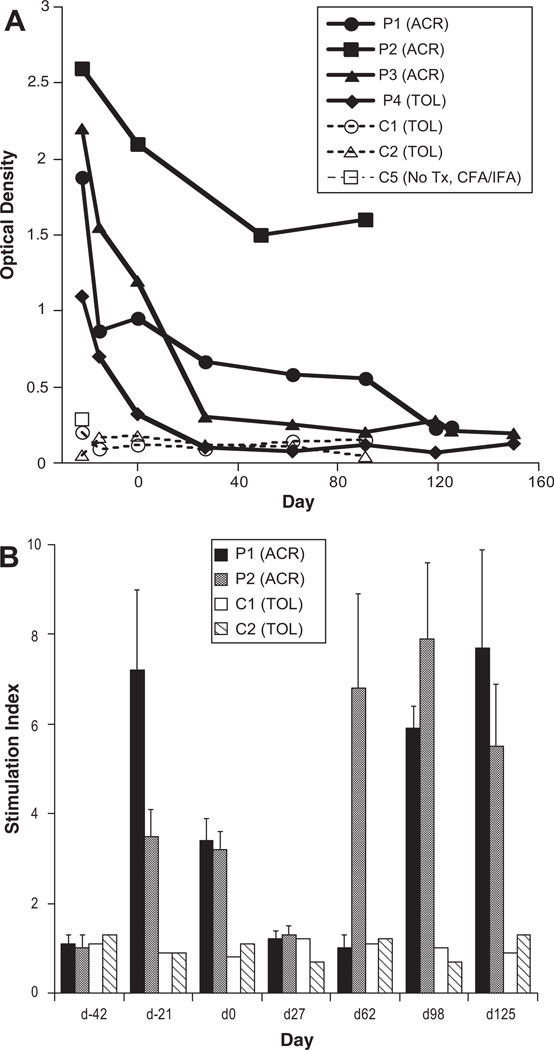
As shown in Figure 3(A), CM-immunized P1 to P3 pigs exhibited high and sustained serum levels of anti-CM antibodies. These antibodies displayed an IgG isotype. In contrast, no CM-specific antibodies were detected in control nonimmunized swine and swine immunized with adjuvant alone (Fig. 3A). The swine P4, characterized by a poor DTH and low grade acute cellular rejection, displayed an early antibody response to CM which subsided rapidly after transplantation as anti-CM antibodies could no longer be detected by day 27 posttransplantation (Fig. 3A).
Figure 3.
Kinetics of antibody and T-cell proliferative responses to cardiac myosin (CM) in swine immunized with CM and transplanted with a minor antigen-mismatched heart. (A) The presence of anti-CM antibodies was detected by enzyme-linked immunosorbent assay in swine that were immunized with CM emulsified in Freund’s adjuvant (on day −42 and −21) and received minor antigen-mismatched heart transplants on day 0. Antibody levels were assessed at different time points pre- and posttransplantation in swine undergoing acute rejection (P1 and P2), and in a tolerant swine (P4) and in control nontransplanted (open square) and nonimmunized (open triangle) swine. (B) T-cell proliferative responses to CM (10 µg/mL) were assessed at different time points in two CM-immunized pigs (P1 and P2) and two control nonimmunized pigs (C1 and C2), which receive a minor-mismatched heart allograft at d0. The stimulation indices: SI correspond to the average count per million (cpm)±standard deviation for a responder stimulated with CM/cpm of the same responder stimulated by medium alone.
Next, we measured T-cell proliferative responses in two CM-immunized swine (P1 and P2) and two control swine (C1 and C2) at different time points pre- and postcardiac transplantation. As shown in Figure 3(B), T-cell proliferation was detected in both immunized pigs, 21 and 42 days after immunization (day –21 and d0). This proliferative response was no longer detectable at day 27. However, interestingly, it reappeared at day 62 posttransplantation and remains high at day 125. This suggests that, during the course of transplant rejection, endogenous CM determinants derived from cardiac myocytes are processed and presented to T cells thereby reactivating some anti-CM memory T cells. No T-cell proliferation was observed in control nonimmunized swine (Fig. 3B).
