Abstract
Background
Childhood maltreatment has been linked to a variety of changes in brain structure and function and stress-responsive neurobiological systems. Epidemiological studies have documented the impact of childhood maltreatment on health and emotional well-being.
Methods
After a brief review of the neurobiology of childhood trauma, we use the Adverse Childhood Experiences (ACE) Study as an epidemiological “case example” of the convergence between epidemiologic and neurobiological evidence of the effects of childhood trauma. The ACE Study included 17,337 adult HMO members and assessed 8 adverse childhood experiences (ACEs) including abuse, witnessing domestic violence, and serious household dysfunction. We used the number of ACEs (ACE score) as a measure of cumulative childhood stress and hypothesized a “dose-response” relationship of the ACE score to 18 selected outcomes and to the total number of these outcomes (comorbidity).
Results
Based upon logistic regression analysis, the risk of every outcome in the affective, somatic, substance abuse, memory, sexual, and aggression-related domains increased in a graded fashion as the ACE score increased (P < 0.001). The mean number of comorbid outcomes tripled across the range of the ACE score.
Conclusions
The graded relationship of the ACE score to 18 different outcomes in multiple domains theoretically parallels the cumulative exposure of the developing brain to the stress response with resulting impairment in multiple brain structures and functions.
Keywords: child development, neurobiology, stress, childhood abuse, domestic violence, substance, mental health
Introduction
The organization and functional capacity of the human brain depends upon an extraordinary set and sequence of developmental and environmental experiences that influence the expression of the genome (Perry and Pollard 1998; Teicher 2000, 2002). Unfortunately, this elegant sequence is vulnerable to extreme, repetitive, or abnormal patterns of stress during critical or circumscribed periods of childhood brain development that can impair, often permanently, the activity of major neuroregulatory systems, with profound and lasting neurobehavioral consequences (Teicher 2000; Heim and Nemeroff 2001; Repetti 2002; Gutman and Nemeroff 2002; Gorman 2002; De Bellis and Thomas 2003a; Bremner and Vermetten 2001). Now, converging evidence from neurobiology and epidemiology suggests that early life stress such as abuse and related adverse experiences cause enduring brain dysfunction that, in turn, affects health and quality of life throughout the lifespan.
An expanding body of evidence from rodent, primate, and human research suggests that early stressors cause long term changes in multiple brain circuits and systems (Sanchez 2001; Bremner 2003a). The amygdala mediates fear responses, and the prefrontal cortex is involved in mood as well as emotional and cognitive responses (Bremner 2003b). The hypothalamic-pituitary-adrenal (HPA) axis plays a critical role in the stress response. There is an important interaction between development and stress, e. g., young infants do not have a fully developed glucocorticoid (cortisol in humans) response to stress, although other markers such as c-fos show that they do respond to stressors (Smith 1997). Substantial research has focused on the relationship between development, early stress, the HPA axis, and the hippocampus, a stress-sensitive brain region that plays a critical role in learning and memory (McEwen 1992; Sapolsky 1990, 1996; Gould and Tanapat 1999). The hippocampus has the capacity to grow new neurons in adulthood (neurogenesis), but stress inhibits neurogenesis (Nibuya 1995; Duman 1997; Gould 1997) and memory function (Diamond 1996; Luine 1994). Early stressors cause long-term increases in glucocorticoid responses to stress (Plotsky and Meaney 1993; Ladd 1996) as well as decreased genetic expression of cortisol receptors in the hippocampus and increased genetic expression of corticotrophin-releasing factor in the hypothalamus, both of which may contribute to dysregulation of the hypothalamic-pituitary-adrenocortical (HPA) system (Ladd 1996; Liu 1997). Early environmental deprivation inhibits hippocampal neurogenesis; conversely, neurogenesis is enhanced by enriched environment (Kempermann 1997), learning (Gould 1999a) and, at times, some antidepressant treatments (Malberg 2000; Czeh 2001). The noradrenergic/locus coeruleus system also plays a key role in stress (Bremner 1996a) and early stressors lead to long-term decreases in genetic expression of alpha-2 noradrenergic receptors in the locus coeruleus, which may lead to loss of feedback inhibition of noradrenergic activity with associated increases in noradrenergic responses to subsequent stressors (Sanchez 2001; Caldji 2000; Francis 1999). Alterations in serotonergic (Rosenblum 1994; Bennett 2002) and GABAergic (Caldji 2000) receptors also contribute to deficits in social attachment and regulation of mood and affect following early stress. Cognitive problems have also been identified in children with PTSD (Beers 2002).
Studies in clinical populations of abuse survivors with posttraumatic stress disorder (PTSD) are consistent with animal studies. Smaller hippocampal volume is found among adults with early abuse-related PTSD (Bremner 1997, 2003a; Stein 1997), adult women with early abuse and depression (Vythilingam 2002), and borderline personality disorder (Driessen 2000; Schmahl 2003) but not in children with PTSD (De Bellis 1999a, 2002; Carrion 2001) suggesting that early abuse with chronic long-term stress-related psychiatric disorder is required for this finding. Consistent with deficits in hippocampal function are deficits in verbal declarative memory (Bremner 1995) and failure of hippocampal activation with memory tasks (Bremner 2003a) in adult women with early abuse-related PTSD. Children with PTSD have smaller whole brain and corpus callosum volume (Carrion and Steiner 2000; De Bellis 2002) and alterations in structure of the cerebellum (Anderson 2002) and frontal cortex. (De Bellis and Thomas 2003b; Carrion 2001). Abused children also show alterations in EEG activity in the frontal cortex (Teicher 1994, 1997; Ito 1998). Studies in adult women with early abuse-related PTSD have shown altered function in the anterior cingulate/medial prefrontal cortex while they were remembering their childhood trauma (Bremner 1999; Shin 1999). Similar to animal studies there is evidence of dysregulation of the sympathetic nervous system in humans; early abuse and PTSD is associated with increased cortisol and norepinephrine levels in children (Carrion 2002; De Bellis 1999, Gunnar 2001), down-regulated platelet alpha-2 adrenergic receptors (Perry 1994), and increased resting heart rate (Perry 2001) while adults with early abuse and PTSD have low baseline (Bremner 2003b) and increased stress-induced cortisol responses (Elzinga 2003; Bremner 2003c) and increased norepinephrine at baseline (Lemieux and Coe 1995; El-Sheikh 2001). Women with early abuse and depression also have increased cortisol reactivity to stress (Heim 2000, 2001).
Deprivation of developmentally appropriate experience may reduce neuronal activity, resulting in a generalized decrease in neurotrophin production, synaptic connectivity, and neuronal survival (Gould and Tanapat 1999; Nibuya 1995; Duman 1997; Gould 1997) resulting in profound abnormalities in brain organization and structure (Perry 2002; Read 2001). Thus, childhood abuse and exposure to domestic violence can lead to numerous differences in the structure and physiology of the brain that expectedly would affect multiple human functions and behaviors (Perry and Pollard 1998; Teicher 2000, 2002).
Numerous studies have established that childhood stressors such as abuse or witnessing domestic violence can lead to a variety of negative health outcomes and behaviors, such as substance abuse, suicide attempts, and depressive disorders (Brodsky 1997; Kingree 1999; van der Kolk 1991; Kendall-Tackett 1993; Osofsky 1999; Hefferman 2000; Kendler 2000; Putnam 2003; Rohsenow 1988). This paper presents a conceptual framework that integrates findings from recent studies of the neurobiological effects of childhood abuse and exposure to domestic violence on brain structure and function (as reviewed above) with epidemiologic data from the Adverse Childhood Experiences (ACE) Study. Although the literature about the effects of childhood maltreatment is extensive (Bremner 2000, 2003a, 2003b; Kendall-Tackett 1993), we use the data and findings from the ACE Study as series of epidemiologic “case examples” in this paper because it simultaneously assessed a wide range of interrelated adverse experiences including abuse (emotional, physical, or sexual); witnessing domestic violence; parental marital discord; growing up with mentally ill, substance abusing, or criminal household members (Dong 2003a; Dube 2004a, 2002b) whereas most prior studies have focused on single forms of abuse. In addition, the ACE Study assessed numerous social, behavioral, and health outcomes (Anda 1999, 2001, 2002a, 2002b; Dube 1999, 2002a, 2003a, 2003b; Felitti 1998; Dietz 1999; Hillis 2000, 2001, 2004; Dong, 2003b; Edwards 2003a, 2003b; Chapman 2004;Whitfield 2003a) that would necessarily involve the performance of multiple brain functions and neuroregulatory systems. These aspects of the study design along with a large sample size allow for the illustration of how the effects of multiple forms of abuse and related stressors are cumulative and affect a wide variety of outcomes that might be expected based upon the neurobiological alterations reviewed above.
We used data from the ACE Study to test the following hypotheses, which have their basis in the neurosciences:
-
●
The damaging effects of adverse childhood experiences (ACEs) would be nonspecific, thereby affecting a variety of functions and behaviors, because abuse/traumatic stress affect a variety of brain structures and functions.
-
●
The likelihood of disturbances in any given function or behavior such as anxiety, sleep disturbances, substance abuse, sexuality, and hyperarousal or aggression would have a cumulative or “dose-response” relationship to the number of ACEs, theoretically paralleling the total exposure of the developing central nervous system to the activated stress response during childhood.
-
●
The number of comorbidities (Lilienfeld 2003) (mean number of human behaviors and functions affected), which theoretically parallels the number of brain systems and associated functions affected, would also have a dose-response relationship to the number of ACEs.
Methods
The ACE Study is an ongoing collaboration between Kaiser Permanente’s Health Appraisal Center (HAC) in San Diego, California, and the U. S. Centers for Disease Control and Prevention. The objective is to assess the impact of numerous, interrelated, ACEs on a wide variety of health behaviors and outcomes and on health care utilization and the methods of the study have been described in detail elsewhere. (Anda 1999; Dube 1999; Felitti 1998).
The study population was drawn from the HAC, which provides preventive health evaluations to adult members of Kaiser Health Plan in San Diego County. All persons evaluated at the HAC complete a standardized questionnaire, which includes health histories and health-related behaviors, a medical review of systems, and psychosocial evaluations which are a part of the ACE Study database.
Two weeks after their evaluation, each person evaluated at the HAC between August 1995 and March 1996 (survey wave 1; response rate 70 %) and June and October 1997 (survey wave 2; response rate 65%) received the ACE Study questionnaire by mail. The questionnaire collected detailed information about ACEs including abuse, witnessing domestic violence, and serious household dysfunction as well as health-related behaviors from adolescence to adulthood. Wave 2 respondents were asked detailed questions about health topics that analysis of wave 1 data had shown to be important (Anda 2003a; Felitti 1998; Dube 2003a; Dong 2003b). The response rate for both survey waves combined was 68%, for a total of 18175 responses.
We excluded 754 respondents who coincidentally underwent examinations during the time frames for both survey waves, leaving an unduplicated total of 17421. After exclusion of 84 respondents with missing demographic information, the final sample included 95% of the respondents (17337/18175); (wave I=8 708, wave II=8 629).
Definitions of Adverse Childhood Experiences (ACEs)
Questions used to define ACEs are listed in Table 1. All questions about ACEs pertained to the respondents’ first 18 years of life (≤ 18 years of age). For questions adapted from the Conflict Tactics Scale (CTS) (Strauss and Gelles 1990) there were 5 response categories: “never”,“once or twice”,“sometimes”,“often”, or “very often”. We defined 3 types of childhood abuse: emotional abuse (2 questions), physical abuse (2 questions), or contact sexual abuse (4 questions) by Wyatt (1985). We defined 5 exposures to household dysfunction during childhood: exposure to alcohol or other substance abuse (defined by 2 questions) (Schoenborn 1991), mental illness (2 questions), violent treatment of mother or stepmother (4 questions) (Strauss 1990), criminal behavior in the household (1 question), and parental separation or divorce (1 question). Respondents were defined as exposed to a category if they responded “yes”to 1 or more of the questions. Despite the sensitivity of these questions, the test-retest reliability for every ACE and the ACE score were in the good to excellent range (range of Cohen’s kappa: 0.46–0.86) (Dube 2004). Furthermore, a comparison of respondents and nonrespondents to the ACE Study questionnaire found no evidence of response rate bias or that respondents were biased toward attributing their health problems to childhood experiences (Edwards and Anda 2001).
Table 1.
Definition and prevalence of each category of adverse childhood experience and the ACE score
| Childhood abuse | Total N = 17,337 |
|---|---|
| Emotional abuse | 10.6 |
(Did a parent or other adult in the household…)
|
|
| Physical | 28.3 |
(Did a parent or other adult in the household…)
|
|
| Sexual | 20.7 |
(Did an adult or person at least 5 years older ever…)
|
|
| Household dysfunction | |
| Substance abuse | 26.9 |
|
|
| Mental illness | 19.4 |
|
|
| Mother treated violently | 12.7 |
(Was your mother (or stepmother)):
|
|
| Incarcerated household member | 4.7 |
|
|
| Parental separation or divorce | 23.3 |
|
|
| Number of adverse childhood experiences (ACE score) | |
| 0 | 36.1 |
| 1 | 26.0 |
| 2 | 15.9 |
| 3 | 9.5 |
| ≥4 | 12.5 |
The number of ACEs (range: 0–8) was summed to create the ACE scores, with scores of 4 or more included as one category (≥ 4). Analyses were conducted treating the ACE score as 4 dichotomous variables (yes or no for scores of ≥ 4, 3, 2, and 1) with a score of 0 (no ACEs) as the referent.
Epidemiological evidence of disordered brain function in adulthood
The data and definitions used for the outcomes that provide evidence of disordered function were selected on an a priori basis using a general framework of health and social problems that likely represent dysfunction of specific brain systems and/or improper integration between systems. We recognize that functional neuroanatomical and physiologic systems are interactive and integrated and that behaviors and health problems cannot generally be attributed to the function of any single or particular system.
To define the health-related behaviors or problem sources, we used information from the medical review of systems (ROS), the physical examination (PE), and the ACE Study questionnaire (ACEQ). In the definitions of these problems that follow, the source of the data is in parentheses.
Mental health disturbances
Panic reactions (ROS)
A “yes” response to the question: “Have you had or do you now have special circumstances in which you find yourself panicked?”
Depressed affect (ROS)
A “yes”to the question,“Have you had or do you now have depression or feel down in the dumps?”
Anxiety (ROS)
A “yes” to the question,“Do you have much trouble with nervousness?”
Hallucination (ROS)
A “yes” response to the question, “Have you ever had or do you have hallucinations (seen, smelled, or heard things that weren’t really there)?”
Somatic disturbances
Sleep disturbance (ROS)
A “yes” to “Do you have trouble falling asleep or staying asleep” or a “yes” to “Tiredness, even after a good night’s sleep?”
Severe obesity (PE)
Body mass index (kg/m2) ≥ 35.
Multiple somatic symptoms (ROS)
A total of 6 or more somatic symptoms in at least 2 different organ systems in the absence of a diagnosis specific to those systems.
Substance abuse
Current Smoking–Nicotine (ACEQ)
A “yes” to the question, “Do you currently smoke cigarettes?”
Self-reported alcoholic (ACEQ)
A “yes” to the question, “Have you ever considered yourself to be an alcoholic?”
Ever used illicit drugs (ACEQ)
A “yes”to the question,“Have you ever used street drugs?”
Injected drug use (ACEQ)
A “yes” to the question,“Have you ever injected street drugs?”
Impaired memory of childhood
Impaired memory of childhood (ACEQ)
A “yes” to the question, “Are there large parts of your childhood after age 4 that you can’t remember?”
Number of age periods affected (ACEQ)
Those who responded “yes” to the previous were asked to check boxes indicating age periods (in years) of impaired memory (4–6, 7–9, 10–12, 13–15, and 16–18). We summed the number of boxes checked to assess the relationship of the ACE score to the mean number of age periods affected. Information about impaired memory was available only for the wave 1 (N = 8708).
Sexuality
Early intercourse (ACEQ)
Age at first intercourse of 14 years or younger.
Promiscuity (ACEQ)
Lifetime sexual partners ≥ 30 (approximately the 90th percentile for males and the 95th percentile for females).
Sexual dissatisfaction (ROS)
A “no” to the question: “Are you currently satisfied with your sex life?”
Perceived stress, anger control, and risk of intimate partner violence
High level of perceived stress (ROS)
A response indicating “high” to the instruction,“Please fill in the circle that best describes your stress level (high, medium, low).”
Difficulty controlling anger (ROS)
A “yes” to the question, “Do you have or have you had reason to fear your anger getting out of control?”
Risk of perpetrating intimate partner violence (ROS)
A “yes” to the question, “Have you ever threatened, pushed, or shoved your partner?” Data about the risk of perpetrating intimate partner violence was available only for wave 2 (N = 8629).
Number of comorbid outcomes
We summed the number of outcomes (range: 0–18) for each respondent to quantitate the amount of comorbidity (mean number of disordered functions) associated with increasing ACE scores.
Statistical analysis
Adjusted odds ratios (OR) and 95% confidence intervals (CI) were obtained from logistic regression models using The SAS System Version 8.2, which assessed the associations between the ACE score (0, 1, 2, 3, or ≥ 4) and each of the 18 outcome measures. We used multiple linear regression to estimate the number of comorbid outcomes by ACE score. Covariates in all multivariate models included age, sex, race (other versus white), and education (high school diploma, some college, or college graduate versus less than high school).