DISCUSSION
De novo induction of autoimmunity to tissue-specific antigens has now been observed in heart, lung, kidney, skin, and liver transplant models (4, 5, 14–16). We have previously shown the involvement of CM-specific immunity in the rejection of MHC disparate cardiac allografts studied in an experimental mouse model (4, 9). It is noteworthy that patients originally diagnosed with chronic myocarditis experience more frequent and severe rejection episodes than patients with other heart diseases (17). In addition, Latif et al. (11) have shown an association between the presence of anti-heart autoantibodies and clinical course after heart transplantation in patients. Actually, increases in the amounts of circulating CM after transplantation have been correlated with poor prognosis for cardiac transplant survival (18). Most importantly, Warraich et al. (13) recently reported the presence of anti-CM autoantibodies during acute rejection of cardiac allografts in patients with dilated cardiomyopathy. Recently, the presence of autoimmune responses to CM has been observed in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy (10, 12). Therefore, autoimmunity to CM represents a general phenomenon in cardiac allotransplantation. The present study shows for the first time that CM-specific autoimmunity can trigger the acute rejection of minor antigen-mismatched cardiac allografts in a large-animal model.
Apparently, the nature of the rejection was dependent on the strength of the response to CM because high responder swine (P1–P3) rejected their allografts acutely whereas the swine displaying low and transient DTH and antibody responses to CM (P4) did not. The suboptimal immune response to CM detected in the fourth experimental animal may simply be due to a poor immunization. Alternatively, it may reflect genetic differences in susceptibility to CM autoimmunity among these swine. This result is reminiscent of the observation that tolerance to CM can be broken only in certain mouse strains that are also susceptible to EAM whereas other strains do not mount autoimmune responses to CM on immunization and are resistant to EAM (7, 19). Finally, this observation may reflect differences in the T- and B-cell repertoires acquired through the pig’s “immunological history.”
Similar to previous observations reported in rodent lung and heart allotransplant models, no signs of tissue injury were detected in the native heart of the swine injected with CM. It is important to note that while CM autoimmunity is known to trigger EAM, CM immunization is insufficient on its own to cause cardiac tissue injury. Serial coinjections with Pertussis toxin are necessary to induce EAM in mice (7, 20, 21). It is likely that Pertussis toxin activates antigen-presenting cells and exacerbates the processing and presentation of CM peptides to autoimmune T cells and thereby promotes local inflammation and cellular infiltration in the otherwise unmanipulated mouse heart tissue. Likewise, it is firmly established that, under normal conditions, individuals do not develop autoimmune diseases, despite the presence of high numbers of potentially pathogenic autoreactive T cell in the periphery. Altogether, this suggests that, in our and other transplant models, the absence of trauma and of local inflammation in the native organ accounts for lack of infiltration by activated lymphocytes and subsequent tissue injury.
Anti-CM autoantibodies have been shown to be pathogenic and play a key role in the initiation of EAM in rodent models (22–24). The mechanisms by which antibodies directed against an intracellular protein can cause tissue damage are still unclear. It is possible that T cells recognizing CM peptides presented by MHC molecules on cardiac antigen-presenting cells cause initial tissue injury and release of soluble CM, thereby inducing an autoantibody response. Subsequently, these antibodies can form immune complexes and opsonize cardiac target cells thus exacerbating the autoimmune process. In addition, it is known that apoptosis is known to reorient intracellular proteins and allow their expression on cell surface blebs (25, 26). This phenomenon may contribute to reveal some CM epitopes for recognition by CM-specific B cells and antibodies.
In summary, our findings further support the view that autoimmune reactivity to tissue-specific antigens expressed by the transplanted graft represents an important component of the process of allograft rejection. Therefore, monitoring the level of CM-specific immunity in the blood of heart-transplanted patients may help to predict the rejection of cardiac allografts.
MATERIALS AND METHODS
Animals
The Massachusetts General Hospital miniature swine preclinical model has been previously described (27). The genotyping was controlled by strict pedigree breeding and confirmed with flow cytometric analysis using indirect allospecific antibodies. All donor-recipient pairs were matched for MHC and mismatched for minor histocompatibility antigens. The animals were 3 to 6 months of age at the time of transplantation. Animal care and procedures were performed in compliance with both the Principles of Laboratory Animal Care formulated by the National Society of Medical Research and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Purification of CM
Swine CM was purified as described by Shiverick et al. (28). The purity of preparations (>95%) was determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis. The myosin concentration was assessed spectrophotometrically using the BCA Protein Assay kit (Pierce, Rockford, IL). Myosin was dissolved in 50 mM sodium pyrophosphate and was stored at −80°C.
Cardiac Transplantation
Heterotopic heart transplantation was performed as previously described (29). Cyclosporine A, generously provided by Novartis (Hanover, NJ), was administered intravenously at 10 to 13 mg/kg per day beginning on the day of surgery (postoperative day 0) and continuing until posttransplant day 11 (29). Cardiac function was monitored by daily palpation and serial electrocardiograms and echocardiograms. The survival of cardiac allografts was analyzed using the Kaplan-Meier method, and survival curves were compared using the log-rank test.
Four control swine (C1–C4) received minor antigen-mismatched heart transplants. Four experimental swine (P1–P4) were injected subcutaneously with 2 mg purified CM emulsified in complete Freund’s adjuvant and in incomplete Freund’s adjuvant 42 days and 21 days, respectively, before receiving a minor antigen-mismatched heart transplant. A fifth control swine (C5) was injected with adjuvant alone (complete Freund’s adjuvant and incomplete Freund’s adjuvant given 42 and 21 days, respectively, before transplantation) before transplantation of a minor histocompatibility-mismatched heart. All animals were treated with cyclosporine A for 12 days after transplantation and monitored for acute and chronic rejection. The animals were tested for DTH and antibody responses against CM before and after transplantation.
Measurement of DTH Responses
DTH responses were evaluated 14 days after CM immunization by re-challenging recipients with 200 µg of purified CM in 0.1 mL phosphate-buffered saline (PBS) injected intradermally. Width of induration was measured at 48 hr after injection by blinded observers using calipers. A response of more than 10 mm of induration was scored as positive, whereas negative responses were less than 10 mm.
Histology and Immunohistochemistry
Formalin-fixed tissue was stained with hematoxylin-eosin. Acute interstitial rejection of heart allografts was scored from 1R to 3R based on the International Society for Heart and Lung Transplantation system (30).
Detection of anti-CM Antibodies by Enzyme-Linked Immunosorbent Assay
Plates were coated with 50 µL of purified CM (2 µg/mL) or PBS and incubated overnight at 4°C. The plates were washed twice with 200 µL of PBS+0.1% Tween20 and then blocked by dispensing 200 µL of PBS+0.05% Tween20 and 1% bovine serum albumin with a 1-hr incubation at room temperature. The plates were then washed three times, and swine serum at 1:10 dilution in PBS+0.1% Tween20 was serially diluted. After a 2-hr incubation at room temperature, plates were washed five more times. Rabbit anti-pig IgG (1:250) and IgM (1:500) in PBS+0.05% Tween20 and 1% bovine serum albumin were added to each well and incubated for 2 hr at room temperature. After five more washes, 50 µL streptavidin horseradish peroxidase developing solution (1:1000) was added to each well and allowed to incubate for 1 hr at room temperature and in the dark. Another five washes were performed, and hydrolysis was measured after adding 2,2′-azino-bis-[3-ethylbenzthiazoline-6-sulfonic acid] peroxidase solution to each well. Product absorbances were measured using an enzyme-linked immunosorbent assay plate reader at 405 nm (BioRad; Hercules, CA).