Results
The final study sample included 9367 (54%) women and 7970 (46%) men. The mean age was 56 years for women and 58 years for men. Seventy-three percent of women and 76% of men were white; 34% of women and 45% of men were college graduates, and another 37 % and 34%, respectively had some college education.
Prevalence of the adverse childhood experiences
At least 1 ACE was reported by 64% of respondents. The prevalence of each ACE is shown in Table 1.
ACE score and the risk of health and behavioral outcomes
The ACE score had a strong, graded relationship to the prevalence and risk (adjusted OR) of affective disturbances (P < 0.001; Table 2, mental health disturbances). For persons with ≥ 4 ACEs, the risk of panic reactions, depressed affect, anxiety, and hallucinations were increased 2.5-, 3.6-, 2.4 and 2.7-fold, respectively (Table 2).
Table 2.
Relationship of the ACE score to the prevalence and relative risk (adjusted odds ratio)* of disturbances in two major domains of dysfunction: mental and somatic health disturbances
| Mental health disturbances | Somatic health disturbances | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Panic reactions | Depressed affect | Anxiety | Hallucinations | Sleep disturbance | Severe obesity | Multiple somatic symptoms | |||||||||
| ACE score |
(N) | % | Adjusted odds ratio |
% | Adjusted odds ratio |
% | Adjusted odds ratio |
% | Adjusted odds ratio |
% | Adjusted odds ratio |
% | Adjusted odds ratio |
% | Adjusted odds ratio |
| 0 | (6255) | 8.3 | 1.0 (referent) | 18.4 | 1.0 (referent) | 7.8 | 1.0 (referent) | 1.3 | 1.0 (referent) | 36.3 | 1.0 (referent) | 5.6 | 1.0 (referent) | 5.1 | 1.0 (referent) |
| 1 | (4514) | 10.9 | 1.3 (1.2–1.5) | 25.2 | 1.5 (1.3–1.6) | 9.1 | 1.2 (1.1–1.4) | 1.5 | 1.1 (0.8–1.5) | 41.6 | 1.2 (1.1–1.3) | 7.4 | 1.3 (1.1–1.5) | 6.4 | 1.3 (1.1–1.5) |
| 2 | (2758) | 13.6 | 1.7 (1.4–1.9) | 34.1 | 2.2 (2.0–2.4) | 12.4 | 1.7 (1.4–1.9) | 2.3 | 1.6 (1.2–2.3) | 47.5 | 1.6 (1.4–1.7) | 8.3 | 1.4 (1.2–1.7) | 8.6 | 1.8 (1.5–2.1) |
| 3 | (1650) | 16.8 | 2.0 (1.7–2.4) | 38.8 | 2.5 (2.2–2.8) | 14.1 | 1.8 (1.6–2.2) | 2.9 | 2.0 (1.4–2.9) | 51.1 | 1.8 (1.6–2.0) | 8.8 | 1.5 (1.2–1.8) | 8.1 | 1.6 (1.3–2.0) |
| ≥4 | (2160) | 20.9 | 2.5 (2.2–2.9) | 49.0 | 3.6 (3.2–4.0) | 19.0 | 2.4 (2.1–2.8) | 4.0 | 2.7 (1.9–3.7) | 56.1 | 2.1 (1.9–2.4) | 11.9 | 1.9 (1.6–2.2) | 13.9 | 2.7 (2.3–3.2) |
| Total | (17337) | 12.2 | – | 28.4 | – | 10.9 | – | 2.0 | – | 43.3 | – | 7.6 | – | 7.4 | – |
All odds ratios are adjusted for age, sex, race, and educational attainment using logistic regression
The ACE score also had a graded relationship to the prevalence and risk (adjusted OR) each of the somatic disturbances (P < 0.001; Table 2, somatic health disturbances). The risk of sleep disturbance, severe obesity, and multiple somatic symptoms were increased 2.1-, 1.9-, and 2.7-fold, respectively, for persons with 4 or more ACEs.
Substance use and abuse also increased as the ACE score increased. The risk of smoking, alcoholism, illicit drug use, and injected drug use were increased 1.8-, 7.2-, 4.5-, and 11.1-fold, respectively, for persons with ≥ 4 ACEs (Table 3, substance abuse).
Table 3.
Relationship of the ACE score to the prevalence and relative risk (adjusted odds ratio)* of disturbances in two domains: substance abuse and sexuality
| Substance abuse | Sexuality | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smoking | Alcoholism | Illicit drug use | Injected drug use | Early intercourse | Promiscuity (≥30 partners) | Sexual dissatisfaction | |||||||||||
| ACE score |
(N) | % | Adjusted odds ratio |
% | Adjusted odds ratio |
% | Adjusted odds ratio |
% | Adjusted odds ratio |
% | Adjusted odds ratio |
% | Adjusted odds ratio |
% | Adjusted odds ratio |
||
| 0 | (6255) | 6.5 | 1.0 (referent) | 2.5 | 1.0 (referent) | 7.9 | 1.0 (referent) | 0.2 | 1.0 (referent) | 2.3 | 1.0 (referent) | 3.9 | 1.0 (referent) | 23.0 | 1.0 (referent) | ||
| 1 | (4514) | 7.6 | 1.1 (0.94–1.3) | 5.1 | 2.0 (1.6–2.4) | 13.8 | 1.6 (1.4–1.8) | 0.6 | 2.3 (1.2–4.4) | 5.1 | 2.1 (1.7–2.6) | 5.2 | 1.3 (1.1–1.5) | 24.8 | 1.1 (1.1–1.2) | ||
| 2 | (2758) | 9.3 | 1.3 (1.1–1.5) | 7.4 | 2.9 (2.4–3.6) | 20.0 | 2.2 (1.9–2.6) | 1.4 | 4.5 (2.4–8.4) | 6.6 | 2.7 (2.2–3.4) | 7.4 | 1.9 (1.5–2.3) | 28.9 | 1.5 (1.3–1.6) | ||
| 3 | (1650) | 11.9 | 1.6 (1.3–1.9) | 10.5 | 4.5 (3.6–5.6) | 24.9 | 2.9 (2.5–3.4) | 1.6 | 5.3 (2.7–10.2) | 8.5 | 3.7 (2.9–4.7) | 8.7 | 2.5 (2.0–3.0) | 28.1 | 1.5 (1.3–1.7) | ||
| ≥4 | (2160) | 14.5 | 1.8 (1.5–2.1) | 15.3 | 7.2 (5.9–8.9) | 35.2 | 4.5 (3.9–5.2) | 3.7 | 11.1 (6.2–19.9) | 14.2 | 6.6 (5.3–8.2) | 10.8 | 3.6 (3.0–4.4) | 32.3 | 2.0 (1.8–2.2) | ||
| Total | (17337) | 8.8 | – | 6.3 | – | 16.5 | – | 1.1 | – | 5.8 | – | 6.1 | – | 26.0 | – | ||
All odds ratios are adjusted for age, sex, race, and educational attainment using logistic regression
Similarly, all three measures of sexuality were associated with the ACE score (Table 3, sexuality). The risk of early intercourse, promiscuity, and sexual dissatisfaction were increased 6.6-, 3.6-, and 2-fold, respectively, for persons with ≥ 4 ACEs (Table 3).
The risk of impaired memory of childhood was increased 4.4-fold for persons with ≥ 4 ACEs (Table 4). The number of age periods affected for memory disturbances increased in a graded fashion as the ACE score increased (P < 0.0001; Table 4).
Table 4.
Relationship of the ACE score to the prevalence and relative risk (adjusted odds ratio)* of problems with memory impairment for childhood and to the mean number of age periods affected
| Prevalence and risk of memory impairment |
Number of age periods affected** |
|||
|---|---|---|---|---|
| ACE score | (N)*** | % | Adjusted odds ratio | Mean** (SD) |
| 0 | (3202) | 9.7 | 1.0 (referent) | 0.19 (0.02) |
| 1 | (2246) | 12.0 | 1.3 (1.1–1.5) | 0.23 (0.02) |
| 2 | (1379) | 18.9 | 2.1 (1.8–2.6) | 0.35 (0.02) |
| 3 | (834) | 22.1 | 2.6 (2.1–3.1) | 0.40 (0.03) |
| ≥4 | (1047) | 34.0 | 4.4 (3.7–5.2) | 0.69 (0.03) |
| Total | (8708) | 15.8 | – | – |
All odds ratios are adjusted for age, sex, race, and educational attainment using logistic regression;
The mean number of age periods affected was adjusted for the same demographic variables using linear regression;
The sample size is 8708 because data about memory impairment were available for the wave 1 survey only
High perceived stress, difficulty controlling anger, and the risk of perpetrating intimate partner violence (IPV) were increased 2.2-, 4.0-, and 5.5-fold, respectively, for persons with ≥ 4 ACEs (Table 5). We found (data not shown) that the adjusted odds ratio (95 % CI) for the relationship between difficulty controlling anger and the risk of perpetrating IPV were 6.3 (4.4–9.0) for men and 7.6 (5.3–11.1) for women (P < 0.001). Similarly (data not shown), the adjusted odds ratio (95 % CI) for the relationship between perceived high stress and the risk of perpetrating IPV was the same for both men and women: 1.8 (1.4–2.3), (P < 0.001).