Proliferation Assays
A total of 4×105 peripheral blood mononuclear cells were cultured for 5 days along with purified CM protein (10 µg/mL). In all proliferation assays, 1 uCi of [3H]thymidine was added to each well and incubated for 5 hr to allow for incorporation. [3H]-incorporation was determined in triplicate samples by β-scintillation counting. Results were expressed as stimulation indices (SI), calculated as stimulation index (SI)=average count per million (cpm)±standard deviation for a responder stimulated with CM/cpm of the same responder stimulated by medium, alone. Average maximum SI of naïve responses was 1.2.
ACKNOWLEDGMENTS
The authors thank Ms. K. Stenger for her assistance in the preparation of the manuscript. They also thank Drs. G. Tocco and H. Winn for helpful discussions and Drs. T. Millington and A. Aoyama for expert review of the manuscript.
This work was supported, in part, by the National Heart Lung and Blood Institute grants RO1HL54211 and PO1HL18646 and the National Institute of Allergy and Infectious Disease grant KO2AI53103 of the National Institutes of Health.
G.R.V., H.S., and K.M.K. participated in performance of the research and data analysis; A.J.M., M.J.W., Y.I., and S.L.H. participated in performance of the research; B.R.R., J.S.A., and D.H.S. participated in research design; and G.B. and J.C.M. participated in research design, data analysis, and in the writing of the manuscript.
REFERENCES
- 1.Benichou G, Fedoseyeva EV. The contribution of peptides to T cell allorecognition and allograft rejection. Int Rev Immunol. 1996;13:231. doi: 10.3109/08830189609061750. [DOI] [PubMed] [Google Scholar]
- 2.Goulmy E. Human minor histocompatibility antigens. Curr Opin Immunol. 1996;8:75. doi: 10.1016/s0952-7915(96)80108-7. [DOI] [PubMed] [Google Scholar]
- 3.Fedoseyeva EV, Tam RC, Popov IA, et al. Induction of T cell responses to a self-antigen following allotransplantation. Transplantation. 1996;61:679. doi: 10.1097/00007890-199603150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Fedoseyeva EV, Zhang F, Orr PL, et al. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162:6836. [PubMed] [Google Scholar]
- 5.Haque MA, Mizobuchi T, Yasufuku K, et al. Evidence for immune responses to a self-antigen in lung transplantation: Role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169:1542. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- 6.Barber LD, Whitelegg A, Madrigal JA, et al. Detection of vimentin-specific autoreactive CD8+ T cells in cardiac transplant patients. Transplantation. 2004;77:1604. doi: 10.1097/01.tp.0000129068.03900.25. [DOI] [PubMed] [Google Scholar]
- 7.Neu N, Rose NR, Beisel KW, et al. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630. [PubMed] [Google Scholar]
- 8.Yasufuku K, Heidler KM, O’Donnell PW, et al. Oral tolerance induction by type V collagen downregulates lung allograft rejection. Am J Respir Cell Mol Biol. 2001;25:26. doi: 10.1165/ajrcmb.25.1.4431. [DOI] [PubMed] [Google Scholar]
- 9.Fedoseyeva EV, Kishimoto K, Rolls HK, et al. Modulation of tissue-specific immune response to cardiac myosin can prolong survival of allogeneic heart transplants. J Immunol. 2002;169:1168. doi: 10.4049/jimmunol.169.3.1168. [DOI] [PubMed] [Google Scholar]
- 10.Laguens RP, Vigliano CA, Argel MI, et al. Anti-skeletal muscle glycolipid antibodies in human heart transplantation as predictors of acute rejection: Comparison with other risk factors. Transplantation. 1998;65:1345. doi: 10.1097/00007890-199805270-00011. [DOI] [PubMed] [Google Scholar]
- 11.Latif N, Rose ML, Yacoub MH, et al. Association of pretransplantation antiheart antibodies with clinical course after heart transplantation. J Heart Lung Transplant. 1995;14(1 part 1):119. [PubMed] [Google Scholar]