Table 5.
Relationship of the ACE score to the prevalence and relative risk (adjusted odds ratio)* of high perceived stress, difficulty controlling anger, and risk of perpetrating intimate partner violence during adulthood
| High level of perceived stress |
Difficulty controlling anger |
Risk of perpetrating intimate partner violence |
||||||
|---|---|---|---|---|---|---|---|---|
| ACE score | (N) | % | Adjusted odds ratio** |
% | Adjusted odds ratio** |
(N)* | % | Adjusted odds ratio** |
| 0 | (6255) | 10.5 | 1.0 (referent) | 3.5 | 1.0 (referent) | (3053) | 1.6 | 1.0 (referent) |
| 1 | (4514) | 13.5 | 1.2 (1.1–1.4) | 4.9 | 1.4 (1.1–1.7) | (2268) | 3.0 | 1.8 (1.2–2.6) |
| 2 | (2758) | 16.0 | 1.4 (1.3–1.6) | 8.0 | 2.2 (1.8–2.7) | (1379) | 4.0 | 2.4 (1.6–3.5) |
| 3 | (1650) | 17.8 | 1.5 (1.3–1.8) | 8.5 | 2.3 (1.9–2.9) | (816) | 5.4 | 3.3 (2.1–5.0) |
| ≥4 | (2160) | 24.7 | 2.2 (1.9–2.5) | 14.4 | 4.0 (3.3–4.8) | (1113) | 8.8 | 5.5 (3.8–7.8) |
| Total | (17337) | 14.6 | – | 6.4 | – | (8629) | 3.6 | – |
All odds ratios are adjusted for age, sex, race, and educational attainment using logistic regression. The adjusted odds ratio (95 % CI) for the relationship between difficulty controlling anger and the risk of perpetrating IPV were: 6.3 (4.4–9.0) for men; 7.6 (5.3–11.1) for women. The adjusted odds ratio (95 % CI) for the relationship between high perceived stress and the risk of perpetrating IPV was the same for both men and women: 1.8 (1.4–2.3).
The sample size is 8629 because data about memory impairment were available for the wave 2 survey only
ACE score and number of comorbid outcomes
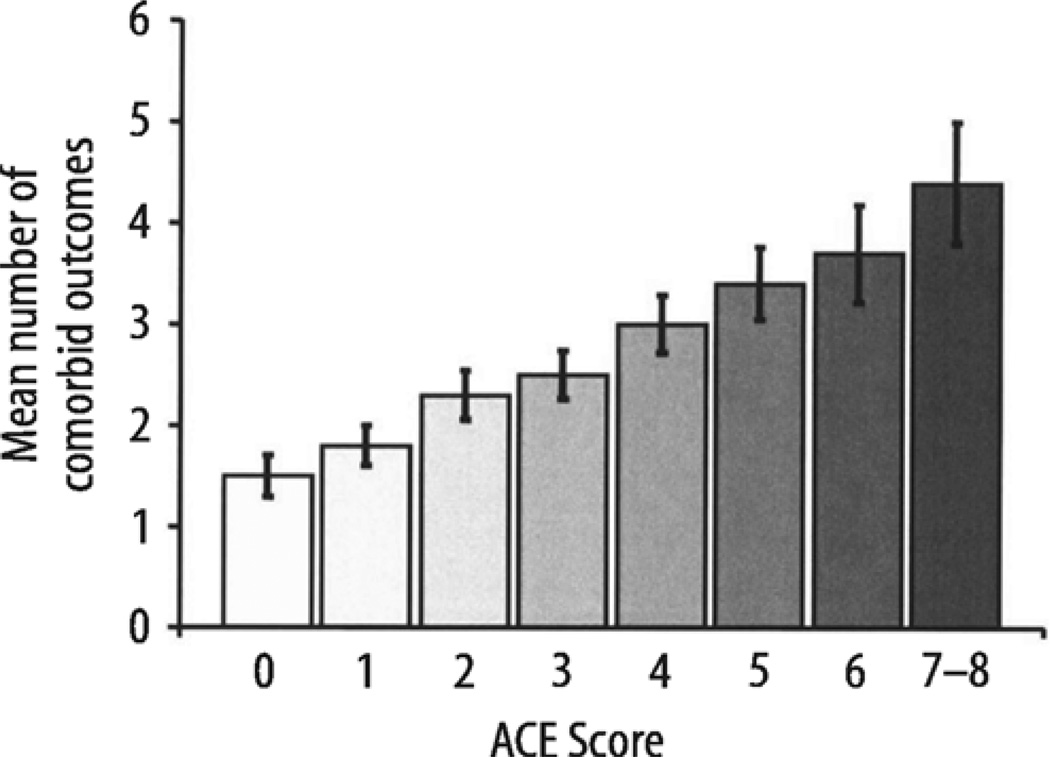
As the ACE score increased, the mean number of comorbid outcomes increased in a graded fashion (Fig. 1), nearly tripling between ACE scores of 0 and ACE scores of 7–8.
Fig. 1.
The mean number of comorbid outcomes in the study sample was 2.1 (range: 0–14); means are adjusted for age, sex, race, and educational attainment. The trend in the means is significant (P < 0.0001); vertical error bars represent 95% confidence intervals
Discussion
These epidemiological findings converge with evidence from neurobiology about numerous effects of childhood stress on brain and physical systems (Glaser 2000). Extreme, traumatic or repetitive childhood stressors such as abuse, witnessing or being the victim of domestic violence, and related types of ACES are common, tend to be kept secret, and go unrecognized by the outside world. Likewise, the fight-or-flight response among children exposed to these types of stressors, and the attendant release of endogenous catecholamines and adrenal corticosteroids are both uncontrollable and invisible (Perry 1998; Teicher 2002; De Bellis 1994, 1997; Scaer 2001). Furthermore, the detrimental effects of traumatic stress on developing neural networks and on the neuroendocrine systems that regulate them have until recently remained hidden even to the eyes of most neuroscientists. However, the information and data that we present herein suggest that this veiled cascade of events represents a common pathway to a variety of important long-term behavioral, health, and social problems (Table 6).
Table 6.
Summary of the convergence between neurobiological effects of childhood maltreatment with ACE study epidemiological findings
| Area of function or dysfunction studied | Demonstrated neurobiological defects from early trauma | ACE study findings |
|---|---|---|
| Anxiety, panic, depressed affect, hallucinations, and substance abuse | Repeated stress & childhood trauma → hippocampus, amygdala & medial prefrontal cortex atrophy and dysfunction that mediate anxiety & mood problems |
Tables 2 and 3 Unexplained panic, depression, anxiety, hallucinations & alcohol & other drug problems |
| Smoking, alcoholism, illicit drug use, injected drug use | Repeated stress & childhood trauma → Increased locuscoeruleus & norepinephrine activity, decreased by heroin & alcohol |
Table 3 Increased smoking, alcohol and other drug use |
| Early intercourse, promiscuity, sexual dissatisfaction, perpetration of intimate partner violence | Repeated stress & childhood trauma → amygdala defects; role in sexual & aggressive behavior and deficits in oxytocin with impaired pair bonding |
Tables 3 and 5 Risky sexual behavior, anger control, risk for aggression against intimate partners |
| Memory storage and retrieval | Hippocampus role in memory storage and retrieval; hippocampal & amygdala size reduction in childhood trauma; deficits in memory function |
Table 4 Impaired memory of childhood and number age periods affected increases as the ACE score increase |
| Body weight and obesity | Repeated stress & distress, via glucocorticoid pathways, leads to increased intra-abdominal & other fat deposits |
Table 2 Increased obesity |
| Sleep, multiple somatic symptoms, high perceived stress | Repeated stress & distress, via several pathways, leads to increase in other physical problems |
Tables 2 and 5 Increased somatic symptoms and disorders, including sleep problems |
| Co-morbidity/Trauma spectrum disorders | Multiple brain and nervous system structure and function defects, including monoamine neurotransmitter systems |
Fig. 1 The graded relationship of the ACE score to psychiatric and physical symptoms or disorders, including multiple co-occurring problems (comorbidity) |
The convergence of evidence from neurobiology and epidemiology calls for an integrated perspective on the origins of health and social problems throughout the lifespan. This constellation of effects from childhood stressors calls to mind the wisdom of Occam’s razor, a celebrated dictum in medicine, which holds that if a single unifying explanation can be found for multiple symptoms and problems, then it is likely that the correct explanation lies in the simplest account (Lo Re and Bellini 2002). In the context of what we present herein, the application of this dictum has the potential to unify and improve our understanding of many seemingly unrelated, but often co-morbid health and social problems that have historically been seen and treated as categorically independent in Western culture.
Certain neurobiological findings are especially congruent with the data from the ACE Study reported herein (Table 6). Magnetic resonance imaging (MRI) has revealed reductions in hippocampus (Bremner 1997, 2003a; Stein 1997), and amygdala (Driessen 2000; Schmahl 2003) volumes as well as deficits in verbal declarative memory measured with neuropsychological testing (Teicher 2000; Heim and Nemeroff 2001) among women who were sexually abused as children. The hippocampus plays a role in memory storage and retrieval; we found that impaired memory of childhood increases as the ACE score increases. Neurobiological evidence supports the hypothesis of dysfunction in hippocampus, amygdala, medial prefrontal cortex, and other limbic structures believed to mediate anxiety and mood dysregulation following early abuse (Teicher 2002). We, in turn, demonstrated a graded relationship of the ACE score to affective symptoms and unexplained periods of panic among our study participants. We found that a history of hallucinations increases as the ACE score increases; these symptoms may be related to alterations in hippocampal and/or prefrontal cortical function. The amygdala plays a critical role in fear responses and probably sexual and aggressive behaviors (Pinchus and Tucker 1978) and in the current study we show strong relationships of the ACE score to sexual behaviors, poor anger control, and the risk for perpetrating intimate partner violence.
The current study adds support for numerous effects of childhood adverse experiences on physical health. Stress is known from animal studies to be associated with a broad range of effects on physical health, including cardiovascular disease, hypertension, hyperlipidemia, asthma, metabolic abnormalities, obesity, infection, and other physical disorders (Musselman 1998; Kaplan 1982; Rozanski, McEwen and Stellar 1993; Anda 1993). Findings of increased obesity as the ACE score increases in this study and reported elsewhere (Williamson 2002) are consistent with animal studies showing that stress, acting through the effects of glucocorticoids on the glucocorticoid receptor on intra-abdominal adipocytes, leads to increased intra-abdominal fat which carries its own independent mortality risk.
We found a strong relationship between early adverse experience and substance use and abuse (illicit drugs, alcohol, and nicotine) later in life. Studies in animals show that early stressors lead to increased activity of the locus coeruleus with resultant increased release of nor-epinephrine in the brain (Abercrombie and Jacobs 1987). Substances such as heroin and alcohol decrease firing of the locus coeruleus, while substance withdrawal has the opposite effect (Bermner 1996). Consistent with this, the onset of substance abuse corresponds to the time of traumatization in PTSD patients, and these patients report that heroin and alcohol decrease symptoms of PTSD (Bremner 1996b). Stress also results in altered release of dopamine in the nucleus accumbens (striatum), the primary reward system within the brain (Deutch and Roth 1990). Smoking causes release of dopamine in this area, which is felt to underlie the addictive properties of nicotine (Volkow 2003). Early adverse experiences may disrupt this dopamine circuit, leading to increased risk of smoking, with its attendant negative health consequences. In summary, findings from animal studies provide a physiological rationale for how early stress can be associated with substance abuse and smoking in later life.
Another interesting finding is the relationship between ACE score and sexuality (early intercourse, promiscuity, sexual dissatisfaction) in adulthood. Animal studies show that early stressors result in long-term changes in peptides such as oxytocin that regulate pair bonding and social attachment (Insel and Winslow 1998; Francis 2002). Early adverse experiences may disrupt the ability to form long-term attachments in adulthood. The unsuccessful search for attachment may lead to sexual relations with multiple partners, with resultant promiscuity and other issues related to sexuality.
The monoamine neurotransmitter systems (norepinephrine, dopamine, serotonin) (Valentsein 1998) act within a primary regulatory system of large neural networks; these monoamine systems help to orchestrate complex neural functions. Their ubiquitous patterns of connectivity originate in the lower regions of the brain and send projections throughout the brain; in addition, they receive input from the autonomic nervous system and peripheral sensory apparatus (Foote 1983). In young animals, experimental manipulation of these systems can create behaviors similar to those seen in abuse victims, including aggression, eating problems, alcohol use, stress-response dysfunction, hyper-reactivity, anergy, and many other behavioral problems. A similar situation exists in humans in whom monoamine dysfunction has been hypothesized in a host of neuropsychiatric syndromes, including aggressive and violent behavior, suicidality, alcoholism, substance abuse and dependence, depression, anxiety disorders, and social/relational problems. We know from several studies that the functioning of these monoamine systems in adults is influenced by childhood experiences (De Bellis 1999b; Whitfield 2003b). In addition, a recent study of a polymorphism for the promoter region of the serotonin transporter (5-HTT) gene found that childhood maltreatment increased the risk of depression in early adulthood for persons with the common “short” allele compared to persons with the long allele; the short allele is associated with lower transcriptional efficiency of the promoter (Caspi 2003). Not surprisingly, many currently prescribed psychoactive drugs act by altering the dynamics of these monoamine systems. In some circumstances, the effects of these drugs may have caused an 182 oversight of the important distinction between understanding intermediary mechanisms (alterations in monoamine neurotransmitter systems) and recognizing the underlying causes of these alterations (childhood traumatic stress).
Numerous studies have shown that early abuse survivors have multiple overlapping psychiatric disorders (Kessler 1995) which have been described as “comorbidity”. The term comorbidity, however, can imply that these represent unique disorders with distinct etiologies (Lillienfeld 2003). An alternative explanation is that several disorders (e. g., depression, PTSD, dissociative disorders, substance abuse, borderline personality disorder) have to varying degrees a common etiology and are modulated by genetics (Caspi 2003) and repeated exposure to stress such as childhood maltreatment. Indeed, the term “trauma spectrum disorders” has been used to describe these overlapping conditions (Bremner 2003b). In addition, the artificial distinction between psychiatric and physical disorders has represented an impediment to the effective treatment of the numerous problems among survivors of childhood maltreatment. Epidemiological findings are consistent with a need to develop more broad based approaches to addressing the wide spectrum of effects of childhood maltreatment (Fig. 1).
There are several potential limitations with retrospective reporting of childhood experiences and self-reporting of the outcome measures. For example, respondents may have had difficulty recalling certain childhood events (Edwards 2001) or may choose not to disclose certain experiences or personal behaviors. Longitudinal follow-up of adults whose childhood abuse was documented has shown that their retrospective reports of childhood abuse are likely to underestimate actual occurrence (DellaFemina 1990; Williams 1995). Interestingly, evidence of the effects of traumatic stress in childhood on the hippocampus provides a neurophysiologic explanation for this phenomenon. Difficulty recalling childhood events likely results in misclassification (classifying persons truly exposed to ACEs as unexposed) that would bias our results toward the null (Rothman and Greenland 1998). Thus, this potential weakness probably resulted in underestimates of the true strength of the relationships between ACEs and the 18 outcomes we examined.
The historical mind-body dichotomy that persists in traditional Western medical training points medical researchers and clinicians away from risk factors that may be judged psychosocial. Thus, the original traumatic pathophysiological insults may be “silent” until much later in life (Brown 2001; Putnam 1998), when they are likely to be overlooked by investigators and clinicians who are understandably prone to focus on proximate determinants of human well-being. This leads to treatment of symptoms without a full understanding of their potential origins in the disruptive effects of ACEs on childhood neurodevelopment.
The argument for a causal relationship between ACEs and a variety of outcomes is strengthened by the combined evidence from neurobiology and epidemiology. This argument is important because evidence of causation affects decisions about prognosis, diagnosis, and treatment and can enhance understanding of the role of the childhood stressors on the developing brain in producing changes in affect, behavior, and cognition (Putnam 1998).
We summarize the application of Sir Bradford Hill’s 9 criteria for establishing an argument for causation (van Reekum 2001) in the context of this converging evidence:
-
●
Demonstration of a strong association between the causative agent and the outcome. The strength of the relationship between ACEs and numerous outcomes is consistently strong as reported herein.
-
●
Consistency of findings across research sites and methods. Numerous studies in different study populations and measures of abuse, neglect, and related experiences have shown relationships of ACEs to a variety of symptoms and behaviors.
-
●
Specificity. In the context of the converging evidence from epidemiology and neurobiology, specificity is lacking, but this in no way detracts from the argument of causation. The ACE score is a combined score representing cumulative stress and was not designed to provide evidence of specificity. Moreover, ACEs would be expected to be associated with multiple outcomes because of their effects on a variety of brain structures and functions.
-
●
Temporal sequence. Most of the outcomes presented herein occurred during adulthood; the exposures (childhood experiences) clearly antedate the outcomes in these cases.
-
●
Biological gradient. The “dose-response” relationship between the number of ACEs and each of the outcomes (as well as the number of comorbid outcomes) is strong and graded. This is consistent with cumulative effects of childhood stress on the developing brain.