- 12.Nath DS, Ilias Basha H, Tiriveedhi V, et al. Characterization of immune responses to cardiac self-antigens myosin and vimentin in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy. J Heart Lung Transplant. 2010;29:1277. doi: 10.1016/j.healun.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warraich RS, Pomerance A, Stanley A, et al. Cardiac myosin autoantibodies and acute rejection after heart transplantation in patients with dilated cardiomyopathy. Transplantation. 2000;69:1609. doi: 10.1097/00007890-200004270-00015. [DOI] [PubMed] [Google Scholar]
- 14.Valujskikh A, Fedoseyeva E, Benichou G, et al. Development of autoimmunity after skin graft rejection via an indirect alloresponse. Transplantation. 2002;73:1130. doi: 10.1097/00007890-200204150-00021. [DOI] [PubMed] [Google Scholar]
- 15.Portugal K, Dozmorov I, Sidorov I, et al. Renal transplant patients show variations in their self-reactive repertoires: A serial study. Int Immunol. 2001;13:747. doi: 10.1093/intimm/13.6.747. [DOI] [PubMed] [Google Scholar]
- 16.Kerkar N, Hadzic N, Davies ET, et al. De-novo autoimmune hepatitis after liver transplantation. Lancet. 1998;351:409. doi: 10.1016/S0140-6736(97)06478-7. [DOI] [PubMed] [Google Scholar]
- 17.Kong G, Madden B, Spyrou N, et al. Response of recurrent giant cell myocarditis in a transplanted heart to intensive immunosuppression. Eur Heart J. 1991;12:554. doi: 10.1093/oxfordjournals.eurheartj.a059938. [DOI] [PubMed] [Google Scholar]
- 18.Uchino T, Belboul A, el-Gatit A, et al. Assessment of myocardial damage by circulating cardiac myosin light chain I after heart transplantation. J Heart Lung Transplant. 1994;13:418. [PubMed] [Google Scholar]
- 19.Kuan AP, Chamberlain W, Malkiel S, et al. Genetic control of autoimmune myocarditis mediated by myosin-specific antibodies [published erratum appears in Immunogenetics 1999; 49: 595. Immunogenetics. 1999;49:79. doi: 10.1007/s002510050466. [DOI] [PubMed] [Google Scholar]
- 20.Rose NR, Neumann DA, Herskowitz A. Autoimmune myocarditis: Concepts and questions. Immunol Today. 1991;12:253. doi: 10.1016/0167-5699(91)90118-D. [DOI] [PubMed] [Google Scholar]
- 21.Smith SC, Allen PM. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. 1991;147:2141. [PubMed] [Google Scholar]
- 22.Kuan AP, Zuckier L, Liao L, et al. Immunoglobulin isotype determines pathogenicity in antibody-mediated myocarditis in naive mice. Circ Res. 2000;86:281. doi: 10.1161/01.res.86.3.281. [DOI] [PubMed] [Google Scholar]
- 23.Liao L, Sindhwani R, Rojkind M, et al. Antibody-mediated autoimmune myocarditis depends on genetically determined target organ sensitivity. J Exp Med. 1995;181:1123. doi: 10.1084/jem.181.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neu N, Beisel KW, Traystman MD, et al. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to Coxsackievirus B3-induced myocarditis. J Immunol. 1987;138:2488. [PubMed] [Google Scholar]
- 25.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clancy RM, Buyon JP. More to death than dying: Apoptosis in the pathogenesis of SSA/Ro-SSB/La-associated congenital heart block. Rheum Dis Clin North Am. 2004;30:589. doi: 10.1016/j.rdc.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Pennington LR, Lunney JK, Sachs DH. Transplantation in miniature swine. VIII. Recombination within the major histocompatibility complex of miniature swine. Transplantation. 1981;31:66. [PubMed] [Google Scholar]
- 28.Shiverick KT, Thomas LL, Alpert NR. Purification of cardiac myosin. Application to hypertrophied myocardium. Biochim Biophys Acta. 1975;393:124. doi: 10.1016/0005-2795(75)90222-6. [DOI] [PubMed] [Google Scholar]
- 29.Madsen JC, Yamada K, Allan JS, et al. Transplantation tolerance prevents cardiac allograft vasculopathy in major histocompatibility complex class I-disparate miniature swine. Transplantation. 1998;65:304. doi: 10.1097/00007890-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 30.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]