-
●
Biological plausibility. The strength of the convergence between epidemiology and neurobiology is most evident here. Recent studies from the neurosciences show that childhood stress can affect numerous brain structures and functions providing convincing biologic plausibility for the epidemiologic findings.
-
●
Coherence.“The term coherence implies that a cause and effect interpretation for an association does not conflict with what is known about the natural history and biology of the disease (Rothman 1998).” In fact, recent research shows that childhood maltreatment interacts with a common functional polymorphism in the promoter region of the serotonin transporter 5-HTT, resulting in higher risk of depression and suicidality (Caspi 2003), both of which are associated with the ACE score. This information is consistent with an effect of early maltreatment on monoamine pathways known to be involved in depressive disorders.
-
●
Experimental evidence. This is the most persuasive evidence, but for ethical reasons randomized experiments depend on animal studies. Evidence from studies in rodents and primates show that stressful exposures induce neuroanatomical and neurophysiologic differences as well as aggression and drug seeking behaviors.
-
●
Analogous evidence. A widely acknowledged analogy for an exposure causing a multitude of outcomes (as seen with ACEs, including a dose-response relationship) is the causal relationship of cigarette smoking to cardiovascular diseases, neoplasms, lung disease, and other health problems (CDC, 2002).
In conclusion, there is a striking convergence of recent findings from the neurosciences with those from a large epidemiologic study of the long-term effects of ACEs which has the potential to open multidisciplinary approaches to studying and improving human well-being. Current practices of medicine and public health are fragmented by categorical funding, organizational boundaries, and a symptom-based system of medical care. Prevention and remediation of our nation’s leading health and social problems is likely to benefit from understanding that many of these problems tend to be co-morbid and may have common origins in the enduring neurodevelopmental consequences of abuse and related adverse experiences during childhood.
Acknowledgments
The ACE study was supported under cooperative agreement #TS-44–10/11 from the CDC through the Association of Teachers of Preventive Medicine and a grant from the Garfield Memorial Fund.
Contributor Information
Robert F. Anda, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Adult and Community Health, 4770 Buford Highway, N.E., MS K-67, Atlanta, Georgia 30341-3717, USA.
Vincent J. Felitti, Dept. of Preventive Medicine, Southern California Permanente Medical Group (Kaiser Permanente), San Diego, CA, USA.
J. Douglas Bremner, Depts. of Psychiatry and Radiology, Emory Center for Positron Emission Tomography, Emory University School of Medicine, Atlanta, GA, Atlanta VA Medical Center, Decatur, GA, USA.
John D. Walker, Dept. of State Health Services, Texas Health and Human Services Commission, Austin, TX, USA.
Charles Whitfield, Private Practice in Addiction and Trauma Medicine, Atlanta, GA, USA.
Bruce D. Perry, The Child Trauma Academy, Houston, TX, USA; Ministry of Children’s Services, Alberta, Canada.
Shanta R. Dube, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Adult and Community Health, 4770 Buford Highway, N.E., MS K-67, Atlanta, Georgia 30341-3717, USA.
Wayne H. Giles, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Adult and Community Health, 4770 Buford Highway, N.E., MS K-67, Atlanta, Georgia 30341-3717, USA.
References
- 1.Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and non-stressful stimuli. J Neurosci. 1987;7:2837–2847. doi: 10.1523/JNEUROSCI.07-09-02837.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anda RF, Chapman DP, Felitti VJ, Edwards VE, Williamson DF, Croft JP, Giles WH. Adverse Childhood Experiences and Risk of Paternity in Teen Pregnancy. Obstet Gynecol. 2002a;100:37–45. doi: 10.1016/s0029-7844(02)02063-x. [DOI] [PubMed] [Google Scholar]
- 3.Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, Giovino GA. Adverse childhood experiences and smoking during adolescence and adulthood. J Am Med Assoc. 1999;282:1652. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- 4.Anda RF, Felitti VJ, Chapman DP, Croft JB, Williamson DF, Santelli J, Dietz PM, Marks JS. Abused boys, battered mothers, and male involvement in teen pregnancy. Pediatrics. 2001;107(2):e19. doi: 10.1542/peds.107.2.e19. [DOI] [PubMed] [Google Scholar]
- 5.Anda RF, Whitfield CL, Felitti VJ, Chapman D, Edwards VJ, Dube SR, Williamson DF. Adverse childhood experiences, alcoholic parents and later risk of alcoholism and depression. Psychiatr Serv. 2002b;53:1001–1009. doi: 10.1176/appi.ps.53.8.1001. [DOI] [PubMed] [Google Scholar]
- 6.Anda RF, Williamson D, Jones D, Macera C, Eaker E, Glassman A, Marks J. Depressed affect, hopelessness and the risk of ischemic heart disease in a cohort of US adults. Epidemiology. 1993;4:285–294. doi: 10.1097/00001648-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Anderson CM, Teicher MH, Polcari A, Renshaw PI. Abnormal T2 relaxation time in the cerebellar vermis of adults sexually abused in childhood: potential role of the vermis in stress-enhanced risk for drug abuse. Psychoneuroendocrinology. 2002;27:231–244. doi: 10.1016/s0306-4530(01)00047-6. [DOI] [PubMed] [Google Scholar]
- 8.Beers SR, De Bellis MD. Neuropsychological function in children with maltreatment-related posttraumatic stress disorder. Am J Psychiatry. 2002;159(3):483–486. doi: 10.1176/appi.ajp.159.3.483. [DOI] [PubMed] [Google Scholar]
- 9.Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- 10.Bremner JD. Long-term effects of childhood abuse on brain and neurobiology. Child Adolesc Psychiatr Clin North Am. 2003a;12:271–292. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 11.Bremner JD. Understanding trauma-based disorders from a neurological perspective. New York: Norton; 2003b. Does Stress Damage the Brain? [Google Scholar]
- 12.Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I Preclinical studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bremner JD, Randall P, Scott TM, Capelli S, Delaney R, McCarthy G, Charney DS. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res. 1995;59:97–107. doi: 10.1016/0165-1781(95)02800-5. [DOI] [PubMed] [Google Scholar]
- 15.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic image resonance-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bremner JD, Southwick M, Darnell MA, Charney DS. Chronic PTSD in Vietnam combat veterans: Course of illness and substance abuse. Am J Psychiatry. 1996b;153:369–375. doi: 10.1176/ajp.153.3.369. [DOI] [PubMed] [Google Scholar]
- 17.Bremner JD, Vermetten E. Stress and development: behavioral and biological consequences. Dev Psychopathol. 2001;13:473–489. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- 18.Bremner JD, Vythilingam M, Anderson G, Vermetten E, McGlashan T, Heninger G, Rasmusson A, Southwick SM, Charney DS. Assessment of the hypothalamic-pituitary-adrenal (HPA) axis over a 24-hour diurnal period and in response to neuroendocrine challenges in women with and without early childhood sexual abuse and posttraumatic stress disorder (PTSD) Biol Psychiatry. 2003;54:710–718. doi: 10.1016/s0006-3223(02)01912-1. [DOI] [PubMed] [Google Scholar]
- 19.Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, Afzal N, McGlashan T, Elzinga B, Anderson GM, Heninger G, Southwick SM, Charney DS. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28:733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- 20.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Duncan JS, Charney DS. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 21.Brodsky BS, Malone KM, Ellis SP, Dulit RA, Mann JJ. Characteristics of borderline personality disorder associated with suicidal behavior. Am J Psychiatry. 1997;154:1715–1719. doi: 10.1176/ajp.154.12.1715. [DOI] [PubMed] [Google Scholar]
- 22.Brown D. (Mis)representations of the long-term effects of childhood sexual abuse in the courts. J Child Sex Abuse. 2001;9:79–107. doi: 10.1300/j070v09n03_05. [DOI] [PubMed] [Google Scholar]
- 23.Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 24.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci. USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrion VG, Steiner H. Trauma and dissociation in delinquent adolescents. J Am Acad Child Adolesc Psychiatry. 2000;39:353–359. doi: 10.1097/00004583-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 27.Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. J Biol Psychiatry. 2002;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- 28.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 29.CDC. Annual smoking-attributable mortality, years of potential life lost, and economic costs – United States, 1995–1999. MMWR. 2002;51:300–303. [PubMed] [Google Scholar]
- 30.Chapman DP, Anda RF, Felitti VJ, Dube SR, Edwards VJ, Whitfield CL. Epidemiology of adverse childhood experiences and depressive disorders in a large health maintenance organization population. J Affect Disord. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Nat Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. AE Bennett Research Award. Developmental traumatology. Part I: Biological stress systems. Biol Psychiatry. 1999;15(45):1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 33.De Bellis MD, Baum AS, Birmaher B, Ryan ND. Urinary catecholamine excretion in childhood overanxious and post-traumatic stress disorders. Ann NY Acad Sci. 1997;821:451–455. doi: 10.1111/j.1749-6632.1997.tb48303.x. [DOI] [PubMed] [Google Scholar]
- 34.DeBellis MD, Chrousos GP, Dorn LD, Burke L, Helmers K, Kling MA, Trickett PK, Putnam FW. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. J Clin Endocrinol Metab. 1994;78:249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- 35.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. AE Bennett Research Award. Developmental traumatology. Part II: Brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 36.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 37.De Bellis M, Thomas L. Biologic findings of post-traumatic stress disorder and child maltreatment. Curr Psychiatry Rep. 2003;5:108–117. doi: 10.1007/s11920-003-0027-z. [DOI] [PubMed] [Google Scholar]
- 38.DellaFemina D, Yeager CA, Lewis DO. Child abuse: adolescent records vs. adult recall. Child Abuse Negl. 1990;14:227–231. doi: 10.1016/0145-2134(90)90033-p. [DOI] [PubMed] [Google Scholar]
- 39.Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–402. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- 40.Diamond DM, Fleshner M, Ingersoll N, Rose GM. Psychological stress impairs spatial working memory: relevance to electrophysiological studies of hippocampal function. Behav Neurosci. 1996;110:661–672. doi: 10.1037//0735-7044.110.4.661. [DOI] [PubMed] [Google Scholar]
- 41.Dietz PM, Spitz AM, Anda RF, Williamson DF, McMahon PM, Santelli JS, Nordenberg DF, Felitti VJ, Kendrick JS. Unintended pregnancy among adult women exposed to abuse or household dysfunction during their childhood. J Am Med Assoc. 1999;282:1359–1364. doi: 10.1001/jama.282.14.1359. [DOI] [PubMed] [Google Scholar]
- 42.Dong M, Anda RF, Felitti VJ, Dube SR, Giles WH. The relationship of exposure to childhood sexual abuse to other forms of abuse, neglect and household dysfunction during childhood. Child Abuse Negl. 2003a;27:625–639. doi: 10.1016/s0145-2134(03)00105-4. [DOI] [PubMed] [Google Scholar]
- 43.Dong M, Dube SR, Felitti VJ, Giles WH, Anda RF. Adverse childhood experiences and self-reported liver disease: new insights into a causal pathway. Arch Int Med. 2003b;163:1949–1956. doi: 10.1001/archinte.163.16.1949. [DOI] [PubMed] [Google Scholar]
- 44.Dube SR, Anda RF, Felitti VJ, Chapman D, Williamson DF, Giles WH. Childhood abuse, household dysfunction and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. J Am Med Assoc. 2001;286:3089–3096. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- 45.Dube SR, Anda RF, Felitti VJ, Croft JB, Edwards VJ, Giles WH. Growing up with Parental alcohol abuse: Exposure to childhood abuse, neglect and household dysfunction. Child Abuse Negl. 2001;25:1627–1640. doi: 10.1016/s0145-2134(01)00293-9. [DOI] [PubMed] [Google Scholar]
- 46.Dube SR, Anda RF, Felitti VJ, Edwards VJ, Croft JB. Adverse childhood experiences and personal alcohol abuse as an adult. Addict Behav. 2002a;27:713–725. doi: 10.1016/s0306-4603(01)00204-0. [DOI] [PubMed] [Google Scholar]
- 47.Dube SR, Anda RF, Felitti VJ, Edwards VJ, Williamson DF. Exposure to abuse, neglect and household dysfunction among adults who witnessed intimate partner violence as children. Violence Vict. 2002b;17:3–17. doi: 10.1891/vivi.17.1.3.33635. [DOI] [PubMed] [Google Scholar]
- 48.Dube SR, Felitti VJ, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect and household dysfunction and the risk of illicit drug use: the Adverse Childhood Experience Study. Pediatrics. 2003a;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- 49.Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. 2003b;37:268–277. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 50.Dube SR, Williamson DF, Thompson T, Felitti VJ, Anda RF. Assessing the Reliability of Retrospective Reports of Adverse Childhood Experiences Among Adult HMO Members Attending a Primary Care Clinic. Child Abuse Negl. 2004;28(7):729–737. doi: 10.1016/j.chiabu.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, Osterheider M, Petersen D. Magnetic resonance imaging volumes of the hippocampus and the Amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 52.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 53.Edwards VJ, Anda RF, Felitti VJ, Dube SR. In: Adverse childhood experiences and health-related quality of life as an adult. Kendall-Tackett K, editor. Victimization and Health, American Psychological Association; 2003a. pp. 81–94. [Google Scholar]
- 54.Edwards VJ, Anda RF, Nordenberg DF, Felitti VJ, Williamson DF, Wright JA. Bias assessment or child abuse survey: factors affecting probability of response to a survey about childhood abuse. Child Abuse Negl. 2001;25:307–312. doi: 10.1016/s0145-2134(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 55.Edwards VJ, Fivush R, Anda RF, Felitti VJ, Nordenberg DF. Autobiographical memory disturbances in childhood abuse survivors. In: Freyd JJ, DePrince AP, editors. Trauma and Cognitive Science: A meeting of minds, science, and human experience. Binghamton, NY: Haworth Press; 2001. Also published in Aggression, Maltreatment, and Trauma. [Google Scholar]
- 56.Edwards VJ, Holden GW, Anda RF, Felitti VJ. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the Adverse Childhood Experiences (ACE) Study. Am J Psychiatry. 2003b;160:1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- 57.El-Sheikh M, Harger J, Whitson S. Exposure to interparental conflict and children’s adjustment and physical health: the moderating role of vagal tone. Child Dev. 2001;72:1617–1636. doi: 10.1111/1467-8624.00369. [DOI] [PubMed] [Google Scholar]
- 58.Elzinga BM, Schmahl CG, Vermetten E, van Dyck R, Bremner JD. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28:1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- 59.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 60.Finkelhor D, Hotaling G, Lewis IA, Smith C. Sexual abuse in a national survey of adult men and women: prevalence, characteristics, and risk factors. Child Abuse Negl. 1990;14:19–28. doi: 10.1016/0145-2134(90)90077-7. [DOI] [PubMed] [Google Scholar]
- 61.Foote SL, Bloom E, Aston-Jones G. Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol Behav. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 62.Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor-norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry. 1999;46:1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- 63.Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopression (V1a) receptors: gender differences. J Neuroendocrinol. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- 64.Glaser D. Child abuse and neglect and the brain: a review. J Child Psychol Psychiatry. 2000;41:97–116. [PubMed] [Google Scholar]
- 65.Gorman JM, Mathew S, Coplan J. Neurobiology of early life stress: nonhuman primate models. Clin Neuropsychiatry. 2002;7:96–103. doi: 10.1053/scnp.2002.31784. [DOI] [PubMed] [Google Scholar]
- 66.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999a;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 67.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999b;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 69.Gutman D, Nemeroff CB. Neurobiology of early life stress: rodent studies. Semin Clin Neuropsychiatry. 2002;7:89–95. doi: 10.1053/scnp.2002.31781. [DOI] [PubMed] [Google Scholar]
- 70.Heffernan K, Cloitre M, Tardiff K, Marzuk PM, Portera L, Leon AC. Childhood trauma as a correlate of lifetime opiate use in psychiatric patients. Addict Behav. 2000;25:797–803. doi: 10.1016/s0306-4603(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 71.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 72.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 73.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. J Am Med Assoc. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 74.Hillis SD, Anda RF, Dube SR, Felitti VJ, Marchbanks PA, Marks JS. The association between adolescent pregnancy, longterm psychosocial outcomes, and fetal death. Pediatrics. 2004;113:320–327. doi: 10.1542/peds.113.2.320. [DOI] [PubMed] [Google Scholar]
- 75.Hillis SD, Anda RF, Felitti VJ, Marchbanks PA. Adverse childhood experiences and sexual risk behaviors in women: a retrospective cohort study. Fam Plan Perspect. 2001;33:206–211. [PubMed] [Google Scholar]
- 76.Hillis SD, Anda RF, Felitti VJ, Nordenberg D, Marchbanks PA. Adverse childhood experiences and sexually transmitted diseases in men and women: a retrospective study. Pediatrics. 2000;106:E11. doi: 10.1542/peds.106.1.e11. [DOI] [PubMed] [Google Scholar]
- 77.Insel TR, Winslow J. Serotonin and neuropeptides in affiliative behaviors. Biol Psychiatry. 1998;44:207–219. doi: 10.1016/s0006-3223(98)00094-8. [DOI] [PubMed] [Google Scholar]
- 78.Ito Y, Teicher MH, Glod CA, Ackerman E. Preliminary evidence for aberrant cortical development in abused children: a quantitative EEG study. J Neuropsychiatry Clin Neurosci. 1998;10:298–307. doi: 10.1176/jnp.10.3.298. [DOI] [PubMed] [Google Scholar]
- 79.Kaplan JR, Manuck SB, Clarkson TB, Luss M. Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1982;2:359–368. doi: 10.1161/01.atv.2.5.359. [DOI] [PubMed] [Google Scholar]
- 80.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 81.Kendall-Tackett KA, Williams LM, Finkelhor D. Impact of sexual abuse on children: a review and synthesis of recent empirical studies. Psychol Bull. 1993;113:164–180. doi: 10.1037/0033-2909.113.1.164. [DOI] [PubMed] [Google Scholar]
- 82.Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance abuse disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- 83.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 84.Kingree JB, Thompson MP, Kaslow NJ. Risk factors for suicide attempts among low-income women with a history of alcohol problems. Addict Behav. 1999;24:583–587. doi: 10.1016/s0306-4603(98)00109-9. [DOI] [PubMed] [Google Scholar]
- 85.Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal separation. Endocrinology. 1996;137:1212–1218. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- 86.Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: Evidence for chronic neuroendocrine activation in women. Psychosom Med. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 87.Lilienfeld SO. Comorbidity between and within childhood externalizing and internalizing disorders: reflections and directions. J Abn Child Psychol. 2003;32:285–291. doi: 10.1023/a:1023229529866. [DOI] [PubMed] [Google Scholar]
- 88.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic- pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 89.Lo Re V, Bellini LM. William of Occam and Occam’s razor (letter) Ann Int Med. 2002;136:634–635. doi: 10.7326/0003-4819-136-8-200204160-00022. [DOI] [PubMed] [Google Scholar]
- 90.Luine V, Villegas M, Mortinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 91.MacMillan HL, Fleming JE, Trocme N, Boyle MH, Wong M, Racine YA, Beardslee WR, Offord DR. Prevalence of child physical and sexual abuse in the community: results from the Ontario Health Supplement. J Am Med Assoc. 1997;278:131–135. [PubMed] [Google Scholar]
- 92.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McEwen BS, Angulo J, Cameron H, Chao HM, Daniels D, Gannon MN, Gould E, Mendelson S, Sakai R, Spencer R. Paradoxical effects of adrenal steroids on the brain: Protection versus degeneration. Biol Psychiatry. 1992;31:177–199. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- 94.McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Int Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 95.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 96.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Osofsky JD. The impact of violence on children. Future Child. 1999;99:33–49. [PubMed] [Google Scholar]
- 98.Perry BD. Neurobiological Sequelae of Childhood Trauma: Post Traumatic Stress Disorders in Children. In: Murburg M, editor. Catecholamine Function in Post Traumatic Stress Disorder: Emerging Concepts. Washington, DC: American Psychiatric Press; 1994. pp. 253–276. [Google Scholar]
- 99.Perry BD. The neurodevelopmental impact of violence in childhood. In: Schetky D, Benedek EP, editors. Textbook of Child and Adolescent Forensic Psychiatry. Washington, DC: American Psychiatric Press, Inc; 2001. pp. 221–238. [Google Scholar]
- 100.Perry BD. Childhood experience and the expression of genetic potential: What childhood neglect tells us about nature and nurture. Brain Mind. 2002;3:79–100. [Google Scholar]
- 101.Perry BD, Pollard R. Homeostasis, stress, trauma, and adaptation. A neurodevelopmental view of childhood trauma. Child Adolesc Psychiatr Clin North Am. 1998;7:33–51. viii. [PubMed] [Google Scholar]
- 102.Pinchus JH, Tucker GJ. Behavioral Neurology. New York: Oxford; 1978. [Google Scholar]
- 103.Plotsky PM, Meaney MJ. Early postnatal experience alters hypothalamic-corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced releasing in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 104.Putnam FW. Developmental pathways in sexually abused girls. Presented at Psychological Trauma: Maturational Processes and Psychotherapeutic interventions; Harvard Medical School; March 20; Boston MA. 1998. [Google Scholar]
- 105.Putnam FW. Ten-year research update review; child sexual abuse. J Am Acad Child Adolesc Psychiatry. 2003;4:269–278. doi: 10.1097/00004583-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 106.Read J, Perry BD, Moskowith A, Connolloy J. The contribution of early traumatic events to schizophrenia in some patients: a traumagenic neurodevelopmental model. Psychiatry. 2001;64:319–345. doi: 10.1521/psyc.64.4.319.18602. [DOI] [PubMed] [Google Scholar]
- 107.Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- 108.Rohsenow DJ, Corbett R, Devine D. Molested as children: a hidden contribution to substance abuse? J Subst Abuse Treat. 1988;5:13–18. doi: 10.1016/0740-5472(88)90032-3. [DOI] [PubMed] [Google Scholar]
- 109.Rosenblum L, Coplan J, Friedman S, Bassoff T, Gormen JM, Andrews MW. Adverse early experiences affect noradrenergic and serotonergic functioning in adult primates. Biol Psychiatry. 1994;35:221–227. doi: 10.1016/0006-3223(94)91252-1. [DOI] [PubMed] [Google Scholar]
- 110.Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia. PA: Lippincott Raven Publications; 1998. [Google Scholar]
- 111.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 112.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 113.Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- 114.Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scaer RC. The neurophysiology of dissociation and chronic disease. Appl Psychophysiol Biofeedback. 2001;26:73–91. doi: 10.1023/a:1009571806136. [DOI] [PubMed] [Google Scholar]
- 116.Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 2003;122:193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 117.Schoenborn CA. Exposure to alcoholism in the family: United States, 1988. Adv Data. 1991;205:1–13. [PubMed] [Google Scholar]
- 118.Shin LH, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am J Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 119.Smith MA, Kim SY, van Oers HJ, Levine S. Maternal deprivation and stress induce immediate early genes in the infant rat brain. Endocrinology. 1997;138:4622–4628. doi: 10.1210/endo.138.11.5529. [DOI] [PubMed] [Google Scholar]
- 120.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:1–9. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 121.Straus M, Gelles J. Physical Violence in American Families: Risk Factors and Adaptations to Violence in 8,145 Families. New Brunswick, NJ: Transaction Press; 1990. [Google Scholar]
- 122.Teicher MH. Wounds that time wouldn’t heal: the neurobiology of childhood abuse. Cerebrum. 2000;2:50–67. [Google Scholar]
- 123.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatr Clin North Am. 2002;25:397–426. vii–viii. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 124.Teicher MH, Ito Y, Glod CA, Andersen SL, Dumont N, Ackerman E. Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Ann N Y Acad Sci. 1997;821:160–175. doi: 10.1111/j.1749-6632.1997.tb48277.x. [DOI] [PubMed] [Google Scholar]
- 125.Teicher MH, Ito Y, Glod CA, Schiffer F, Ackerman E. Possible effects of early abuse on human brain development, as assessed by EEG coherence. Am J Neuropsychopharmacol. 1994;33:52. [Google Scholar]
- 126.Valenstein ES. Blaming the Brain: The Truth About Drugs and Mental Health. Basic Books, NY: 1998. [Google Scholar]
- 127.van der Kolk BA, Perry JC, Herman JL. Childhood origins of self-destructive behavior. Am J Psychiatry. 1991;148:1665–1671. doi: 10.1176/ajp.148.12.1665. [DOI] [PubMed] [Google Scholar]
- 128.van Reekum R, Streiner DL, Conn DK. Applying Bradford Hill’s criteria for causation to neuropsychiatry: challenges and opportunities. J Neuropsychiatry Clin Neurosci. 2001;12:318–325. doi: 10.1176/jnp.13.3.318. [DOI] [PubMed] [Google Scholar]
- 129.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vythilingam M, Heim C, Newport CD, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Whitfield CL, Anda RF, Dube SR, Felitti VJ. Violent childhood experiences and the risk of intimate partner violence in adults: assessment in a large health maintenance organization. J Interpersonal Violence. 2003a;18:166–185. [Google Scholar]
- 132.Whitfield CL. The Truth about Depression: Choices for Healing. Deerfield Beach, FL: Health Communications; 2003b. [Google Scholar]
- 133.Williams LM. Recovered memories of abuse in women with documented child sexual victimization histories. J Trauma Stress. 1995;8:649–673. doi: 10.1007/BF02102893. [DOI] [PubMed] [Google Scholar]
- 134.Williamson DF, Thompson TJ, Anda RF, Dietz WH, Felitti VJ. Body weight, obesity, and self-reported abuse in childhood. Int J Obesity. 2002;26:1075–1082. doi: 10.1038/sj.ijo.0802038. [DOI] [PubMed] [Google Scholar]
- 135.Wyatt GE. The sexual abuse of Afro-American and white-American women in childhood. Child Abuse Negl. 1985;9:507–519. doi: 10.1016/0145-2134(85)90060-2. [DOI] [PubMed] [Google Scholar]